Abstract
We achieved possibility of isolation, characterization human umbilical cord blood endothelial progenitor cells (EPCs), examination potency of EPCs to form new blood vessels and differentiation into cardiomyoctes in canines with acute myocardial infarction (AMI). EPCs were separated and cultured from umbilical cord blood. Their phenotypes were confirmed by uptake of double stains dioctadecyl tetramethylindocarbocyanine-labeled acetylated LDL and FITC-labeled Ulex europaeus agglutinin 1 (DILDL-UEA-1). EPCs of cord blood were counted. Human VEGFR-2 and eNOS from the cultured EPCs were assessed by qPCR. Human EPCs was transplanted intramyocardially in canines with AMI. ECG and cardiac enzymes (CK-MB and Troponin I) were measured to assess severity of cellular damage. Histopathology was done to assess neovascularisation. Immunostaining was done to detect EPCs transdifferentiation into cardiomyocytes in peri-infarct cardiac tissue. qPCR for human genes (hVEGFR-2, and eNOS) was done to assess homing and angiogenic function of transplanted EPCs. Cultured human cord blood exhibited an increased number of EPCs and significant high expression of hVEGFR-2 and eNOS genes in the culture cells. Histopathology showed increased neovascularization and immunostaining showed presence of EPCs newly differentiated into cardiomyocyte-like cells. Our findings suggested that hEPCs can mediate angiogenesis and differentiate into cardiomyoctes in canines with AMI.
Abbreviations: CTO, chronic total occlusion; CAG, coronary angiography; AMI, acute myocardial infarction; DILDL-FITC labeled UEA-11, 1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine-labeled acetylated LDL (DiLDL,) and FITC-labeled Ulex europaeus agglutinin-1; MVD, multivessel disease; CFU, colony forming unit
Keywords: Human EPCs, Neovascularization, Canine, Acute myocardial infarction
Introduction
Chronic total occlusion (CTO) is diagnosed in patients with coronary artery disease during angiography [1]. Multivessel disease (MVD) effects are due to the presence of CTO in a noninfarct-related artery [2]. CTO lesion in a non-infarct related artery was a high risk factor for mortality after acute myocardial infarction (AMI) [3].
Endothelial progenitor cells (EPCs), described as a heterogeneous population of circulating cells in peripheral blood [4]. Their origin is found in multiple precursors, such as hemangioblasts, non-hematopoietic precursors, monocytic cells, or tissue-resident stem cells. EPCs play an important role in vasculogenesis because of their capacity to proliferate, migrate, differentiate in vivo and in vitro into endothelial cells, and incorporate into the preexisting endothelium. Thus, phenotypically, they have morphofunctional characteristics of both hematopoietic and mature endothelial cells [5]. EPCs are rare, representing approximately 0.01%–0.0001% of the mononuclear fraction in peripheral blood. However, several stimuli, such as physical exercise, can mobilize them from bone marrow, temporarily increasing their number in peripheral circulation. EPCs neovasculogenesis function was due to secretion of pro-angiogenic factors such as vascular endothelial growth factor (VEGF) and granulocyte colony stimulating factor (G-CSF) [6]. Early EPCs (present in the BM or directly after reaching the bloodstream) are CD133+/CD34+/VEGFR2+ cells, whereas circulating EPCs are CD34+ and VEGFR-2+, CD133− and start to express membrane molecules typical to mature ECs [7].
Experimental studies revealed that intravascular or intramyocardial administration of EPCs may enhance functional regeneration of infracted myocardium and neovascularization of ischemic myocardium [8]. Clinical studies suggested that intracoronary infusion of progenitor cells is accessible and may greatly affect left ventricular contractile function or decrease infarct size in patients with AMI. Previous results either experimental or clinical provide important evidence about use of progenitor cell in cell therapy of chronic coronary artery disease [9].
Studies showed an increase in capillary density associated with an improvement of ventricular function and a reduction in ventricular size three months after stem cell transplantation into the under perfused myocardial segments compared to control group [10,11]. These effects could be increased by preincubation of the stem cells with cardiomyogenic growth factors leading to a cardiomyogenic differentiation. Applying these modified stem cells in an infraction model; an improved functional recovery was obvious when compared with the transplantation of unmodified stem cells [12]. EPCs in healthy individuals may be a biologic marker for vascular function. Moreover, low levels of circulating EPCs may predict early atherosclerosis, occurrence of cardiovascular disorders, death from cardiovascular disorders [13] and prognosis after ischemic stroke [14]. The previous finding indicates that EPCs play an important part in the pathogenesis of atherosclerotic disease and assessment of EPCs may improve risk of cardiovascular disorders. This study aimed to prove that human EPCs can differentiate into cardiac myocytes after intramyocadial transplantation into canine with AMI. Phenotypic and functional biomarkers were assessed to prove myocardial differentiation.
Methodology
EPCs isolation from human umbilical cord blood
Five samples of human cord blood were enrolled in our after taken informed consents from women during caesarean sections labor. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008. The blood mononuclear cell fraction (MNCs) was isolated from the buffy coats through density-gradient centrifugation with 20 ml Ficoll-Paque (Gibco-Invitrogen, Grand Island, NY). Centrifugation was for 35 min at 400g. The interphase layer of MNC was carefully aspirated and washed in PBS containing 2 mM EDTA and further centrifuged for 10 min at 200g°. The cell pellet was resuspended in 300 μl buffer and cultured for further cells propagation.
EPCs culture, propagation, labeling and counting based assay
EPCs were identified in culture by formation of a Colony Forming Unit (CFU) [5]. CFUs were formed after MNCs culturing for 7 days. For the EPCs counting assay, 5 × 106 MNCs were cultured onto fibronectin coated 96-well plates in M199 medium supplemented with 20% fetal calf serum (FCS), 0.1% human vascular endothelial growth factor-1 (VEGF-1) and 0.1% insulin-like growth factor (IGF-1) at 37 °C for 48 h. After seven days, EPCs were stained and further labeled with 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine-labeled acetylated LDL (DiLDL,) and FITC-labeled Ulex europaeus agglutinin 1 (UEA-1, Sigma Chemical Company) Lectin. This double staining is specific for EPCs identification. EPCs were counterstained with 4′,6-diamidino-phenylindole (DAPI) for 10 min and visualized as a distinct blue cytoplasm under inverted fluorescent microscope (Lieca, Germany). DAPI staining was to ensure cells viability. Only double stained cells (DILDL-FITC labeled UEA-1) with a distinctly blue cytoplasm (DAPI positive cells) were counted in a five random fields [15].
EPCs function assessment
Total RNA was isolated from collected cultured human EPCs using Qiagene cells/tissue extraction kit (Qiagene, USA) according to instructions of manufacture. The purity (A260/A280 ratio) and the concentration of RNA were obtained using spectrophotometry (dual wave length Beckman, Spectrophotometer, USA). The extracted and purified RNA samples were subjected to RNase inhibitor at 37 °C for 20 min and stored at –80 °C for further use.
Two μg RNA was reversed into cDNA using high capacity cDNA reverse transcription kit (#K1621, Fermentas, USA). The cDNA 25 μl master mix was prepared; first strand buffer (10×) 5 μl, 10 mM dNTP’s, RNase inhibitor (40 U/μl), MMLV-RT enzyme (50 U/μl), and DEPC-treated water. RT mix was incubated for one hour at 37 °C followed by inactivation of enzymes at 95 °C for 10 min, and cooled at 4 °C. qPCR was performed using an Applied Biosystem with software version 3.1 (StepOne™, USA). cDNA including previously prepared samples (for VEGFR-2 and eNOS genes expression), internal control (for GAPDH gene expression as housekeeping gene), and non-template control (to assure absence of DNA contamination), were in duplicate. Each 25 μL of reaction mix contained 12.5 μL of SYBR Green (Fermentas), 1 μL of each primers (10 μmol/L), and cDNA (1 μg/mL) for sample determination. The thermal reaction was initiated by activation of Taq polymerase at 95 °C for 5 min, followed by 40 amplification cycles: 10 s denaturizing at 95 °C, 50 s annealing at 59 °C (VEGF-R2) or 61.2 °C (eNOS). After the RT-PCR run the data were expressed in Cycle threshold (Ct) of assessed genes (VEGFR-2 and eNOS) and the house keeping gene (GAPDH). Therefore, Relative quantitation (RQ) of target genes expression was assessed and related to housekeeping gene by previously published method RQ = 2−(ΔΔCt) [16,17]. Sequence of primers was designed as in Table 1.
Table 1.
Shows the primers sequence for the VEGF-R2&eNOS genes.
| Gene | Forward primer | Reverse primer | Accession number |
|---|---|---|---|
| VEGF-R2 | GATGTGGTTCTGAGTCCGTCT | CATGGCTCTGCTTCTCCTTTG | NT_022853.15 |
| eNOS | ATTATATCCTACACAAGACTCCAG | TCTTCAAGTTGCCCATGTTAC | NT_007914.15 |
| GAPDH | CCTCTACTGGCGCTGCCAAGGCT | GTCCACCACTGACACGTTGG | NT_009759.16 |
Induction of experimental AMI model
The study comprised 12 mongrels canines, aged from 1–2 years old weighed between 17–26 kg. All Institutional and National Guidelines for the care and use of animals were followed. Dogs were subjected to anesthesia with ketamine (10 mg/kg IV) and diazepam (1 mg/kg IV). After intubation, anesthesia was maintained with all dogs, AMI was performed by left thoracotomy followed by ligation of the left anterior descending coronary artery (LAD) distal to the first diagonal branch (Fig. 1). Experimental animals were two groups: group 1: EPCs-AMI treated canines were subjected to intramyocardial transplantation of pooled EPCs (5 × 106 cells/2 ml infusion saline) isolated from 5 human umbilical cord blood samples (Fig. 2) and group 2: AMI canines were subjected to 2 ml intramyocardial saline injection as control placebo group.
Fig. 1.
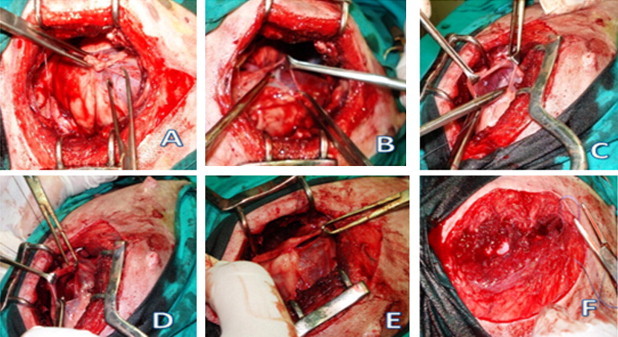
This figure represents the steps of LAD ligation operation: (A) thoracotomy, (B) Opening of the pericardium, (C) Lifting of LAD, (D) Ligation and suturing of LAD, (E) Closure of pericardium, (F) Closure of thorax.
Fig. 2.
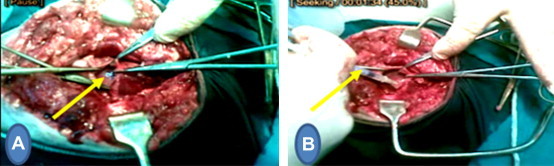
This figure represents steps of the second operation, (A) one week after LAD ligation: myocardial infarction (B) injection of EPCs (group 1) or saline (group 2) in the peri-infarct area one week after LAD ligation.
Assessment and follow up
Vital signs (blood pressure, heart rate, temperature and O2 saturation) as well as laboratory tests (blood count to assess inflammatory response, troponin I and creatin kinase-MB fraction to assess myocardial damage) were obtained one day after LAD ligation, seven and 26 ± 4 days after cell/placebo infusion. Cardiac noninvasive monitoring, including lead II electrocardiograms (ECGs) was recorded before and at various time intervals after cell infusion.
Animal scarification
At 26 ± 4 days after cell or placebo infusion, the animals were sacrificed with euthanasia. The hearts were dissected after median sternotomy. After gross examination, the hearts were divided into two parts:
One part was fixed in 10% formaldehyde for 48 h for paraffin embedding. Some fixed sections (5 μm) were further stained with hematoxylin and eosin for qualitative histopathological analysis specifically targeted to assess for scar tissue, new blood vessels development, inflammation and/or infarction lesions. Other paraffin sections were stained with Trichrome to evaluate fibrosis and collagen deposition. Further sections were cryosectioned and remained unstained for fluorescence examination of labeled EPCs, to assess homing, transdifferentiation and tracing of transplanted cells.
Immunostaining for detection of EPCs differentiation
The survival of engrafted cells was identified by labeled DiLDL- and DAPI-positive cells in frozen unstained sections made from the heart tissues. Differentiation to cardiac-like cells from injected EPCs was identified by antibody immunostaining for cardiac troponin I. Briefly, frozen cutting unstained tissue sections were fixed in acetone at 4 °C for 10 min and further incubated with a goat polyclonal immunoglobulin G anti-troponin I antibody (Santa Cruz Biotechnology, Inc.) for 60 min at room temperature. Sections were washed in PBS solutions and incubated with a biotinylated secondary antibody and streptavidin-FITC (both Vector Laboratories) immunoglobulin G for troponin I. Human endothelial cells differentiated to cardiac like cells were identified with a phycoerythrin-conjugated anti-human HLA-DR antibody (Caltag) [18].
Homing assessment of transplanted EPCs
The other part of heart was further processed for RNA extraction followed by RT (for cDNA synthesis) and qPCR for human VEGF-R2 and eNOS. qPCR for these expressed genes to assess homing of cells and estimate their function in neovasularization and cardiac repair in peri-infarct cardiac tissue [19]. The same steps as described previously at the in vitro part for RNA isolation from EPCs followed by real time PCR using SYBER Green I.
Statistical analysis
The data were presented as mean ± SD. Computerized data were analyzed using SPSS 15.0 software (SPSS Inc.). ANOVA was used to determine significant differences between different groups. Significant differences were considered when P value < 0.05.
Results
EPCs isolation, culture, and propagation
EPCs in culture
Human umbilical cord blood EPCs were isolated, cultured and propagated for 7 days on fibronectin coated wells using media supplemented with specific growth factors as; VEGF-1 and IGF-1 (Fig. 3a and b). They were rounded in shapes and adhere to fibronectin plates.
Fig. 3.
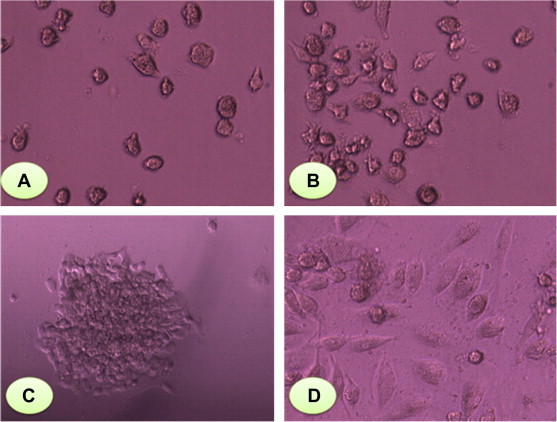
(A) EPCs at 0 day of culture, EPCs were rounded in their shape and adherent on fibronectin plate. (B) EPCs were more confluent in culture on 4th day (200× magnification). (C) EPCs-CFU at 24 h cultured on fibronectin plate and was as central core of rounded cells surrounded by elongated spindled-shaped cells (200× magnification). (D) at 7 days of culture more confluent (400× magnification).
EPCs-CFU characterization in culture
EPCs were characterized in culture by formation of CFU (Fig. 3c and d). CFU was identified as a central rounded cells surrounded by elongated spindled-shaped cells.
EPCs characterization by specific fluorescent stains
EPCs were characterized by their specific double fluorescent staining with DiLDL-UEA-1 stains (Fig. 4a–c). EPCs stained with DAPI to ensure their viability and survival in vitro (Fig. 5).
Fig. 4.

This figure represents picture for specific DiLDL-UEA-1 double staining of EPCs in culture for characterization. (A) DiLDL staining (200× magnification) and (B) UEA-1 staining (200× magnification). (C) merged picture for DiLDL-UEA-1 double staining of EPCs (200× magnification).
Fig. 5.
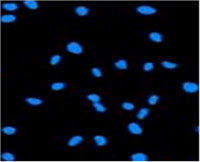
This figure represents picture for characterization of viability of cultured EPCs by positive-DAPI blue cytoplasm staining (200× magnification).
EPCs counting and functions
EPCs were significantly higher as regarding count and function at early (2 days) and late (7 days) times of culture as shown in Table 2.
Table 2.
h EPCs counting & function in relation to time of culture.
| Parameter | hEPCs at 2 days culture | hEPCs at 7 days culture | P-value |
|---|---|---|---|
| EPCsX106 count/mL | 1.17(3.1) | 2.45 (1.9) | 0.06 |
| eNOS gene expression | 6.4(5.1) | 7.1 (2.3) | 0.1 |
| VEGF-R2 gene expression | 4.2(4.4) | 5.65(2.2) | 0.04⁎ |
Data were expressed as median (range), comparison between early and late cultures was done by paired sample Wilcoxon signed rank test.
P value <0.05 was significant.
Results
Biochemical analysis of troponin I and CK-MB
24 h. after LAD ligation, CK-MB & Troponin I levels showed marked elevation indicating occurrence of myocardial infarction. CK-MB & Troponin I levels were measured one week after intramyocardial infusion and at scarification, showed residing of their levels at Table 3.
Table 3.
CK-MB &Troponin I levels in EPCs-AMI treated canines in relation to time after ligation.
| EPCs-AMI treated canines (n = 6) | CK-MB U/L | Troponin ng/ml |
|---|---|---|
| After ligation(24 h) | 60(16) | 0.41(0.15) |
| After injection (1 week after ligation) | 39(5)⁎ | 0.19(0.05)⁎ |
| At scarification (26 ± 4 days) | 31(3)⁎ | 0.14(0.02)⁎ |
Data were expressed as median (range), comparison was done by paired sample Wilcoxon signed rank test.
Statistically significantly different from (after ligation). P-value < 0.05
Electrocardiogram tracings at the indicated time intervals after intramyocardial infusion of the EPCs
ECG revealed ST segment elevation after 24 h of AMI which was resolved after one week of EPCs transplantation (Fig. 6).
Fig. 6.
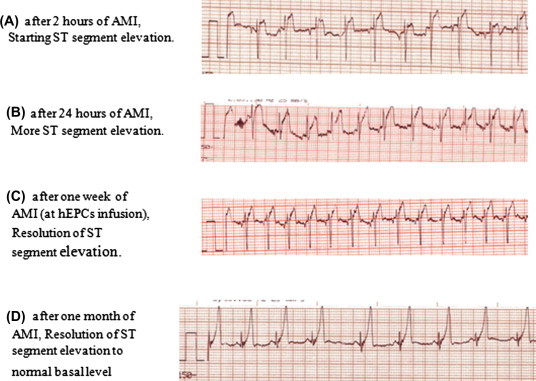
Electrocardiogram tracings before and after intramyocardial infusion of the hEPCs.
EPCs homing
Fluorescent cardiac tissue analysis of group II canine showed no fluorescence indicating no cells transplantation (Fig. 7A). Di-labeled EPCs transplanted to cardiac tissues of canine with LAD were identified as fluorescent collected cells between striated cardiac muscles (Fig. 7B). The survival of EPCs was observed as positive-DAPI cells as shown in Fig. 8).
Fig. 7.
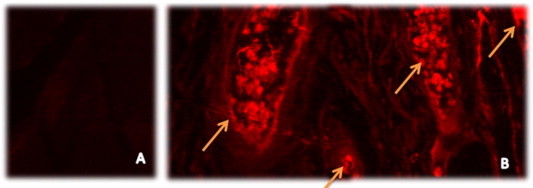
Confocal microscopy showed Fluorescent cardiac tissue analysis. (A) In vivo control for cardiac tissues of group II canine with no fluorescence of hEPCs. (B) Arrows showed in vivo localization of injected Di-labeled hEPCs for cells tracing and probably developed neovascularization between striated cardiac muscle tissues. (Magnification 200×).
Fig. 8.
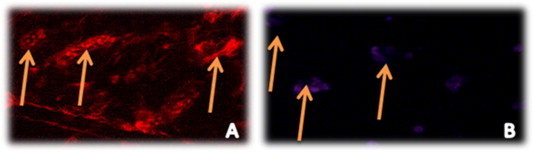
Confocal microscopy shows (A) homing and localization of injected Di-labeled hEPCs within cardiac striated muscles of canine. (B) positive-DAPI staining of injected EPCs to document their viability after transplantation (100× magnification).
Immunostaining for detection of EPCs differentiation
Transplanted EPCs were differentiated into cardiomyocyte-like cells and were observed within the fibrotic area of the infarction and identified by staining with a specific cardiac marker as troponine I (Fig. 9A and B).
Fig. 9.
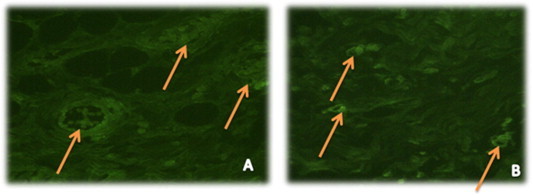
Confocal microscopy image (A) showed transdifferentiation of injected hEPCs into cardiomyocyte-like cells and their green staining for troponin I as a cardiac marker. (B) showed another view angle to document the localization of differentiated injected hEPCs stained with troponinI cardiac marker between cardiac striations (magnification 100×).
Heart histopathological examination
The neighboring non-infracted cardiac tissue in both groups I and II showed normal striated musculature, stained either with HE and MT as shown in Fig. 10. Pathological changes in infracted cardiac muscle were observed as following: (i) Myocyte necrosis in the form of empty cells (sarcolysis) and esosinophilic granular necrosis with loss of central nuclei. (ii) Atrophy of myocytes surrounding necrotic zones. (iii) Granulation tissue rich in fibroblasts & inflammatory cells (macrophages more than lymphocytes & few polymorph nuclear leucocytes). (iv) Congestion of blood vessels, hemorrhage in infarction area and nearby intramuscular zones. (v) Fibrous tissue is loose and edematous (Fig. 11). Pathological changes in infracted cardiac muscle transplanted with EPCs; in addition to previous changes observed in acute MI group, the following changes were added: (i) Many congested capillaries, congested large blood vessels with budding & branching indicating neovascularization of area (Fig. 12A). (ii) Fibrosis was enhanced & denser than in control Fig. 12B) (iii) Collections of dark oval small cells slightly larger than lymphocytes in a perivascular localization suggested as EPCs collections (Figs. 13 and 14).
Fig. 10.
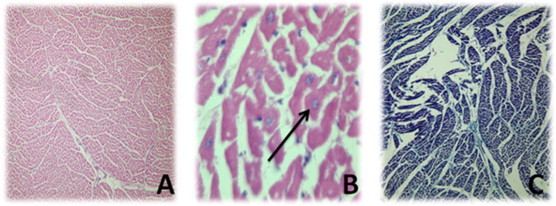
Showed normal striated cardiac musculature; (A) stained with HE with 100× magnification, (B) stained with HE with 1000× magnification and arrow showed preserved nuclei, and (C) stained with MT with 100× magnification.
Fig. 11.
Showed infarction cardiac muscle; (A) stained with MT with 200× magnification and arrow showed fibrous tissue, edema & inflammatory cells, (B) stained with HE with 1000× magnification and arrow showed eosinophylic necrosis with loss of nuclei and (C) stained with MT with 200× magnification and arrow showed congested vessel, RBCs & edema.
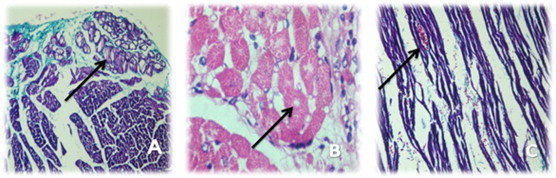
Fig. 12.
Showed infarction cardiac muscle transplanted with human EPCs; (A) cardiac tissue stained with HE and arrow indicates collection of large vessels & many budding capillaries within fibrosed infarction (magnification 200×). (B) cardiac tissue stained with MT and arrow indicates dense fibrosis with many newly formed capillaries surrounding necrotic muscle (magnification 200×).
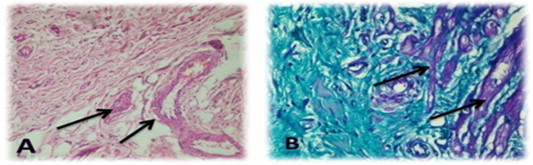
Fig. 13.
Showed infarction cardiac muscle transplanted with human EPCs; (A) cardiac tissue stained with HE and arrow indicates neovascularization & branched congested blood vessels within fibrosed infarction (magnification 10×). (B) cardiac tissue stained with MT and arrow indicates congestion, hemorrhages & increased intramuscular vessels (magnification 100×). (C) Dil-labeled human EPCs within cardiac tissue of canine were branching into newly formed blood vessel.
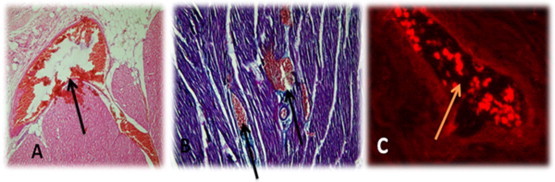
Fig. 14.
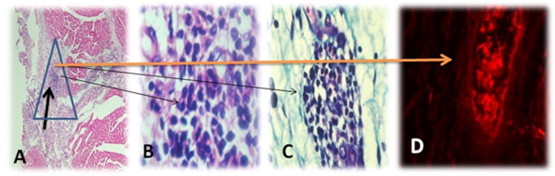
Showed infarction cardiac muscle transplanted with human EPCs; (A) cardiac tissue stained with HE and arrow indicates EPCs collection within fibrosed infarction (magnification 10×). (B) cardiac tissue stained with HE and arrow indicates EPCs collection (magnification 1000×). (C) cardiac tissue stained with MT and arrow indicates EPCs collection (magnification 1000×). (D) DiLDL-UEA-1-labeled human EPCs collection within cardiac tissue of canine was branching into newly formed blood vessel.
QRT-PCR gene expression of human VEGF-R2 and eNOS (after animal scarification) to assess homing and functional neovascularisation in canine heart tissues
There is high significant difference between VEGFR-2 and eNOS genes expression on infractioned tissues & VEGFR-2 and eNOS genes expression on adjacent tissues in group 1; EPCs-AMI treated canines at Table 4. This indicates homing and localization of transplanted EPCS and may indicate cardiac repair.
Table 4.
Comparison of gene expressions between infracted tissues and adjacent normal tissues in EPCs-AMI treated canines (n = 6).
| Median(range) | |
|---|---|
| VEGFR-2 gene expression on adjacent tissues | 0.19 (0.019) |
| VEGFR-2 gene expression on infracted tissues | 0.26 (0.043)⁎ |
| eNOS gene expression on adjacent tissues | 0.113 (0.02) |
| eNOS gene expression on infracted tissues | 0.175 (0.025)⁎ |
Data were expressed as median (range), comparison was done by paired sample Wilcoxon signed rank test.
Statistically significantly different from adjacent normal tissues. P-value < 0.05
Human VEGFR-2 and eNOS genes expressions were not expressed by QRT-PCR in group 2 canine heart tissues as they were not injected with human EPCs.
Discussion
The objective of the present study aimed isolation and characterization of EPCs from human umbilical cord blood and examination the role of EPCs in neovascularization and cardiac regeneration by intramyocardial transplantation of human EPCs in an experimental model (canines) with AMI. We reported here careful characterization of the investigated cell population. Our results were agreed with other reports [20]. The cultured MNCs on fibronectin coated plates with endothelium based medium were double-positive for DILDL-UEA-1 uptake and lectin binding. FACS data revealed that about 30% of isolated and cultured MNCs can be analyzed as EPCs, in accordance with other published data [21]. To exclude that these cells are attached monocytes or macrophages, which are also capable for LDL uptake and lectin binding [22] further identification was necessary. The cultured cells were analyzed and quantified by RT-PCR and they exhibited an endothelial phenotype, positive gene expression of VEGFR-2 and eNOS.
In our present study we found that EPCs at 7 days culture have a significance high number of endothelial precursor cells, and increase in the quantitative gene expression of VEGFR-2 and eNOS expressed by endothelial precursor cells compared to those with EPCs at 2 days of culture. These findings agreed with several studies as Matsuo et al. [15] who demonstrated that EPCs from subjects with good collateral formation had lower rates of in vitro senescence, and more CFUs compared to those with poor collateral formation. These results indicate that EPC-mediated angiogenesis might be related to coronary collateral development to some degree even in humans. In another recent study, Tokgözoğlu et al. [23] showed that number of the circulating EPCs were higher among patients with normal coronary vessels compared to patients with CAD for CD133+/34+ and CD34+/KDR+ cells. In contrast, Güven et al. [24] revealed that the number of EPCs was more among patients with significant CAD especially in those requiring coronary intervention, and EPC numbers correlated with the angiographic stenosis severity. The controversy between these two studies might be attributable to the degree of ischemia as more severe ischemia required intervention could be responsible for EPC mobilization.
There are several potential explanations for the association between low EPCs counts and severity of CAD. First was the presence of other cardiac risk factors. Several studies have identified an inverse relation between EPCs counts and cardiac risk factors. Second explanation was that both low EPCs and sever CAD were associated with older age. We can say that harmful processes that contribute to atherosclerosis (diabetes, aging, inflammation, etc.) can also suppress bone marrow production of EPCs, without any relationship between EPCs levels and CAD. It may be hypothesized that EPCs counts decreased with age, and that low EPCs and atherosclerosis coexist in the elderly without cause and effect relationship [25].
As regards the quantitative values of genes expression by Real-time PCR and analysis, we found that there was a significant elevation of eNOS gene expression & VEGFR-2 (KDR) gene expression in good collateral group of patients as compared to poor collateral group of patients. Murohara et al. [26] identified that cell clusters, spindle-shaped and cord-like structures could develop from cultures of CB MNCs. These cells have property incorporation of acetylated-LDL, released nitric oxide and expressed VEGFR-2, VE-cadherin, CD31 and vWF but not CD45. Locally transplanted cells had multiple endothelial phenotypes, survived and participated in capillary networks in the ischemic tissues of immunodeficient nude rats in vivo.
Umbilical cord blood EPCs transplanted via tail vein into nude mice, they incorporated into capillary networks in ischemic hind limb and augmented neovascularisation. In addition, in ischemic tissues, there were elevated expressions of VEGF and stromal-derived factor 1-α, both of which had chemotactic effect on EPCs [27].
In our vivo study demonstrated that intramyocardial transplantation of EPCs in canine model of AMI was associated with neovascularization and improvement of cardiac function. The localization and homing of transplanted DILDL-UEA-1human EPCs in canine cardiac tissues were identified by detection of these labeled EPCs as fluorescent collected cells between striated cardiac muscles. The survival of human EPCs in the transplanted cardiac tissue was observed as positive-DAPI cells. Immunofluorescence studies done by Silva et al. [28] identified that DAPI- and DILDL-UEA-1-positive cells were localized primarily in the anterolateral wall of cardiac tissue of canine chronic ischemia model. However, in every transplanted dog, some lateral and posterior sections showed labeled cells suggesting migration of transplanted cells.
Our in vivo results suggested that injected EPCs into ischemic myocardium improved myocardial function and increased vascularity. Histopathological cardiac tissue analysis of infarctioned cardiac muscle transplanted with human EPCs showed many congested capillaries, congested large blood vessels with budding & branching, indicating neovascularization in the area of infarction.
In vivo transplantation of bone marrow-derived cells has shown regenerated areas of infracted myocardium and new coronary capillaries formation and this led to limiting functional impairment after myocardial infarction [29]. Transendocardial injection of autologous bone marrow MNCs has shown increase in myocardial contractility and perfusion in swine [30]. In vitro study showed evidence of bone marrow stem cells differentiation into cardiomyocytes, endothelium, and smooth muscle cells [31]. Autologous bone marrow CD34+ cells transplantation in surgically induced experimental hindlimb ischemia revealed cells incorporation into the capillary network among preserved skeletal myocytes [32]. Comparative Study between transendocardial (TE) delivery of bone marrow MSCs after AMI and intracoronary (IC) delivery. The TE group showed higher cell retention (clusters even in the injury center of the infarct) with an increased vascularity and greater functional improvement than the IC group (no clusters; cells at the border of the infarct). The higher local cell density in the TE group may be important for therapeutic effectiveness [33]. Human cord blood MNCs transplantation in ischemic animal models increased the number of capillaries, angiogenic genes expression and factors [34]. Our findings suggested that EPCs express VEGFR-2, which is linked to both neoangiogenesis and stem cell homing and migration, also support a contribution of paracrine stimulation of endogenous repair.
In our study we detected transplanted EPCs were differentiated into cardiomyocyte-like cells within the fibrotic area of the infarction and identified by staining with a specific cardiac marker as troponine I. Our results agreed with Badorff et al. [18] who stated that EPCs from healthy volunteers and CAD patients can transdifferentiate in vitro into functionally active cardiomyocytes when cocultivated with rat cardiomyocytes. According to cell-to-cell contact but not cellular fusion mediates EPC transdifferentiation. Autologous EPCs transplantation may help cardiomyocyte regeneration in patients with ischemic heart disease. Koyanagi et al. [35] proved a novel type of cell-to-cell communication between EPCs and cardiomyocytes in vitro. This communication might enable EPCs through autocrine action to acquire a cardiomyogenic phenotype without permanent cellular or nuclear fusion.
Silva et al. [28] identified that transplanted MSCs in a canine chronic ischemia model were detectable in vessel walls and were positive for α-smooth muscle actin, suggesting their transdifferentiation into smooth muscle cells. MSCs were usually predominant in the luminal face of the endothelium of several vessels and expressed factor VIII, highly suggesting their transdifferentiation into endothelial cells. This transdifferentiation contributed to the significantly higher capillary density in the anterolateral wall of stem cell-treated animals. Tagged human vascular progenitor cell (VPCs) was injected in immunosuppressed dogs proximal to created critical stenosis. VPCs are self-renewing, can differentiate predominantly into ECs, SMCs and partly into cardiomyocytes. One month later, there was an increase in coronary blood flow (CBF) distal to the stenotic artery, resulting in functional improvement of the ischemic myocardium [36]. Murasawa et al. [37] evaluated nich-dependent expression profile of EPCs in vitro. Coculture of human EPCs with rat cardiac myoblast cell lineage (H9C2) revealed that EPCs can contribute not only to vasculogenesis but also to myogenesis in ischemic myocardium in vivo.
To detect homing, localization of transplanted EPCs and their role of in cardiac repair, gene expression of human VEGFR-2 gene and human eNOS gene were measured by Real-time qPCR on infarctioned tissues & on adjacent infarct tissues in group 1; EPCs-AMI treated canines. Quantitative gene expression of both human VEGFR-2 and human eNOS genes showed high significant difference between expressions on infarctioned tissues compared to expressions on adjacent infarct tissues. Human VEGFR-2 and eNOS genes expression was not expressed by QRT-PCR in group 2 canine heart tissues as they were not injected with human EPCs. Our results were supported by Tei et al. [38] who transplanted peripheral mobilized human granulocyte colony-stimulating CD34+ with atelocollagen to injured medial collateral ligament in immunodeficient rats. RT-PCR and immunohistochemical staining at the injury site demonstrated that molecular and histological expression of human-specific markers for endothelial cells such as human VEGF was increased in transplanted CD34+ group compared with the other groups transplanted with MNCs at one week.
Conclusions
The intracardiac administration of EPCs enhances neovascularisation after induced experimental AMI. Therefore, EPCs might be useful for cell therapy as potentially promote both neovascularization and cardiac regeneration in ischemic heart disease. In conclusion, EPCs can be isolated from the human umbilical cord blood and transdifferentiated into cardiomyocyte like cells in AMI experimental model. Large-scale studies should be induced to examine the potential effects of this novel approach on the risk of death and complications in patients with large acute myocardial infarctions and depressed left ventricular contractile function.
Conflict of interest
The authors have declared no conflict of interest.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Acknowledgment
Funding source was from Faculty of Medicine, Cairo University, Egypt.
Footnotes
Peer review under responsibility of Cairo University.
This submitted manuscript was previously presented at two conferences at 2012. (1) Cardiovascular Research Technologies (CRT) 2012 conference. (2) EuroPCR and the European Association of Percutaneous Cadiovascular Interventions (EAPCI) 2012 conference.
References
- 1.Christofferson R.D., Lehmann K.G., Martin G.V., Every N., Caldwell J.H., Kapadia S.R. Effects of chronic total coronary occlusion on treatment strategy. Am J Cardiol. 2005;95:1088–1091. doi: 10.1016/j.amjcard.2004.12.065. [DOI] [PubMed] [Google Scholar]
- 2.Claessen B.E., Hoebers L.P., van der Schaaf R.J., Kikkert W.J., Engstrom A.E., Vis M.M. Prevalence and impact of a chronic total occlusion in a non-infarct-related artery on longterm mortality in diabetic patients with ST elevation myocardial infarction. Heart. 2010;96:1968–1972. doi: 10.1136/hrt.2010.197673. [DOI] [PubMed] [Google Scholar]
- 3.Lee J.H., Park H.S., Ryu H.M., Lee H., Bae M.H., Lee J.H. Impact of multivessel coronary disease with chronic total occlusion on one-year mortality in patients with acute myocardial infarction. Korean Circ J. 2012;42(2):95–99. doi: 10.4070/kcj.2012.42.2.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asahara T., Murohara T., Sullivan A., Silver M., van der Zee R., Li T. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275(5302):964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 5.Silva J.F., Rocha N.G., Nóbrega A.C. Mobilization of endothelial progenitor cells with exercise in healthy individuals: a systematic review. Arq Bras Cardiol. 2012;98(2):182–191. [PubMed] [Google Scholar]
- 6.Asahara T., Kawamoto A., Masuda H. Concise review: circulating endothelial progenitor cells for vascular medicine. Stem Cells. 2011;29(11):1650–1655. doi: 10.1002/stem.745. [DOI] [PubMed] [Google Scholar]
- 7.Mangi A.A., Noiseux N., Kong D., He H., Rezvani M., Ingwall J.S. Mesenchymal stem cells modified with Akt prevents remodeling and restore performance of infarcted hearts. Nat Med. 2003;9:1195–1201. doi: 10.1038/nm912. [DOI] [PubMed] [Google Scholar]
- 8.Fernandez-Aviles F., San Roman J.A., Garcia-Frade J., Fernandez M.E., Penarrubia M.J., de la F.L. Experimental and clinical regenerative capability of human bone marrow cells after myocardial infarction. Circ Res. 2004;95:742–748. doi: 10.1161/01.RES.0000144798.54040.ed. [DOI] [PubMed] [Google Scholar]
- 9.Kendziorra K., Barthel H., Erbs S., Emmrich F., Hambrecht R., Schuler G. Effect of progenitor cells on myocardial perfusion and metabolism in patients after recanalization of a chronically occluded coronary artery. J Nucl Med. 2008;49:557–563. doi: 10.2967/jnumed.107.046706. [DOI] [PubMed] [Google Scholar]
- 10.Liu J.F., Wang B.W., Hung H.F., Chang H., Shyu K.G. Human mesenchymal stem cells improve myocardial performance in a splenectomized rat model of chronic myocardial infarction. J Formos Med Assoc. 2008;107:165–174. doi: 10.1016/S0929-6646(08)60130-8. [DOI] [PubMed] [Google Scholar]
- 11.Tran N., Franken P.R., Maskali F., Nloga J., Maureira P., Poussier S. Intramyocardial implantation of bone marrow-derived stem cells enhances perfusion in chronic myocardial infarction: Dependency on initial perfusion depth and follow-up assessed by gated pinhole SPECT. J Nucl Med. 2007;48:405–412. [PubMed] [Google Scholar]
- 12.Bartunek J., Croissant J.D., Wijns W., Gofflot S., de Lavareille A., Vanderheyden M. Pretreatment of adult bone marrow mesenchymal stem cells with cardiomyogenic growth factors and repair of the chronically infarcted myocardium. Am J Physiol Heart Circ Physiol. 2007;292:H1095–H1104. doi: 10.1152/ajpheart.01009.2005. [DOI] [PubMed] [Google Scholar]
- 13.Werner L., Deutsch V., Barshack I., Miller H., Keren G., George J. Transfer of endothelial progenitor cells improves myocardial performance in rats with dilated cardiomyopathy induced following experimental myocarditis. J Mol Cell Cardiol. 2005;39(4):691–697. doi: 10.1016/j.yjmcc.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 14.Yip H.K., Chang L.T., Chang W.N., Lu C.H., Liou C.W., Lan M.Y. Level and value of circulating endothelial progenitor cells in patients after acute ischemic stroke. Stroke. 2008;39:69–74. doi: 10.1161/STROKEAHA.107.489401. [DOI] [PubMed] [Google Scholar]
- 15.Matsuo Y., Imanishi T., Hayashi Y., Tomobuchi Y., Kubo T., Hano T. The effect of endothelial progenitor cells the development of collateral formation in patients with coronary artery disease. Int Med. 2008;47(3):127–134. doi: 10.2169/internalmedicine.47.0284. [DOI] [PubMed] [Google Scholar]
- 16.Pala L., Cresci B., Manuelli C., Maggi E., Yamaguchi Y.F., Cappugi P. Vascular endothelial growth factor receptor-2 and low affinity VEGF binding sites on human glomerular endothelial cells: biological effects and advanced glycosilation end products modulation. Microvasc Res. 2005;70(3):179–188. doi: 10.1016/j.mvr.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 17.Jiang Z.L., Zhu X., Diamond M.P., Abu-Soud H.M., Saed G.M. Nitric oxide synthase isoforms expression in fibroblasts isolated from human normal peritoneum and adhesion tissues. Fertil Steril. 2008;290(3):769–774. doi: 10.1016/j.fertnstert.2007.07.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Badorff C., Brandes R.P., Popp R., Rupp S., Urbich C., Aicher A. Transdifferentiation of blood-derived human adult endothelial progenitor cells into functionally active cardiomyocytes. Circulation. 2003;107:1024–1032. doi: 10.1161/01.cir.0000051460.85800.bb. [DOI] [PubMed] [Google Scholar]
- 19.Minguell J.J., Florenzano F.M., Ramírez M.R., Martínez R.F., Lasala G.P. Intracoronary infusion of a combination of bone marrow-derived stem cells in dogs. Exp Clin Cardiol. 2010;15(2):17–20. [PMC free article] [PubMed] [Google Scholar]
- 20.Tepper O.M., Galiano R.D., Capla J.M., Kalka C., Gagne P.J., Jacobowitz G.R. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation. 2002;106:2781–2786. doi: 10.1161/01.cir.0000039526.42991.93. [DOI] [PubMed] [Google Scholar]
- 21.Vasa M., Fichtlscherer S., Aicher A., Adler K., Urbich C., Martin H. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;89:E1–E7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- 22.Gbaguidi F.G., Chinetti G., Milosavljevic D., Teissier E., Chapman J., Olivecrona G. Peroxisome proliferator-activated receptor (PPAR) agonists decrease lipoprotein lipase secretion and glycated LDL uptake by human macrophages. FEBS Lett. 2002;512:85–90. doi: 10.1016/s0014-5793(02)02223-8. [DOI] [PubMed] [Google Scholar]
- 23.Tokgözoğlu L., Yorgun H., Gürses K.M., Canpolat U., Ateş A.H., Tülümen E. The association between circulating endothelial progenitor cells and coronary collateral formation. Atherosclerosis. 2011;219(2):851–854. doi: 10.1016/j.atherosclerosis.2011.08.045. [DOI] [PubMed] [Google Scholar]
- 24.Güven H., Shepherd R.M., Bach R.G., Capoccia B.J., Link D.C. The number of endothelial progenitor cell colonies in the blood is increased in patients with angiographically significant coronary artery disease. J Am Coll Cardiol. 2006;48:1579–1587. doi: 10.1016/j.jacc.2006.04.101. [DOI] [PubMed] [Google Scholar]
- 25.Kunz G.A., Liang G., Cuculi F., Gregg D., Vata K.C., Shaw L.K. Circulating endothelial progenitor cells predict coronary artery disease severity. Am Heart J. 2006;152:190–195. doi: 10.1016/j.ahj.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 26.Murohara T., Ikeda H., Duan J., Shintani S., Sasaki Ki, Eguchi H. Transplanted cord blood-derived endothelial precursor cells augment postnatal neovascularization. J Clin Invest. 2000;105(11):1527–1536. doi: 10.1172/JCI8296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamaguchi J., Kusano K.F., Masuo O., Kawamoto A., Silver M., Murasawa S. Stromal cell-derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation. 2003;107:1322–1328. doi: 10.1161/01.cir.0000055313.77510.22. [DOI] [PubMed] [Google Scholar]
- 28.Silva G.V., Litovsky S., Assad J.A., Sousa A.L., Martin B.J., Vela D. Mesenchymal stem cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a canine chronic ischemia model. Circulation. 2005;111:150–156. doi: 10.1161/01.CIR.0000151812.86142.45. [DOI] [PubMed] [Google Scholar]
- 29.Orlic D., Kajstura J., Chimenti S., Jakoniuk I., Anderson S.M., Li B. Bone marrow cells regenerate infracted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 30.Fuchs S., Baffour R., Zhou Y.F., Shou M., Pierre A., Tio F.O. Transendocardial delivery of autologous bone marrow enhances collateral perfusion and regional in pigs with chronic experimental myocardial ischemia. J Am Coll Cardiol. 2001;37:1726–1732. doi: 10.1016/s0735-1097(01)01200-1. [DOI] [PubMed] [Google Scholar]
- 31.Toma C., Pittenger M.F., Cahill K.S., Byrne B.J., Kessler P.D. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105:93–98. doi: 10.1161/hc0102.101442. [DOI] [PubMed] [Google Scholar]
- 32.Zhang H.K., Zhang N., Wu L.H., Jin W., Li M., Feng H. Therapeutic neovascularization with autologous bone marrow CD34+ cells transplantation in hindlimb ischemia. Zhonghua Wai Ke Za Zhi. 2005;43(19):1275–1278. [PubMed] [Google Scholar]
- 33.Perin E.C., Silva G.V., Assad J.A., Vela D., Buja L.M., Sousa A.L. Comparison of intracoronary and transendocardial delivery of allogeneic mesenchymal cells in a canine model of acute myocardial infarction. J Mol Cell Cardiol. 2008;44(3):486–495. doi: 10.1016/j.yjmcc.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 34.Kim A.K., Kim M.H., Kim S., Oh W., Hong H.K., Kang K.S. Stem cell therapy for peripheral arterial occlusive disease. Eur J Vasc Endovasc Surg. 2011;42(5):667–675. doi: 10.1016/j.ejvs.2011.06.026. [DOI] [PubMed] [Google Scholar]
- 35.Koyanagi M., Urbich C., Chavakis E., Hoffmann J., Rupp S., Badorff C. Differentiation of circulating endothelial progenitor cells to a cardiomyogenic phenotype depends on E-cadherin. FEBS Lett. 2005;579(27):6060–6066. doi: 10.1016/j.febslet.2005.09.071. [DOI] [PubMed] [Google Scholar]
- 36.Bearzi C., Leri A., Lo Monaco F., Rota M., Gonzalez A., Hosoda T. Identification of a coronary vascular progenitor cell in the human heart. Proc Natl Acad Sci. 2009;106(37):15885–15890. doi: 10.1073/pnas.0907622106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 37.Murasawa S., Kawamoto A., Horii M., Nakamori S., Asahara T. Niche-dependent translineage commitment of endothelial progenitor cells not cell, fusion in general into myocardial lineage cells. Arterioscler Thromb Vasc Biol. 2005;25:1388–1394. doi: 10.1161/01.ATV.0000168409.69960.e9. [DOI] [PubMed] [Google Scholar]
- 38.Tei K., Matsumoto T., Mifune Y., Ishida K., Sasaki K., Shoji T. Administrations of peripheral blood CD34-positive cells contribute to medial collateral ligament healing via vasculogenesis. Stem Cells. 2008;26(3):819–830. doi: 10.1634/stemcells.2007-0671. [DOI] [PubMed] [Google Scholar]



