Abstract
This study examined whether human bone marrow mesenchymal stromal/stem cells (BMMSCs) could alleviate the secondary pathology in the thalamus after middle cerebral artery occlusion (MCAO) in rats. Atypical accumulation of both amyloid-β (Aβ) and calcium in the thalamus was significantly higher in rats receiving the BMMSCs infusion 48 hours after MCAO as compared with the vehicle MCAO group. The elevated Aβ/calcium accumulation correlated with the level of impaired sensorimotor function. Although secondary pathology in the thalamus seems to be rodent specific, it needs to be taken into account because it may impair long-term behavioral recovery and negate therapeutic treatment effects.
Keywords: β-amyloid, calcium, cell therapy, cerebral ischemia, secondary pathology
Introduction
At present therapeutic options in stroke are extremely limited and thus much effort is being directed at developing innovative treatments, such as cell therapies. In particular, intravascular cell therapy is a promising approach for the treatment of stroke and is claimed to achieve an efficient and wide cell engraftment in case of large or multiple lesions.1 Since it is relatively noninvasive, application of this approach would be feasible in stroke therapy.
We have previously shown that intravenous infusion of human umbilical cord blood cells in rats after middle cerebral artery occlusion (MCAO) results in the accumulation of cells primarily in the lungs.2 The lung entrapment can be circumvented by intraarterial cell infusion, which can target cells to the ischemic brain as shown by intraarterial infusion of human bone marrow-derived stem cells (BMMSCs).3
The most effective therapeutic time window for intraarterial cell transplantation is not known. Acute cell infusion after stroke has been postulated to be neuroprotective, whereas cell delivery at later time points (>24 hours) may enhance brain repair mechanisms.4 Late cell delivery is also thought to target the delayed secondary degeneration occurring in remote areas of the brain such as the ventroposterolateral and ventroposteromedial thalamic nuclei.5, 6 In most animal models, the thalamus is spared from the acute ischemic damage but the region is affected because it has synaptic connections with the primary injury site. The widespread corticostriatal damage results in retrograde and anterograde degeneration,7 which is also associated with atypical accumulation of amyloid precursor protein (APP) and amyloid-β (Aβ) in the thalamus.6 The initially diffuse APP and Aβ staining becomes later transformed into dense plaque-like deposits in the thalamus. Interestingly, the APP/Aβ depositions show an overlapping distribution with calcium deposits.8
The secondary pathology in the thalamus takes place in a delayed manner after the ischemic event and thus represents a novel target for stroke therapy. The aim of the present work was to study whether human BMMSCs (1 × 106) infused 2 or 7 days after MCAO could provide neuronal and/or axonal support and prevent the secondary pathology in the thalamus.
Materials and methods
Animals
Sixty male Han:Wistar rats (Harlan, Rehovot, Israel) weighing 313 to 426 g were used in this study. The rats were housed individually in a controlled environment (humidity 50% to 60%, temperature 21±1°C, 12:12 hours light/dark conditions) with free access to fresh water and food. Animals were handled according to European legislation (86/609/EEC) and all efforts were made to minimize the number used and animal suffering, by adhering to the ARRIVE guidelines. The Animal Ethics Committee (Hämeenlinna, Finland) approved this study.
Transient Middle Cerebral Artery Occlusion
The intraluminal filament technique was used to evoke cerebral ischemia in the rats.9 Anesthesia was induced by inhalation of 5% halothane in 30% O2/70% N2O for 2 to 3 minutes and maintained at 0.5% to 1.5% with a nose mask. Briefly, after a midline neck incision, the right common carotid artery, internal carotid artery, and external carotid artery (ECA) were exposed. A heparinized nylon filament (diameter Ø 0.28 mm) was introduced via the ECA stump into the internal carotid artery until resistance was experienced (1.8 to 2.1 cm). After a 60-minute occlusion period, the filament was gently removed and the ECA was carefully closed by electro-coagulation, leaving a long ECA stump for cell infusion.3
Total Infarct Volume
Magnetic resonance imaging was performed on postoperative days 1 and 43 with a Bruker 7 T horizontal scanner (Bruker, Ettlingen, Germany) used for imaging. The ImageJ program was used to analyze infarct volumes from T2-weighted images.10
Study Design and Experimental Groups
Two rats did not meet pretraining criteria and were excluded. Five of the operated rats died. Twenty-four hours after surgery, behavioral impairment of the remaining rats was assessed by the forelimb placing test and then the animals were subjected to magnetic resonance imaging. Twelve rats were excluded because of minor behavioral impairment, the presence of hemorrhage, a very large lesion, or no cortical damage. The exclusion/inclusion criteria were defined before the experiment. The rest of the animals were sequentially assigned into the following experimental groups such that the extent of behavioral impairment and infarct size did not differ between groups: SHAM+vehicle (n=11), MCAO+vehicle (n=12), MCAO+48 hours BMMSC (n=11), and MCAO+7 days BMMSC (n=7). The cryopreserved and thawed p2 BMMSCs (1 × 106) in 500 μL saline were infused over a period of 2 minutes into anesthetized rats directly into the stump of the ECA.3
Sticky Label Test
The sticky label test was used to evaluate sensory function and motor learning, being performed as previously described.10 Behavioral testing was performed in a blinded manner. Before testing, the animals were familiarized with handling and the testing cage. In the test, a white colored circular label (Ø 9 mm, Tough-Spots, Diversified Biotech, Dedham, MA, USA) was placed on the distal–radial region of both wrists and rat was moved to a test cage. The time until the first contact to the label and the time to remove the label were assessed as the total time. A maximum time of 120 seconds was set if the rat was not able to remove the label.
Histology
On postoperative day 43, rats were anesthetized with an overdose of Equithesin (intraperitoneally) followed by perfusion with 0.9% NaCl and 4% paraformaldehyde in 0.1 mol/L phosphate buffer. The brains were carefully removed from the skull, postfixed and cryoprotected. Brain sections (35 μm) were cut using a sliding microtome and stored in antifreeze solution at −20°C. To assess the thalamic accumulation of Aβ, immunohistochemistry using rabbit anti-rodent antibody (Aβ3-16, #9151, Covance, Dedham, MA, USA) was performed as previously described.6 Calcium accumulation in the thalamus after MCAO was assessed by the Alizarin red method.8
Analysis
Stained brain sections were analyzed in a blind manner with the Adobe Photoshop CC software (San Jose, CA, USA). Images were captured under x5 magnification using Axio Imager.M2 upright microscope (Carl Zeiss GmbH, Göttingen, Germany) equipped with AxioCam ERc5s camera. In the analyses, the images were merged together, converted into gray scale, the thalamus was outlined and area with staining intensity above the threshold (background) was extracted and measured.
Statistics
The statistical analyses were performed with the SPSS software (IBM, Armonk, NY, USA). Statistical differences in Aβ and calcium staining between groups were analyzed using one-way analysis of variance with LSD post hoc test, if needed. Correlations between sticky label test and Aβ and calcium deposition and total infarct size were determined using Pearson correlation coefficients.
Results
Infarct Size
Variable degrees of cortical infarction usually along with complete striatal damage were observed in MCAO rats. There were no differences in the total infarct size detected between the MCAO groups (100.8±12.7 mm3 in vehicle, 94.5±16.6 mm3 in 48h cells, and 112.4±20.3 mm3 in 7day cells).
Amyloid-β Accumulation in the Ipsilateral Thalamus
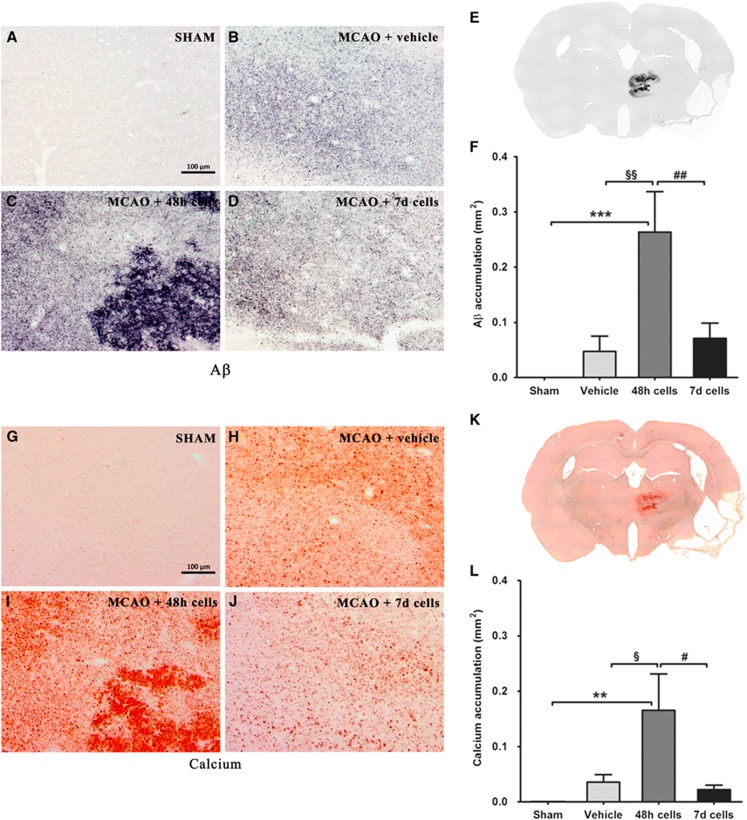
We used a rodent-specific antibody (Aβ3-16) to analyze the deposition of Aβ in the brains of the experimental animals. The antibody revealed Aβ-positive deposits varying in size from small, diffuse granules to large formation (Figures 1B to 1E). There was a significant overall group effect in the Aβ load (F3,32=6.481, P<0.01). Quantification of the area of the deposits showed a highly significant increase in Aβ deposition in the thalamus after infusion of BMMSCs at 48 hours after MCAO (Figure 1F).
Figure 1.
High-power photomicrographs demonstrating atypical amyloid-β (Aβ) (B to D) and calcium (H to J) deposits in the thalamus after middle cerebral artery occlusion (MCAO). No staining was observed in the sham-operated rats (A and G). Only the thalamus was affected (E and K). Semiquantitative analysis revealed a significant increase of Aβ (F) and calcium (L) staining in the ipsilateral thalamus 43 days after MCAO in the group receiving cells 48 hours after occlusion as compared with the other MCAO groups. Data are represented as mean±s.e.m. Statistical significances: §,#P<0.05; **,§§,##P<0.01; ***P<0.001. Scale bar: 100 μm.
Calcium Accumulation in the Ipsilateral Thalamus
Adjacent brain sections were stained for calcium with the Alizarin red method. Calcium staining showed an overlapping distribution pattern in the thalamus as Aβ deposition (Figures 1H to 1K). Also in the case of the calcium load, there was a significant overall group effect (F3,32=3.595, P<0.05), the levels being elevated by infusion of BMMSCs at 48 hours after MCAO (Figure 1L).
Correlations between Amyloid-β/Calcium and Sensorimotor Functions
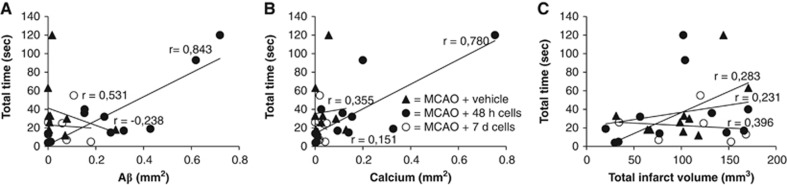
In rats treated with cells 48 hours after MCAO, there was a clear correlation between impaired forelimb performance on postoperative day 42 and Aβ (Figure 2A) and calcium (Figure 2B) accumulation in the thalamus. The total time (the time to first contact plus the removal time) correlated with the levels of accumulation of both Aβ (r=0.843, P<0.001) and calcium (r=0.780, P<0.01), i.e., more prominent behavioral impairments were associated with more severe thalamic pathology. No such correlations were found in the other MCAO groups. There were no significant correlations between total infarct size, Aβ/calcium load in the thalamus or total time in the sticky label test (Figure 2C).
Figure 2.
Correlations between total removal time in the sticky label test and amyloid-β (Aβ) (A) and calcium (B) load in the thalamus and total infarct size (C) after middle cerebral artery occlusion (MCAO) in rats. Correlation coefficients are given separately for ischemic rats treated with vehicle and cells 48 hours or 7 days after occlusion.
Discussion
As far as we are aware, this is the first study to have investigated the effect of cell therapy on delayed secondary pathology after focal cerebral ischemia. We examined whether intraarterial infusion of human BMMSCs 48 hours or 7 days after MCAO could prevent the secondary pathology in the rat thalamus. To our surprise, we observed an exacerbation of Aβ/calcium aggregation in the thalamus, particularly when the cells were infused 48 hours after the MCAO, which may impair long-term behavioral recovery and mask potentially beneficial therapeutic effects.
This phenomenon was independent of primary lesion size, but one cannot completely exclude the possibility that there were secondary lesions caused by BMMSC entrapment in small capillaries. Instead, it is speculated that altered APP expression, maturation, and trafficking may contribute to the present results. Two days after MCAO, APP expression and processing is enhanced in the perilesional cortex,11 which may be a cellular response to stress exerting neurotrophic and protective functions.12 Perilesional APP/Aβ labeling disappears with longer follow-up (≥7 days).6 In addition, APP maturation is enhanced during the acute phase, indicative of elevations in APP trafficking from the perilesional cortex via the descending axons to the thalamus.11 Thus, after effective cell homing to ischemic hemisphere after intraarterial infusion,3 it may be that the presence of BMMSCs at this particular time point (48 hours) further activates perilesional corticothalamic neurons, resulting in Aβ and calcium overflow in the thalamus in turn worsening the secondary pathology. However, one should note that this phenomenon is seen only in rodent stroke models and thus should not affect patient studies.13
An exacerbation of the secondary pathology in the thalamus is likely to have detrimental consequences. This is supported by studies showing treatments that can reduce Aβ accumulation in the thalamus, e.g., a nonselective calcium channel blocker (bepridil), are associated with an improved behavioral outcome in MCAO rats14 and furthermore that thalamus pathology is linked with the late sensory deficit in MCAO rats.15 The present study is also consistent with this concept, i.e., the more prominent the sensorimotor impairment, the more severe the thalamic pathology. More importantly, the intensified secondary pathology shown here may mask the therapeutic benefits provided by cell therapy. Indeed, in our previous study, administration of BMMSCs did not improve the behavioral recovery in MCAO rats.10
The thalamus is often overlooked in experimental studies of ischemia. Nonetheless, this region represents a potential target for therapeutic interventions since it provides an extended time window for delivery. The present results emphasize the importance of timing of such approaches. While subacute cell therapy (48 hours) may enhance early brain repair after focal cerebral ischemia, it also seems to result in a paradoxical exacerbation of the secondary pathology. This unexpected effect in the rat MCAO model should be taken into consideration when studying the optimal therapeutic window as well as in assessments of the efficacies of cell therapies for stroke.
In summary, the lack of treatment effects in MCAO rats may be attributable to the abnormal accumulation of Aβ and calcium in the thalamus, impairing sensory information flow and behavioral recovery after subacute BMMSC treatment, thus complicating the assessment of cell therapies in rodent stroke models.
Acknowledgments
The authors thank Joonas Khabbal, Pasi Miettinen, Elina Hämäläinen, and Lotta Sankkila for their excellent technical assistance.
The authors declare no conflict of interest.
Footnotes
This study was supported by the Health Research Council of the Academy of Finland.
References
- Hicks A, Jolkkonen J. Challenges and possibilities of intravascular cell therapy in stroke. Acta Neurobiol Exp (Warsz) 2009;69:1–11. doi: 10.55782/ane-2009-1724. [DOI] [PubMed] [Google Scholar]
- Mäkinen S, Kekarainen T, Nystedt J, Liimatainen T, Huhtala T, Närvänen A, et al. Human umbilical cord blood cells do not improve sensorimotor or cognitive outcome following transient middle cerebral artery occlusion in rats. Brain Res. 2006;1123:207–215. doi: 10.1016/j.brainres.2006.09.056. [DOI] [PubMed] [Google Scholar]
- Mitkari B, Kerkelä E, Nystedt J, Korhonen M, Mikkonen V, Huhtala T, et al. Intra-arterial infusion of human bone marrow-derived mesenchymal stem cells results in transient localization in the brain after cerebral ischemia in rats. Exp Neurol. 2013;239:158–162. doi: 10.1016/j.expneurol.2012.09.018. [DOI] [PubMed] [Google Scholar]
- Vu Q, Xie K, Eckert M, Zhao W, Cramer SC. Meta-analysis of preclinical studies of mesenchymal stromal cells for ischemic stroke. Neurology. 2014;82:1277–1286. doi: 10.1212/WNL.0000000000000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iizuka H, Sakatani K, Young W. Neural damage in the rat thalamus after cortical infarcts. Stroke. 1990;21:790–794. doi: 10.1161/01.str.21.5.790. [DOI] [PubMed] [Google Scholar]
- Van Groen T, Puurunen K, Mäki HM, Sivenius J, Jolkkonen J. Transformation of diffuse beta-amyloid precursor protein and beta-amyloid deposits to plaques in the thalamus after transient occlusion of the middle cerebral artery in rats. Stroke. 2005;36:1551–1556. doi: 10.1161/01.STR.0000169933.88903.cf. [DOI] [PubMed] [Google Scholar]
- Ross DT, Ebner FF. Thalamic retrograde degeneration following cortical injury: An excitotoxic process. Neuroscience. 1990;35:525–550. doi: 10.1016/0306-4522(90)90327-z. [DOI] [PubMed] [Google Scholar]
- Mäkinen S, van Groen T, Clarke J, Thornell A, Corbett D, Hiltunen M, et al. Coaccumulation of calcium and beta-amyloid in the thalamus after transient middle cerebral artery occlusion in rats. J Cereb Blood Flow Metab. 2008;28:263–268. doi: 10.1038/sj.jcbfm.9600529. [DOI] [PubMed] [Google Scholar]
- Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- Mitkari B, Nitzsche F, Kerkelä E, Kuptsova K, Huttunen J, Nystedt J, et al. Human bone marrow mesenchymal stem/stromal cells produce efficient localization in the brain and enhanced angiogenesis after intra-arterial delivery in rats with cerebral ischemia, but this is not translated to behavioral recovery. Behav Brain Res. 2014;259:50–59. doi: 10.1016/j.bbr.2013.10.030. [DOI] [PubMed] [Google Scholar]
- Hiltunen M, Mäkinen P, Peräniemi S, Sivenius J, van Groen T, Soininen H, et al. Focal cerebral ischemia in rats alters APP processing and expression of Abeta peptide degrading enzymes in the thalamus. Neurobiol Dis. 2009;35:103–113. doi: 10.1016/j.nbd.2009.04.009. [DOI] [PubMed] [Google Scholar]
- Mucke L, Abraham CR, Masliah E. Neurotrophic and neuroprotective effects of hAPP in transgenic mice. Ann NY Acad Sci. 1996;777:82–88. doi: 10.1111/j.1749-6632.1996.tb34405.x. [DOI] [PubMed] [Google Scholar]
- Aho L, Jolkkonen J, Alafuzoff I. Beta-amyloid aggregation in human brains with cerebrovascular lesions. Stroke. 2006;37:2940–2945. doi: 10.1161/01.STR.0000248777.44128.93. [DOI] [PubMed] [Google Scholar]
- Sarajärvi T, Lipsanen A, Mäkinen P, Peräniemi S, Soininen H, Haapasalo A, et al. Bepridil decreases Abeta and calcium levels in the thalamus after middle cerebral artery occlusion in rats. J Cell Mol Med. 2012;16:2754–2767. doi: 10.1111/j.1582-4934.2012.01599.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freret T, Chazalviel L, Roussel S, Bernaudin M, Schumann-Bard P, Boulouard M, et al. Long-term functional outcome following transient middle cerebral artery occlusion in the rat: correlation between brain damage and behavioral impairment. Behav Neurosci. 2006;120:1285–1298. doi: 10.1037/0735-7044.120.6.1285. [DOI] [PubMed] [Google Scholar]