Abstract
The aim of the present study was extended from obtaining information about the interaction of gamma rays with Makrofol DE 7-2 track detector to introduce the basis that can be used in concerning simple sensor for gamma irradiation and bio-engineering applications. Makrofol polymer samples were irradiated with 1.25 MeV 60Co gamma radiations at doses ranging from 20 to 1000 kG y. The modifications of irradiated samples so induced were analyzed using UV–vis spectrometry, photoluminescence spectroscopy, and the measurements of Vickers’ hardness. Moreover, the change in wettability of irradiated Makrofol was investigated by the contact angle determination of the distilled water. UV–vis spectroscopy shows a noticeable decrease in the energy band gap due to gamma irradiation. This decrease could be attributed to the appearance of a shift to UV spectra toward higher wavelength region after irradiation. Photoluminescence spectra reveal a remarkable change in the integrated photoluminescence intensity with increasing gamma doses, which may be resulted from some matrix disorder through the creation of some defected states in the irradiated polymer. The hardness was found to increase from 4.78 MPa for the unirradiated sample to 23.67 MPa for the highest gamma dose. The contact angle investigations show that the wettability of the modified samples increases with increasing the gamma doses. The result obtained from present investigation furnishes evidence that the gamma irradiations are a successful technique to modify the Makrofol DE 7-2 polymer properties to use it in suitable applications.
Keywords: Gamma irradiation, Makrofol DE 7-2, UV–vis, Photoluminescence, Vicker’s hardness, Wettability
Introduction
Polymers are a popular part of everyday life that are included in various applications among several domains. Solid State Nuclear Track Detectors (SSNTDs) have been modified for many applications, such as detection of ion beam, biological filters, sensors and dosimetry [1], [2]. Makrofol DE (a bisphenol-A polycarbonate) is a well-known polymer widely used as SSNTDs. Makrofol DE is transparent material which has high compact strength. This makes it an advantageous material for several applications such as microelectronics and biosensor production technologies [3]. Nowadays, irradiation of polymers by high (i.e., ion beam) and low (gamma-ray and electron beam irradiations) linear energy transfer radiation is widely used in improving the physical and chemical properties of the polymeric material for high technology applications [4], [5]. Ionizing radiation by way of matter, deposits energy inside the target material that causes irreversible modifications in its structure at the macromolecular scale. The gamma-ray loses their energy in several ways, but each type includes electronic liberating which deposit its energy during the interaction with other atomic electrons [6], [7]. Moreover, the gamma ray interaction with polymers leads to complex phenomena such as chain scission, radical composition, bond breaking, creation of unsaturated bonds, intermolecular cross-linking, free radicals formation, hydrogen release and some oxidation reactions. The gamma irradiations of polymer may lead to irreversible changes in their physical properties. Part of these changes are attributed to the chain scission of polymers due to irradiation, cross-linking, breaking of some covalent bonds, and formation of carbon clusters or even libration of free radicals that may also induced the formation of new chemical bonds [8], [9], [10], [11], [12], [13], [14]. During the gamma-irradiated polymers, the release of volatile species almost takes place [15], [16], [17]. However, to be best of our knowledge a few published papers have been carried out in investigating the optical, photoluminescence, as well as the Vicker’s hardness and wettability properties of Makrofol DE 7-2 SSNTDs for the unirradiated and gamma irradiated samples. The obtained results, in this work, shed light on the effect of gamma irradiations of Makrofol DE 7-2 SSNTDs to suitable industrial applications and to modify the optical, photoluminescence, hardness properties and surface wettability through gamma-induced modifications of the polymer structure.
Material and methods
Makrofol DE 7-2 introduced by Farbenfabriken Bayer A.G., Leverkusen (Germany), is a bisphenol-A polycarbonate where its chemical composition is C16H14O3 with thickness of 300 μm and its density of 1.2 g cm−3. The irradiations were obtained at room temperature with 1.25 MeV 60Co gamma source up to 1000 kG y, which is available in NCRRT, Atomic Energy Authority, Cairo, Egypt. UV–vis measurements of pristine and irradiated Makrofol DE 7-2 samples were performed with Jasco V-576 (Japan) model double-beam spectrophotometer where its wavelength range is from 190 to 1000 nm. The induced defects in the irradiated Makrofol samples were determined with photoluminescence measurements at Nuclear Research Center (NRC), Atomic Energy Authority, Cairo, Egypt. The instrument has an RF-1501 SHIMADZU double monochromator spectrometer. The excitation wavelength for the pristine and that irradiated with gamma rays was 346 nm. The hardness was measured using a Shimadzu HMV-2000 micro-hardness tester at NRC. Vicker’s diamond pyramid indenter was used with a square base and 360° pyramid angle to determine the Vicker’s hardness, Hv. The measurements of the hardness were performed on the surfaces of all Makrofol samples at room temperature at load 100 mN and for duration time 40 s. The wettability of both pristine and irradiated samples was performed by the contact angle measurements using drop-distilled water on the surface of the samples. The surface roughness Ra was measured with TR110 Surface Roughness Tester. Three measurements were made for all samples and the roughness Ra values were calculated as the average of the three values.
Results and discussion
UV–vis analysis
The nature changes in the UV–vis absorption spectra of unirradiated and gamma irradiated Makrofol DE 7-2 samples are shown in Fig. 1. A remarkable increase can be observed in the optical absorption for irradiated samples compared with unirradiated one. These increases may be due to the formation of some electronic levels inside the forbidden gap after irradiation with gamma rays [18], [19]. One can notice the existence of a shift in the spectra toward the higher wavelength for irradiated samples. This shift may be attributed to irradiation-induced defects in the polymeric materials [5], [20], [21], [22]. In addition, the changes observed are attributed to unsaturation and the presence of hydroxyl and carbonyl groups [23]. Furthermore, after irradiation with gamma rays, a broadening of the absorption edge is observed. This broadening may be due to the formation of some defects due to gamma irradiation, i.e. the formation of conjugated bonds [19]. From the absorption spectra, the optical energy gap of the modified and unmodified polymers can be determined using the inter-band absorption theory using the relation [24]:
| (1) |
where α is the absorption coefficient and hv is the incident photon energy. B is a constant, Eg is the optical band gap and n is an empirical exponent index that characterizes the transition mode. The exponent n has value of 1/2 for allowed direct transition, while it takes the value 3/2 for the direct forbidden transition. However, it has the values 2 and 3 for the indirect allowed and forbidden transitions, respectively [25]. In our case, the transition is allowed direct transition, where the electron transition is vertical from the top of the valence band to the bottom of the conduction band. The non-vertical transitions are forbidden in this case. Fig. 2a illustrates (αhv)2 as a function of the photon energy hv for unirradiated and gamma irradiated samples. The values of the optical energy gap Eg are normally obtained by extrapolation of the linear part of the curve to intersect with the hv-axis at zero absorption [22]. Fig. 2b shows the variation in energy band gap Eg with the irradiation dose. A considerable decrease can be observed in the value of energy gap for irradiated polymers. The energy gap exhibits a decrease from 3.7 eV for pristine sample to 2.7 eV for that irradiated with gamma ray at the highest irradiation dose, respectively. This decrease may be due to irradiation induce defects and/or increase the number of conjugated bonds (—C C—) and/or attributed to the formation of carbon enriched clusters resulted from hydrogen release from molecules [19], [13]. Moreover, the decrease in the optical energy gap means some enhancements in the electrical conductivity of the polymers after gamma irradiation. This could be attributed to the energy transferred to non-bonding electrons by the gamma radiation, which, in turn, may be due to the creation of free radicals or ions inside the conduction band. The increase in either the number of carriers on localized states or the carrier mobility due to the creation of free radicals or ions will lead to an enhancement in the electrical conductivity of the Makrofol samples, which could be utilized in industrial applications. We are observed that the degree of colorless of the Makrofol after irradiation with gamma rays became yellowish as the gamma dose increases. It could assure the formation of free radical and ions that may be trapped within the Makrofol polymer and are responsible for the coloring in the irradiated samples [22], [26] and/or release of gases from the polymeric material during the irradiation (i.e., hydrogen gas as hydrogen molecules and carbonized the polymeric material). The carbonaceous cluster is playing an important role to enhance the optical properties of the polymeric material. This is attributed to the carbonaceous clusters that are rich with charge carrier, which improves the conductivity of the polymers. One can determine the atoms number of carbon per conjugation length (N) according to Fink et al. [27]:
| (2) |
where 2β gives the energy of the band structure of a pair of adjacent π sites. The value of β is considered to be 2.9 eV for π–π* optical transitions in —C C— structure. The observed shift of the absorption edge (Fig. 1) can be attributed to an enhancement of the conjugation length [28]. The change in the number of carbon atoms in the cluster N as the gamma dose increases is illustrated in Fig. 2b. It is clear that an increase in the number of the carbon atoms in the clusters is recorded as gamma dose increases. The increase may be due to the escape of hydrogen as a result to the breakage of C—H bonds after irradiation with gamma rays. The variation in the logarithm of the absorption coefficient α as a function of the photon energy hν was observed by Urbach and is given by relation [29]:
| (3) |
where αo is a constant, E is the photon energy and Eu is Urbach’s energy, which represents the inverse logarithmic slope of the absorption coefficient. Fig. 3a shows the variation in the logarithm of the absorption coefficient as a function of the photon energy for all unirradiated and gamma irradiated Makrofol samples. The Urbach energy is normally calculated from Fig. 3a using the slope of the linear portion of the curve. Fig. 3b shows the variation in the Urbach energy versus the gamma doses. It is observed from Fig. 3b that the Eu decreases with increasing the gamma doses which may be attributed to the improvement of disorderness in the Makrofol polymer [22].
Fig. 1.
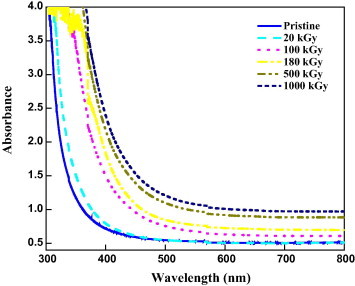
Variation in UV–vis spectra of gamma irradiated Makrofol at different doses.
Fig. 2.
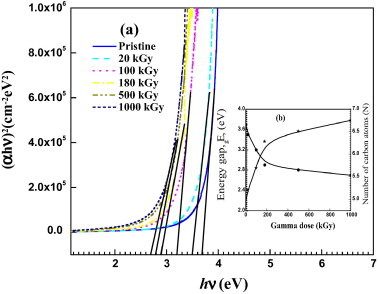
Variation in the (αhv)2 versus (hv) (a) and the optical energy gap Eg and number of carbon atoms N (b) for pristine and Makrofol samples irradiated with different doses of gamma rays.
Fig. 3.
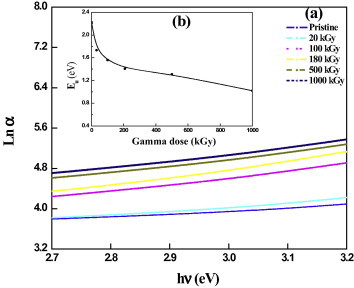
Relation between the absorption coefficient (ln α) and the photon energy (hν) (a) and the Urbach’s energy Eu (b) for pristine and Makrofol samples irradiated with different doses of gamma rays.
Photoluminescence spectra
Fig. 4 shows the photoluminescence emission spectra in the range of 200–900 nm for all Makrofol DE 7-2 track detectors with excitation wavelength 346 nm. It is clear that a considerable change was observed for the PL intensity of irradiated samples with different doses compared with the pristine one. These changes may be due to the sensitivity of the PL intensity to the creation defects formed in the polymers by the gamma rays [30], [31], results the change in molecular structure and compositional transformation to the surface layer attributed to deposited energy by gamma ray in the polymers [19]. The measured values of the peak position of the PL intensity are tabulated in Table 1. As we note from Fig. 4 and Table 1, the photoluminescence peak intensity position shift toward lower energy at doses 20 and 100 kG y compared with the pristine one. However, at 180, 500 and 1000 kG y the PL peak intensity position shifts toward shorter wavelength region. This PL peak shift could be resulted from the formation of defected states and/or destruction of chemical species the gamma dose increases. Also, the photoluminescence intensity decreases with increasing gamma dose. The reduction in the PL intensity compared with the pristine sample at low gamma doses could be due to the formation of defects in the irradiated samples. At 180 kG y, the PL intensity increases, which may be indicated to the increasing in the defects concentration in the irradiated Makrofol polymers. These results are consistent in principle with the results obtained by Kumar et al. [32]. Furthermore, at the higher doses of gamma ray, the PL intensity returns to decrease slightly and the polymeric sample becomes saturated with defects, which may be act as non-radiative recombination centers or traps [30].
Fig. 4.
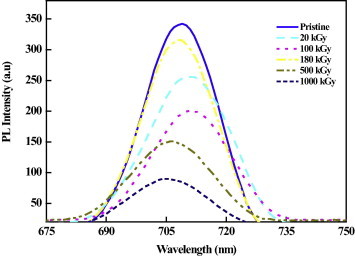
Photoluminescence emission spectra measured at λexc = 346 nm of gamma irradiated Makrofol polymer as a function of different doses.
Table 1.
Photoluminescence emission and surface wettability parameters of pristine and gamma irradiated Makrofol DE 7-2 polymer.
| Gamma dose (kG y) | PL emission | Surface wettability |
|
|---|---|---|---|
| Relative PL intensity | Contact angle (°) | Roughness Ra (mm) | |
| Pristine | 342.21 | 77.6 | 0.87 |
| 20 | 255.67 | 73.3 | 0.91 |
| 100 | 200.72 | 68.2 | 0.85 |
| 180 | 316.20 | 64.7 | 0.64 |
| 500 | 151.14 | 59.6 | 0.73 |
| 1000 | 89.91 | 47.8 | 0.58 |
Hardness measurements
Fig. 5 shows the variation in the Vicker’s hardness (Hv) of pristine and those irradiated with gamma rays at different doses. The Vicker’s hardness was estimated using the relation [33]:
| (4) |
where F is the applied load (mN) and D is the indentation diameter (μm). As shown in Fig. 5, a noticeable increase in the Vicker’s hardness with increasing in gamma dose is observed . The hardness increases from 4.78 MPa to 23.67 MPa for the pristine and the highest dose of irradiated sample, respectively. The increase in the Vicker’s hardness may be due to the formation species that interacts with the surface of the Makrofol samples, leading to cross-linking which would be the direct cause to increase the hardness property of the irradiated Makrofol samples. It is known that, the increase in the hardness surface of the irradiated polymers may be also attributed to the transfer the irradiated polymer into hydrogenated amorphous carbon that enhances the hardness of irradiated Makrofol samples. In addition, the hydrogen-depleted carbon networks cause the polymer to harden due to the cross-linking effects, which suggests an increase in molecular weight. Furthermore, the increase in the hardness may be caused by the enrichment of carbon atoms due to the bond breaking and escape the gas from the polymer surface [28], [34].
Fig. 5.
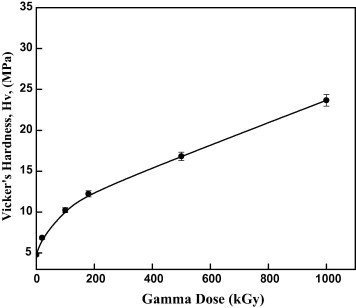
Variation in Vicker’s hardness (Hv) of irradiated Makrofol polymer as a function of gamma doses.
Surface wettability
It is well known that one of the deceiving factors in evaluating the wetting phenomena of polymeric surfaces is the contact angle. Fig. 6 shows the variation in the contact angle of pristine and irradiated Makrofol samples. A remarkable significant decrease in the contact angle of irradiated Makrofol samples with respect to the pristine one is obtained. The contact angle decreases from ∼77.6° for pristine sample to ∼47.8° for that irradiated with the highest gamma dose. The decrease in contact angle means that the wettability of irradiated samples increases with increasing gamma doses. The decrease in the contact angles of the test liquid on Makrofol polymer as the irradiation dose increases means that the wettability of Makrofol is modified by gamma irradiation. It is well known that the surface roughness affects on the wettability through the formation of micro-pores [19]. For more understanding the reason of the decreasing in the contact angle, the roughness Ra of the surface samples was measured and is enlisted in Table 1. One notices that the roughness Ra measurements are independent on the irradiation gamma doses [19]. This means that the noticeable decrease in the contact angle cannot be clarified by the change in roughness of the irradiated sample surface. Therefore, the decrease in the contact angle could be ascribed to the formation of hydrophilic groups and/or oxidation induced [28], [35] by gamma rays on the irradiated Makrofol surface.
Fig. 6.
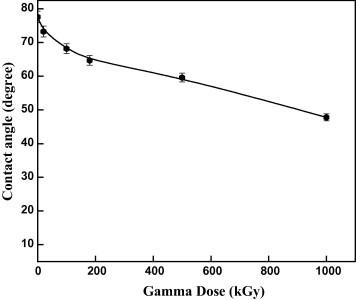
Dependence of water contact angle on the gamma ray doses of irradiated Makrofol samples.
Conclusions
UV–vis reveals a shift toward the lower photon energy (longer wavelength) with increasing the gamma dose. The results of the values of the direct band gap (Eg) show a decrease with increasing gamma doses as estimated from the linear part of optical spectra. Meanwhile the number of carbon atoms (N) raises when gamma dose increases. However, the Urbach’s energy is significantly reduced, which may be attributed to the disorderness of Makrofol samples after gamma irradiation. The Photoluminescence intensity spectra decrease at higher dose of gamma rays, which could be due to the formation of defects. After irradiation with gamma rays, it is found that the Vicker’s hardness is raised from 4.78 MPa for the unirradiated sample up to 23.67 MPa for irradiated Makrofol samples. This increase in the hardness property of irradiated samples is due to the crosslinking of the polymers, which as a result of releasing the gas from the polymer during irradiation. Such promising results can be considered as a step to fabricate shook absorber material able to overcome the problem of fragile of polymeric material at high dynamic load. The decrease in the contact angles in the irradiated samples is because of the formation of defects on the polymer surface i.e. the formation of some polar groups and carbon clusters close to the surface of the irradiated samples.
Conflict of interest
The authors have declared no conflict of interest.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Steckenreiter T., Balanzat E., Fuess H., Trautmann C. Chemical modifications of PET induced by swift heavy ions. Nucl Instrum Meth B. 1997;13:159–166. [Google Scholar]
- 2.Srivastava A., Singh T.V., Mule S., Rajan C.R., Ponrathnam S. Study of chemical, optical and thermal modifications induced by 100 MeV silicon ions in a polycarbonate film. Nucl Instrum Meth B. 2002;192:402–406. [Google Scholar]
- 3.Sharma T., Aggarwal S., Kumar S., Mittal V.K., Kalsi P.C., Manchanda V.K. Effect of gamma irradiation on the optical properties of CR-39 polymer. J Mater Sci. 2007;42:1127–1130. [Google Scholar]
- 4.Radwan R.M. Study of the optical properties of gamma irradiated high-density polyethylene. J Phys D Appl Phys. 2009;42:015419. 5pp. [Google Scholar]
- 5.Singh S., Prasher S. The optical, chemical and spectral response of gamma-irradiated Lexan polymeric track recorder. Radiat Meas. 2005;40:50–54. [Google Scholar]
- 6.Abdul-Kader A.M., El-Gendy Y.A., Awad Al-Rashdy A. Improve the physical and chemical properties of biocompatible polymer material by MeV He ion beam. Radiat Phys Chem. 2012;81:798. [Google Scholar]
- 7.Bieliń ski D., Lipiński P., Ślusarski L., Grams J., Paryjczak T., Jagielski J., et al. Surface layer modification of ion bombarded HDPE. Appl Surf Sci. 2004;564:179–186. [Google Scholar]
- 8.Clough R.L. High-energy radiation and polymers: a review of commercial processes and emerging applications. Nucl Instrum Meth B. 2001;185:8–33. [Google Scholar]
- 9.Hazarik J., Nath C., Kumar A. 160 MeV Ni12+ ion irradiation effects on the dielectric properties of polyaniline nanotubes. Nucl Instrum Meth B. 2012;88:74–80. [Google Scholar]
- 10.Abdul-Kader A.M., Turos A., Radwan R.M., Kelany A.M. Surface free energy of ultra-high molecular weight polyethylene modified by electron and gamma irradiation. Appl Surf Sci. 2009;255:5016–5020. [Google Scholar]
- 11.Siddhartha S., Dev Aarya K., Raghuvanshi S.K., Krishna J.B.M., Wahab M.A. Effect of gamma radiation on the structural and optical properties of Polyethyleneterephthalate (PET) polymer. Radiat Phys Chem. 2012;81(4):458. [Google Scholar]
- 12.Kumar V., Sonkawade R.G., Chakarvarti S.K., Singh P., Dhaliwal A.S. Carbon ion beam induced modifications of optical, structural and chemical properties in PADC and PET polymers. Radiat Phys Chem. 2012;81(6):652. [Google Scholar]
- 13.Šiljegović M., Kacˇarević-Popović Z.M., Bibić N., Jovanović Z.M., Maletić S., Stchakovsky M., et al. Optical and dielectric properties of fluorinated ethylene propylene and tetrafluoroethylene–perfluoro (alkoxy vinyl ether) copolymer films modified by low energy N4+ and C4+ ion beams. Radiat Phys Chem. 2011;80(12):1378. [Google Scholar]
- 14.Abdel Moez A., Aly S.S., Elshaer Y.H. Effect of gamma radiation on low density polyethylene (LDPE) films: optical, dielectric and FTIR studies. Spectrochim Acta Part A Mol Biomol Spectrosc. 2012;93:203. doi: 10.1016/j.saa.2012.02.031. [DOI] [PubMed] [Google Scholar]
- 15.Qureshi A., Singh D., Singh N.L., Ataoglu S., Gulluoglu Arif N., Tripathi A., et al. Effect of irradiation by 140 Mev Ag11+ ions on the optical and electrical properties of polypropylene/TiO2 composite. Nucl Instrum Meth B. 2009;267(20):3456–3460. [Google Scholar]
- 16.Verma R., Dhar R., Rath M.C., Sarkar S.K., Dabrowski R. Electron beam irradiation induced changes in the dielectric and electro-optical properties of a room temperature nematic display material 4-(trans-4′-n-hexylcyclohexyl) isothiocyanatobenzoate (6CHBT) J Phys Chem Solids. 2012;73(2):288–295. [Google Scholar]
- 17.Radwan R.M., Fawzy Y.H.A., El-Hag Ali A. Electrical behaviour of butyl acrylate/methyl methacrylate copolymer films irradiated with 1.5 MeV electron beam. Radiat Phys Chem. 2008;77(2):179. [Google Scholar]
- 18.Grodzinski J.J. Biomedical application of functional polymers. React Funct Polym. 1999;39:99. [Google Scholar]
- 19.Abdul-Kader A.M. The optical band gap and surface free energy of polyethylene modified by electron beam irradiations. J Nucl Mater. 2013;435:231–235. [Google Scholar]
- 20.Kalsi P.C., Agarwal C. UV-irradiation effects on polyester nuclear track detector. Radiat Phys Chem. 2010;79:844–847. [Google Scholar]
- 21.Zakia M.F., Abdul-Kader A.M., Nada Afaf, El-Badry Basm A. Surface modification of Makrofol-DE induced by α-particles. Philos Mag. 2013;93:4276–4285. [Google Scholar]
- 22.Zaki M.F. Gamma-induced modification on optical band gap of CR-39 SSNTD. J Phys D Appl Phys. 2008;41:175404. [Google Scholar]
- 23.Moura E.A.B., Ortiz A.V., Wiebeck HPaula A.B.A., Silva A.L.A., Silva L.G.A. Effects of gamma radiation on commercial food packaging films-study of changes in UV/VIS spectra. Radiat Phys Chem. 2004;71:201–204. [Google Scholar]
- 24.Abdel-Fattah A.A., Abdel-Hamid H.M., Radwan R.M. Changes in the optical energy gap and ESR spectra of proton-irradiated unplasticized PVC copolymer and its possible use in radiation dosimetry. Nucl Instrum Meth. 2002;B 196:279–285. [Google Scholar]
- 25.Singh S., Prasher S. The etching and structural studies of gamma irradiated induced effects in CR-39 plastic track recorder. Nucl Instrum Meth B. 2004;222:518–524. [Google Scholar]
- 26.Kumar V., Ali Y., Sonkawadeb R.G., Dhaliwal A.S. Effect of gamma irradiation on the properties of plastic bottle sheet. Nucl Instrum Meth B. 2012;287:10–14. [Google Scholar]
- 27.Fink D., Chung W.H., Klett R., Schmoldt A., Cardoso J., Montiel R., et al. Carbonaceous clusters in irradiated polymers as revealed by UV–vis spectrometry. Radiat Effect Defect Solids. 1995;133:193–208. [Google Scholar]
- 28.Abdul-Kader A.M., El-Badry Basma A., Zaki M.F., Hegazy Tarek M., Hashem Hany M. Ion beam modification of surface properties of CR-39. Philos Mag. 2010;90(19):2543–2555. [Google Scholar]
- 29.Urbach F. The long-wavelength edge of photo-graphic sensitivity and of the electronic absorption of solids. J Phys Rev. 1953;92:1324. [Google Scholar]
- 30.Kumar V., Sonkawade R.G., Dhaliwal A.S. Gamma irradiation induced chemical and structural modifications in PM-355 polymeric nuclear track detector film. Nucl Instrum Meth B. 2012;290:59–63. [Google Scholar]
- 31.El-Badry B.A., Zaki M.F., Abdul Kader A.M., Hegazy T.M., Morsy A.A. Ion bombardment of poly-allyl-diglycol-carbonate (CR-39) Vacuum. 2009;83:1138–1142. [Google Scholar]
- 32.Kumar V., Sonkawade R.G., Chakarvarti S.K., Kulriya P., Kant K., Singh N.L., et al. Study of optical, structural and chemical properties of neutron irradiated PADC film. Vacuum. 2011;86:275–279. [Google Scholar]
- 33.Smith L., Sandland G.E. An accurate method of determining the hardness of metals with particular reference to those of a high degree of hardness. Proc Inst Mech Eng. 1922;I:623. [Google Scholar]
- 34.Sun Y.M., Zhu Z.Y., Jin Y.F., Liu C.L., Wang Z.G., Zhang Q.X. The effects of high electronic energy loss on the chemical modification of polyimide. Nucl Instrum Meth B. 2002;193:214–220. [Google Scholar]
- 35.Wang H.L., Huang F., Mac Diarmid A.G., Wang Y.Z., Gebler D.D., Epstein A.J. Application of aluminum, copper and gold electrodes in a.c. polymer light-emitting devices. Synthetic Met. 1996;80:97. [Google Scholar]



