Abstract
We report a case of a 38-year-old mold-allergic patient who developed episodes of generalized urticaria and systemic anaphylactic shock immediately after ingesting button mushrooms. A manganese-dependent superoxide dismutase (MnSOD) and a NADP-dependent mannitol dehydrogenase (MtDH) from Agaricus bisporus mushroom were identified as patient-specific IgE-binding proteins. Cross-reactivity between A. bisporus MnSOD and mold aeroallergens was confirmed. We conclude that prior sensitization to mold aeroallergens might explain severe food reactions to cross-reacting homologs mushroom proteins.
Keywords: Agaricus bisporus, Anaphylaxis, Cross-reactivity, Food allergy, Mold allergy
1. Introduction
Fungi are recognized as one of the principal causes of type I allergies, which lead to severe respiratory and cutaneous allergic diseases [1, 2]. Exposure to fungal allergens can occur by inhalation of mold spores, skin contact with saprophytic species or ingestion of edible mushrooms [3]. The most important known allergenic fungi are molds belonging to the genera Alternaria, Aspergillus and Cladosporium [4]. An association between sensitization to airborne molds and systemic reactions caused by ingestion of mushrooms have been suggested but not extensively investigated [5, 6]. Among edible mushroom species, Agaricus bisporus is the most extensively cultivated and consumed throughout the world [7].
Here, we describe a case of a woman with bronchial asthma who experienced episodes of urticaria and anaphylaxis after ingesting a button mushroom. The allergens implicated were identified using the patient's serum.
2. Case
A 38-year-old woman was referred because she developed generalized urticaria immediately after ingesting chicken with mushrooms. One month later and a few minutes after the intake of fried champignon mushrooms, she suffered an episode of anaphylaxis, which needed treatment in the Emergency Department.
She had a previous history of perennial childhood asthma treated with immunotherapy to Alternaria alternata. Seven years before food allergy reaction to mushroom, this patient revealed positive skin prick tests (SPT) to Alternaria, Penicillium, Aspergillus, dog, mites and grass pollen. Specific IgE tests were positive for Aspergillus (RAST class 3), Penicillium (RAST class 3) and mites (RAST class 2). During recent years, she has been stable without asthma exacerbations, and only minimal medication with anti-asthmatic drugs has been necessary.
Currently, she related oral itching after the ingestion of several fruits belonging to the family Rosaceae, and she was tolerant of banana and tropical fruits (pineapple or mango).
Serum from the patient was obtained with a written consent agreeing to participate in the study.
After the anaphylactic episode, SPT to commercially available common inhalants (ALK-Abello SA, Madrid, Spain) were positive for airborne molds (A. alternata, Cladosporium herbarum, Penicillium notatum and Aspergillus fumigatus) but negative for other common inhalants, including mite, dander and pollens. SPT using extracts of Pleurotus ostreatus, Lentinus edodes, Boletus edulis and A. bisporus (Bial-Aristegui SA, Spain) were also positive. Total serum IgE was 271 kU/L. Specific IgE by Fluoro Enzyme ImmunoAssay (ImmunoCAP, Thermo-scientific, Sweden) to the different species and allergens gave the following results (kU/L): A. alternata, 3.7; A. bisporus, 2.1; P. ostreatus, 1.2; L. edodes, 0.5; B. edulis, <0.35; rAsp f 6, 2.3 and Pru p 3, 20.4. Component-resolved diagnosis using the ISAC method revealed the following specific IgE results (ISU): rAlt a 1, 1.3; rAsp f 3, 2.0; rAsp f 6, 2.9 and nPru p 3, 14.
Two dimensional electrophoresis IgE immunoblotting revealed two spots with apparent molecular weights of approximately 24 and 27 kDa and approximated pIs of 5.2 and 3.2, respectively (Fig. 1). Both protein spots were excised and identified by liquid chromatography coupled to mass spectrometry as a manganese-dependent superoxide dismutase (MnSOD) and NADP-dependent mannitol dehydrogenase (MtDH) with significantly high scores (Fig. 1C).
Fig. 1.
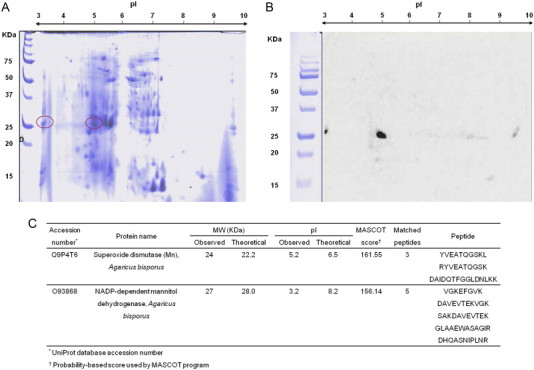
(A) Two-dimensional electrophoresis and (B) Two-dimensional IgE-immunoblotting of Agaricus bisporus crude extract. Immunoblotting was performed using the patient's serum. (C) Proteins identified by liquid chromatographic electron spray ionization tandem mass spectrometric analysis. MW, molecular weight, pI, isoelectric point.
BLAST alignment between MnSOD and MtDH protein sequences from A. bisporus (GenBank accession nos. Q9P4T6 and AAC79985, respectively) and fungal aeroallergen homologs revealed significant homologies (Table 1).
Table 1.
Homologies of Agaricus bisporus manganese-dependent superoxide dismutase and NADP-dependent mannitol dehydrogenase protein sequences with officially recognized WHO/IUIS mold allergens.
| Allergen | Source | Accession number Swissprot/NCBI/PIR | % Identity | E score |
|---|---|---|---|---|
| Manganese-dependent superoxide dismutases | ||||
| Asp f 6 | Aspergillus fumigatus | Q92450 | 49 | 8e−61 |
| Alt a 14 | Alternaria alternata | AGS80276 | 43 | 1e−40 |
| NADP-dependent mannitol dehydrogenase | ||||
| Alt a 8 | Alternaria alternata | P0C0Y4 | 36 | 9e−47 |
| Cla h 8 | Cladosporium herbarum | AAO91801 | 35 | 4e−45 |
ImmunoCap-inhibition results demonstrated that the A. bisporus crude extract was able to strongly inhibit IgE-binding to rAsp f 6 (68%) but not to rPru p 3 (Table 2).
Table 2.
ImmunoCAP inhibition assay results. Recombinant Prup 3 and Asp f 6 were used in the solid phase and different concentrations of Agaricus bisporus as liquid phase inhibitor.
| Inhibitor concentrations (A. bisporus crude extract) (mg/ml) | % of inhibition (rPru p 3 in solid-phase) | % of inhibition (rAsp f 6 in solid-phase) |
|---|---|---|
| 0 | 0 | 0 |
| 0.0001 | 0 | 10 |
| 0.001 | 0 | 30 |
| 0.01 | 0 | 45 |
| 0.1 | 0 | 56 |
| 1.0 | 0 | 68 |
3. Discussion
Strong allergic reactions to mushrooms have been increasingly recognized [8–10], and there are few studies reporting an association between aeroallergens and mushroom ingestion-related symptoms. In this regard, Herrera et al. in some of their works found a relationship between allergenicity to airborne molds (A. alternata, C. herbarum and A. fumigatus) and food allergies to A. bisporus mushrooms and spinach, demonstrating the existence of some non-identified cross-reacting proteins [6–11]. Another study attributed one form of oral allergy syndrome in adults to heat labile mushroom proteins of 43–67 kDa in size that also seem cross-react to molds [5]. In fact, knowledge of allergenic proteins that cause recognized clinically relevant cross-reactions between molds and food is still limited, and research in this field is needed to identify the causative allergens and to understand the immunological events that take place.
In this work, we describe a case of a patient with a previous clinical history of respiratory symptoms associated with mold aeroallergens and treated by immunotherapy with A. alternata extract. Years later, this patient suffered anaphylactic shock after intake of mushrooms. IgE-mediated sensitization to A. alternata, C. herbarum, P. notatum, A. fumigatus, A. bisporus, P. ostreatus, and L. edodes was confirmed, and circulating IgE levels reactive to Alt a 1, Asp f 3, Asp f 6 and Pru p 3 were detected. Moreover, two IgE-binding components from A. bisporus were identified as a MnSOD and a MtDH. To date, six MnSODs have been identified and officially recognized as allergens in A. alternata, A. fumigatus, Hevea brasiliensis, Olea europaea, Malassezia sympodialis and Pistacia vera, whereas only two MtDH from A. alternata and C. herbarum are listed in the WHO/IUIS allergen database (www.allergen.org). Although both families of proteins are described as important cross-reactive allergens [1–12], cross-reactivity between MnSODs from different sources has been more intensively studied and reported [13–15].
Looking at the specific IgE levels, skin prick test results, and the course of development of allergic symptoms in this patient, it is assumed that her mushroom-related symptoms could be due to cross-reactive IgE initially raised against airborne mold homolog allergens. However, because this patient also reported symptoms to several fruits belonging to Rosaceae, probably because of cross-reactive non-specific lipid transfer protein (LTP)-specific IgE antibodies, a relationship between LTP sensitization and allergy to mushrooms was also not excluded. With this in mind, to determine whether immunologic phenomenon that originated in systemic reactions upon ingestion of A. bisporus were due to the presence of cross-reacting antibodies to MnSODs or LTPs, IgE-inhibition assays using Asp f 6 and Pru p 3 in the solid-phase were performed. Blast alignment and inhibition assay results suggest that the A. bisporus MnSOD homolog has some common epitopes causing cross-reactivity between mushrooms and common airborne molds.
In conclusion, our findings suggest that at least the cross-reactive A. bisporus MnSOD may lead to severe food allergic reaction in mold-sensitized patients, justifying the well-recognized relationship between sensitization to airborne molds and allergy to mushrooms ingestion. However, more in-depth knowledge of the role of MnSOD and MtDH as causal agents of severe food reactions is necessary to improve diagnosis and to allow effective avoidance and prevention of further systemic reactions.
Conflict of interest
There are none.
Ethical form
Written informed consent was obtained from the patient for publication of this Case report.
Acknowledgements
This study was funded by the Ministry of Education and Innovation of Spain (Project code: SAF2011-29744) and the Department of Education, Universities and Research of the Basque Country Government (Project code: IT787-13). M.F.G. was granted by the Portuguese Foundation for Science and Technology, Portugal (fellowship: SFRH /BD/82265 /2011).
References
- 1.Simon-Nobbe B., Denk U., Poll V., Rid R., Breitenbach M. The spectrum of fungal allergy. Int. Arch. Allergy Immunol. 2008;145:58–86. doi: 10.1159/000107578. [DOI] [PubMed] [Google Scholar]
- 2.Scalabrin D.M., Bavbek S., Perzanowski M.S., Wilson B.B., Platts-Mills T.A., Wheatley L.M. Use of specific IgE in assessing the relevance of fungal and dust mite allergens to atopic dermatitis: a comparison with asthmatic and nonasthmatic control subjects. J. Allergy Clin. Immunol. 1999;104:1273–1279. doi: 10.1016/s0091-6749(99)70024-2. [DOI] [PubMed] [Google Scholar]
- 3.Mari A., Schneider P., Wally V., Breitenbach M., Simon-Nobbe B. Sensitization to fungi: epidemiology, comparative skin tests, and IgE reactivity of fungal extracts. Clin. Exp. Allergy. 2003;33:1429–1438. doi: 10.1046/j.1365-2222.2003.01783.x. [DOI] [PubMed] [Google Scholar]
- 4.Crameri R., Weichel M., Flückiger S., Glaser A.G., Rhyner C. Fungal allergies: a yet unsolved problem. Chem. Immunol. Allergy. 2006;91:121–133. doi: 10.1159/000090276. [DOI] [PubMed] [Google Scholar]
- 5.Dauby P.L., Whisman B.A., Hagan L. Cross-reactivity between raw mushroom and molds in a patient with oral allergy syndrome. Ann. Allergy Asthma Immunol. 2002;89:319–321. doi: 10.1016/S1081-1206(10)61962-X. [DOI] [PubMed] [Google Scholar]
- 6.Herrera-Mozo I., Ferrer B., Rodriguez-Sanchez J.L., Juarez C. Description of a novel panallergen of cross-reactivity between moulds and foods. Immunol. Investig. 2006;35:181–197. doi: 10.1080/08820130600616599. [DOI] [PubMed] [Google Scholar]
- 7.Giri S.K., Prasad S. Drying kinetics and rehydration characteristics of microwave-vacuum and convective hot-air dried mushrooms. J. Food. 2007;78:512–521. [Google Scholar]
- 8.Toda T., Yamaguchi M., Nakase Y., Sugimoto N., Suzukawa M., Nagase H. A case of anaphylactic reaction following matsutake mushroom ingestion: demonstration of histamine release reaction of basophils. Allergol. Int. 2010;59:417–419. doi: 10.2332/allergolint.10-CR-0205. [DOI] [PubMed] [Google Scholar]
- 9.Helbling A., Bonadies N., Brander K.A., Pichler W.J. Boletus edulis: a digestion-resistant allergen may be relevant for food allergy. Clin. Exp. Allergy. 2002;32:771–775. doi: 10.1046/j.1365-2222.2002.01400.x. [DOI] [PubMed] [Google Scholar]
- 10.Hegde V.L., Das J.R., Venkatesh Y.P. Anaphylaxis caused by the ingestion of cultivated mushroom (Agaricus bisporus): identification of allergen as mannitol. Allergol. Int. 2002;51:121–129. [Google Scholar]
- 11.Herrera I., Moneo I., Caballero M.L., de Paz S., Perez Pimiento A., Rebollo S. Food allergy to spinach and mushroom. Allergy. 2002;57:261–262. doi: 10.1034/j.1398-9995.2002.1n3547.x. [DOI] [PubMed] [Google Scholar]
- 12.Schneider P.B., Denk U., Breitenbach M., Richter K., Schmid-Grendelmeier P., Nobbe S. Alternaria alternata NADP-dependent mannitol dehydrogenase is an important fungal allergen. Clin. Exp. Allergy. 2006;36:1513–1524. doi: 10.1111/j.1365-2222.2006.02582.x. [DOI] [PubMed] [Google Scholar]
- 13.Wagner S., Sowka S., Mayer C., Crameri R., Focke M., Kurup V.P. Identification of a Hevea brasiliensis latex manganese superoxide dismutase (Hev b 10) as a cross-reactive allergen. Int. Arch. Allergy Immunol. 2001;125:120–127. doi: 10.1159/000053805. [DOI] [PubMed] [Google Scholar]
- 14.Flückiger S., Scapozza L., Mayer C., Blaser K., Folkers G., Crameri R. Immunological and structural analysis of IgE-mediated cross-reactivity between manganese superoxide dismutases. Int. Arch. Allergy Immunol. 2002;128:292–303. doi: 10.1159/000063862. [DOI] [PubMed] [Google Scholar]
- 15.Postigo I., Gutierrez-Rodriguez A., Fernandez J., Guisantes J.A., Sunen E., Martinez J. Diagnostic value of Alt a 1, fungal enolase and manganese-dependent superoxide dismutase in the component-resolved diagnosis of allergy to Pleosporaceae. Clin. Exp. Allergy. 2011;41:443–451. doi: 10.1111/j.1365-2222.2010.03671.x. [DOI] [PubMed] [Google Scholar]


