Abstract
Background
Tramalol overdose is disproportionately more common in Iran. In recent years, Tramadol overdose has become one of the most common causes of poisoning admissions to emergency departments in this country. To the best of our knowledge, there is little or no information regarding the toxicokinetic properties of Tramadol such as its half life. Given the fact that poisoning management should be based on the toxicokinetic of substances, we aimed at investigating the half life of Tramadol in man as a critical toxicokinetic variable in overdose.
Methods
Blood samples of each patient were collected on admission and repeated later. Plasma was harvested after separation from blood cells by centrifugation and quantified using HPLC method. Calculations were performed on Tramadol blood concentration quantities.
Findings
Demographic: Most of cases were men (81.81%). Mean (Standard Deviation (SD), min-max) age was 23 (8.142, 17-40). Serum Tramadol levels: Mean (SD, min-max) first Tramadol concentration was 786.91 (394.53, 391-1495). Mean (SD, min-max) second Tramadol concentration was 433.09 (269.63, 148-950). Mean (SD, min-max) of Tramadol half life was calculated as 9.24 hour (2.310, 4.99-13.45) Associations: Half life was associated with higher concentrations (r=0.708 Sig=0.015).
Conclusion
We report the mean half life of tramadol in overdose to be 9.24 hours which is remarkably higher than that measured in previous pharmacokinetic studies. We also concluded that Tramadol half life is dose dependent in overdose which may explain the further consequences of severe overdoses.
Keywords: Tramadol, Toxicokinetic, Half-Life, Overdose
Findings
Background
Tramadol is a synthetic, centrally acting analgesic opioid. It is mainly metabolized to O-desmethyl Tramadol (M1), which is also active [1]. Tramadol has been approved in some countries since 1980 and become the most frequently prescribed opioid around the globe [2]. Tramadol is rapidly and almost fully absorbed after single or multiple oral administrations. Nevertheless, the mean absolute bioavailability of Tramadol is reported to be 65–70% which is due to its extensive first-pass hepatic metabolism [3]. The bioavailability rises to >90% with multiple doses. The large volume of distribution (306 liters) after oral administration suggests its high tissue affinity [4]. Although frequently prescribed, few data on kinetic of Tramadol in humans are available. In a study performed on horses, half-life after intravenous and intramuscular administration was 82 ± 10 and 92 ± 14 min, respectively [5]. In the rat, the terminal elimination half-life of Tramadol is approximately 3 hours [6]. The distribution and elimination half-lives in humans were 1.02 and 141.9 min respectively [7].
Medicinal toxicities are common in Iran [8]. In recent years, Tramadol overdose has become one of the most common causes of poisoning admissions to emergency departments in this country [9,10]. The increasing number of Tramadol abuse and overdoses provokes the need for a more profound knowledge about the kinetic/dynamic profile of Tramadol in overdose. Several studies have been performed to measure the pharmacokinetic profile of this drug [11]. However, to the best of our knowledge, there is little information regarding the toxicokinetic properties of Tramadol such as its half life.
We previously reported the clinical manifestations of Tramadol overdose in relation to alleged dose [12]. The unique feature of this study is defining the half life of Tramadol as a critical toxicokinetic variable, in man in overdose.
Method
This prospective cross-sectional study was performed in Imam Reza University Hospital Poison Center, (Mashhad, Iran) [13] from July 2012 to September 2012. Totally, 25 patients who were admitted to the hospital with Tramadol overdose and were confirmed to have ingested more than the recommended therapeutic dose (primarily by urinary test and secondarily by blood test) met the entry requirement. Among them, 11 patients consented to give at least two blood samples. Exclusion criteria were met and the treatment of patients [14,15] was not influenced by the process of data collecting. Ethics approval was obtained from ethics committee of the Mashhad University of Medical Sciences (MUMS/89/1876).
Blood samples, collected in heparinised glass tubes on admission and repeated later were investigated for Tramadol serum concentration using a high-performance liquid chromatographic system (HPLC) (Knauer, Germany) with the method explained in [16]. Intra- and interday variabilities were measured to determine the precision of the HPLC method. The relative standard deviation (RSD) of intraday and interday variations for Tramadol was 1.7% and 1.4% respectively, indicating that the method was repeatable. To measure the Half Life of Tramadol, first order kinetic (Formula: Nt = N0(1/2)t/t1/2) was assumed. Calculations were performed on blood concentration quantities. N0 denotes the first quantity, Nt is the quantity that is remained at the time of t and t1/2 denotes half-life.
All calculations were performed manually with the help of Microsoft® Office Excel 2003 and SPSS version 13.0 (SPSS Inc., Chicago, IL, USA).
Findings
Most of patients (9) were men (81.81%). Mean (Standard Deviation (SD), min-max) age was 23 years (8.142, 17–40). Mean (SD, min-max) first Tramadol concentration was 786.91 mg/dL (394.53, 391–1495). Mean (SD, min-max) second Tramadol concentration was 433.09 mg/dL (269.63, 148–950). Mean time interval from 1st to 2nd blood sampling was 8.23 hours (1.421, 5.5-11). Mean (SD, min-max) of Tramadol half life was calculated as 9.24 hours (2.310, 4.99-13.45). Half life was associated with higher concentrations (r = 0.708 Sig = 0.015) (Figure 1).
Figure 1.
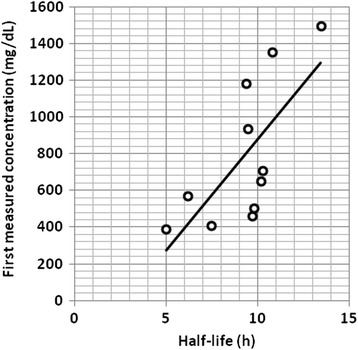
Calculated Tramadol Half-life in relation to first measured blood concentration of Tramadol in overdose patients. Half life is associated with higher concentrations (r = 0.708 Sig = 0.015).
Discussion
In this study, we found the half life of Tramadol to be 9.24 ± 2.310 hours in man in overdose which is comparable to that reported by Ardakani et al. [17]. They demonstrated the half life of Tramadol in healthy humans to be approximately 7 hours. Importantly, we demonstrated that Tramadol half life is dose dependent in overdose which may explain the further consequences of severe overdoses.
Tramadol is mostly metabolised by O- and N-demethylation and conjugation reactions which result in glucuronides and sulphates [18]. The wide variability in the pharmacokinetic properties of Tramadol is partly due to cytochrome P450 (CYP) polymorphism. In fact, Tramadol is mostly metabolized by the highly polymorphic enzyme CYP 2D6, meaning patients with different CYP2D6 genotypes may experience different degrees of pain relief and side effects [19,20]. To obtain more accurate results, we suggest that the toxicokinetic of Tramadol and its metabolites be studied in specific phenotypes. We also believe that in order to determine the definitive toxicokinetic profile of Tramadol, further investigations should be performed on larger groups.
Acknowledgments
This study has been supported by a grant from Mashhad University of Medical Sciences (MUMS). We would like to acknowledge the support of Vice chancellor for Research.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
HK: Lab and clinical work GAT: Data analysis and drafting the manuscript GHD: Lab work SSS: Data collection AA: Data collection RA: Research design, data interpretation, revising the manuscript. All authors read and approved the final manuscript.
Contributor Information
Hamid Khosrojerdi, Email: Hkhosrojerdi7@gmail.com.
Ghazal Alipour Talesh, Email: ghazaal.alipour@gmail.com.
Gholam Hassan Danaei, Email: toxdanay@gmail.com.
Sara Shokooh Saremi, Email: ShokoohS871@mums.ac.ir.
Afrouz Adab, Email: Adaba871@mums.ac.ir.
Reza Afshari, Email: AfshariR@mums.ac.ir.
References
- 1.Raffa RB, Friderichs E, Reimann W, Shank RP, Codd EE, Vaught JL, et al. Complementary and synergistic antinociceptive interaction between the enantiomers of tramadol. J Pharmacol Exp Ther. 1993;267(1):331–340. [PubMed] [Google Scholar]
- 2.Loughrey M, Loughrey C, Johnston S, O’Rourke D. Fatal hepatic failure following accidental tramadol overdose. Forensic Sci Int. 2003;134(2):232–233. doi: 10.1016/S0379-0738(03)00132-4. [DOI] [PubMed] [Google Scholar]
- 3.Gibson TP. Pharmacokinetics, efficacy, and safety of analgesia with a focus on tramadol HCl. Am J Med. 1996;101:S47–S53. doi: 10.1016/S0002-9343(96)00138-6. [DOI] [PubMed] [Google Scholar]
- 4.Lee CR, McTavish D, Sorkin EM. Tramadol. Drugs. 1993;46(2):313–340. doi: 10.2165/00003495-199346020-00008. [DOI] [PubMed] [Google Scholar]
- 5.Shilo Y, Britzi M, Eytan B, Lifschitz T, Soback S, Steinman A. Pharmacokinetics of tramadol in horses after intravenous, intramuscular and oral administration. J Vet Pharmacol Ther. 2008;31(1):60–65. doi: 10.1111/j.1365-2885.2007.00929.x. [DOI] [PubMed] [Google Scholar]
- 6.Matthiesen T, Wöhrmann T, Coogan T, Uragg H. The experimental toxicology of tramadol: an overview. Toxicol Lett. 1998;95(1):63–71. doi: 10.1016/S0378-4274(98)00023-X. [DOI] [PubMed] [Google Scholar]
- 7.Ho S-T, Wang J-J, Liaw W-J, Ho C-M, Li J-H. Determination of tramadol by capillary gas chromatography with flame ionization detection: Application to human and rabbit pharmacokinetic studies. J Chromatogr B Biomed Sci Appl. 1999;736(1):89–96. doi: 10.1016/S0378-4347(99)00434-X. [DOI] [PubMed] [Google Scholar]
- 8.Afshari R, Majdzadeh R, Balali-Mood M. Pattern of acute poisonings in Mashhad, Iran 1993–2000. J Toxicol Clin Toxicol. 2004;42:965–975. doi: 10.1081/CLT-200042550. [DOI] [PubMed] [Google Scholar]
- 9.Shadnia S, Soltaninejad K, Heydari K, Sasanian G, Abdollahi M. Tramadol intoxication: a review of 114 cases. Hum Exp Toxicol. 2008;27(3):201–205. doi: 10.1177/0960327108090270. [DOI] [PubMed] [Google Scholar]
- 10.Talaie H, Panahandeh R, Fayaznouri MR, Asadi Z, Abdollahi M. Dose-independent occurrence of seizure with tramadol. J Med Toxicol. 2009;5(2):63–67. doi: 10.1007/BF03161089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giorgi M. Pharmacokinetic differences of tramadol in several animal species and human beings. J Vet Res. 2008;63(Specia):1–4. [Google Scholar]
- 12.Afshari R, Ghooshkhanehee H. Tramadol overdose induced seizure, dramatic rise of CPK and acute renal failure. J Pak Med Assoc. 2009;59(3):178. [PubMed] [Google Scholar]
- 13.Shokoohizadeh M, Liaghat AR, Marashi H, Mihandoust A, Attaran AR. The Cost and Length of a Stay in Different Hospital Departments: An Analytical Study in Iran. Journal of Mashhad Medical Council. 2013;17:81–84. [Google Scholar]
- 14.Khosrojerdi H, Afshari R, Mehrpour O. Should activated charcoal be given after tramadol overdose. DARU. 2013;21(1):46. doi: 10.1186/2008-2231-21-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanaei-Zadeh H. Is prophylactic administration of the anticonvulsants necessary in tramadol-intoxicated patients after an initial seizure? DARU J Pharm Sci. 2013;21(1):60. doi: 10.1186/2008-2231-21-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yeh G-C, Sheu M-T, Yen C-L, Wang Y-W, Liu C-H, Ho H-O. High-performance liquid chromatographic method for determination of tramadol in human plasma. J Chromatogr B Biomed Sci Appl. 1999;723(1):247–253. doi: 10.1016/S0378-4347(98)00514-3. [DOI] [PubMed] [Google Scholar]
- 17.Ardakani YH, Rouini MR. Pharmacokinetics of tramadol and its three main metabolites in healthy male and female volunteers. Biopharm Drug Dispos. 2007;28(9):527–534. doi: 10.1002/bdd.584. [DOI] [PubMed] [Google Scholar]
- 18.Grond S, Sablotzki A. Clinical pharmacology of tramadol. Clin Pharmacokinet. 2004;43(13):879–923. doi: 10.2165/00003088-200443130-00004. [DOI] [PubMed] [Google Scholar]
- 19.Gan SH, Ismail R, Adnan WAW, Zulmi W. Impact of CYP2D6 genetic polymorphism on tramadol pharmacokinetics and pharmacodynamics. Mol Diagn Ther. 2007;11(3):171–181. doi: 10.1007/BF03256239. [DOI] [PubMed] [Google Scholar]
- 20.García-Quetglas E, Azanza JR, Sádaba B, Muñoz MJ, Gil I, Campanero MA. Pharmacokinetics of tramadol enantiomers and their respective phase I metabolites in relation to CYP2D6 phenotype. Pharmacol Res. 2007;55(2):122–130. doi: 10.1016/j.phrs.2006.11.003. [DOI] [PubMed] [Google Scholar]


