Abstract
Mucormycosis is a rare life threatening fungal infection predominately seen in immunocompromised or diabetic patients. The following case is of a known type II diabetic patient who presented with sepsis and sudden unilateral loss of vision secondary to infective rhino-orbito-cerebral mucormycosis. Treatment of the condition required extensive surgical intervention and medical management for a life saving outcome.
Keywords: Rhinocerebral mucormycosis, Mucorales, Rhizopus arrhizus, Liposomal amphotericin B (AmBisome), Posaconazole, Deferasirox
1. Introduction
Mucormycosis refers to a spectrum of diseases caused by infection with fungi in the order of mucorales. Mucorales with 7 genera: Rhizopus, Mucor, Absidia, Saksenaea, Rhizomucor, Apophysomyces, and Cunninghamella are documented to be pathogenic organisms that produce invasive disease in humans, with the most common causative agent being of the rhizopus species. Endemic worldwide, mucorales are predominantly saprobic soil organisms found on decaying organic material. However, these organisms also act as opportunistic pathogens causing an acute angioinvasive infection seen primarily in the immunocompromised [1].
Over the last few decades there has been an increase in the number of reported cases of infections caused by mucorales [2]. This has been attributed to a rise in awareness of the disease and improvement in methods of identifications. The rise however has also coincided with an increase in risk factors such as immunosuppression, malignancy and diabetes [3].
Patients with poorly controlled diabetes and ketoacidosis are at high risk of developing rhinocerebral mucormycosis, with systemic acidosis creating an ideal environment for the growth of Rhizopus. However initial presentation of rhinocerebral mucormycosis infection can often appear non-specific making correct diagnosis extremely difficult until the disease has caused significant morbidity, aggressive fungal invasion of the paranasal sinuses, orbit, hard palate and brain [4,5].
The basis of mucormycosis treatment remains a combination of extensive surgical debridement and amphotericin B for a protracted period of 4–6 weeks [6]. Although not currently used as first line treatment, the concurrent use of posaconazole, a triazole antifungal drug has been shown to be effective against mucormycosis and use has been increasingly reported when amphotericin B has had to be discontinued due to adverse side effects [7,8].
The following case report illustrates the challenges of managing rhinocerebral mucormycosis in diabetic patients with concurrent co-morbidities, limiting the use of medical therapies.
2. Case
A 62 year old man presented to hospital (day 0) with a one week history of left sided facial hyperaesthesia, retro-orbital discomfort and blurred vision. He had complained of suffering from a dull persistent headache for the past five weeks. He had a past medical history of insulin dependent type two diabetes, chronic kidney disease with stage 3 renal failure, hypertension, chronic pancreatitis and ischaemic heart disease.
Referral to the local ear, nose and throat (ENT) department was made on day +2, after the patient had been seen by the ophthalmology department due to progressive visual loss. On examination the patient was found to have complete ptosis of the left eye. The left pupil was found to be fixed and dilated with paralysis of cranial nerves III, IV and VI. Visual acuity in the left eye was reduced to counting fingers at one metre. Slit lamp examination of the left retina demonstrated a “cherry red spot” and cattle trucking sign, consistent with a central retinal artery occlusion and optic neuritis. Flexible nasal endoscopy revealed brown discharge and eschar on the lateral wall of the left nasal cavity (Fig. 1).
Fig. 1.
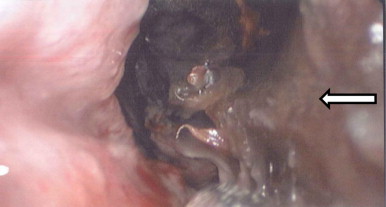
Endoscopic photography of left nasal cavity demonstrating black eschar (dead tissue) indicated by the arrow on the left lateral nasal wall.
Laboratory results on admission (day 0) showed an elevated white cell count 31.7 (×109/l) and C-reactive protein 396 (mg/l0); Creatinine 354 (μmol/L) (elevated from baseline 271 μmol/L 4 months previously); blood sugars 14.3–25.5 (mmol/L) and potassium 6.0 (mmol/L), all of which were consistent with hyperglycemic hyperosmolar state, acute kidney injury (AKI) and hyperkalaemia.
An urgent computed tomography scan (CT) of the head was performed revealing mucosal thickening in the left ethmoid, maxillary and frontal sinuses with disease involving the medial wall of the left orbit, soft tissue swelling around left orbit with slight displacement of the medial extraocular muscles (Fig. 2).
Fig. 2.
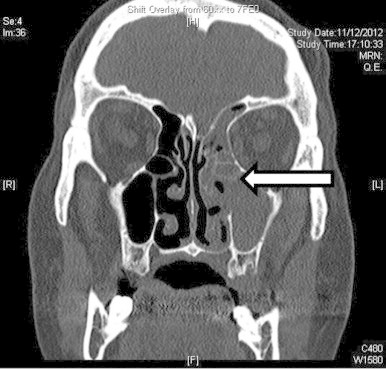
Coronal CT image without contrast showing thickening of mucosa and opacification of left ethmoidal and maxillary sinuses (indicated by the arrow).
Magnetic resonance imaging (MRI) scan showed collection/mass within the left ethmoidal air cells which had breached through the left orbital plate and encroached into the left orbit posteriorly (Fig. 3). At this stage there were no signs of intracranial spread of disease.
Fig. 3.
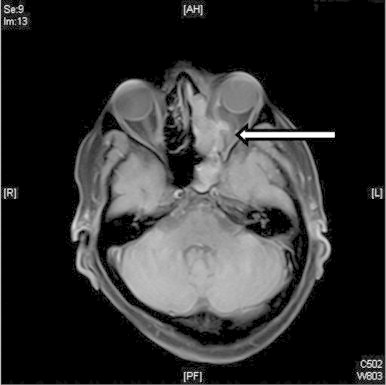
Diffusion weighted MRI axial imaging showing extensive inflammatory changes in the left nasal cavity and paranasal sinuses with mass encroaching into left orbital plate (indicated by arrow).
On day +2, upon assessment by the ENT consultant the patient underwent immediate and extensive débridement of all infected and necrotic tissue. Left infundibulectomy was performed opening up the ethmoid, maxillary and frontal sinuses for drainage of the sinuses and any collections. A left orbital decompression was performed by incising the orbital periosteum in an attempt to preserve the remaining vision.
Tissue samples from debridement macroscopically grew colonies of woolly, white and subsequently grey fungi, which grew with such rapidity they fitted with colloquial description of fungi as ‘lid lifters’ (Fig. 4). Microscopic examination showed broad hyphae with irregular branching and fungus was identified as Rhizopus arrhizus (Fig. 5). Diagnosis of R. arrhizus was made by the colonial appearance and microscopic structure-confirmed by the Public Health England Mycology Reference Laboratory, Myrtle Road Bristol.
Fig. 4.
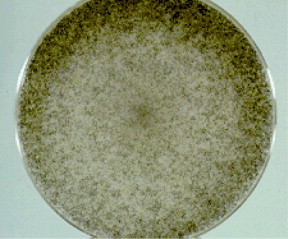
Woolly colonies, known colloquially as ‘lid lifters’.
Fig. 5.
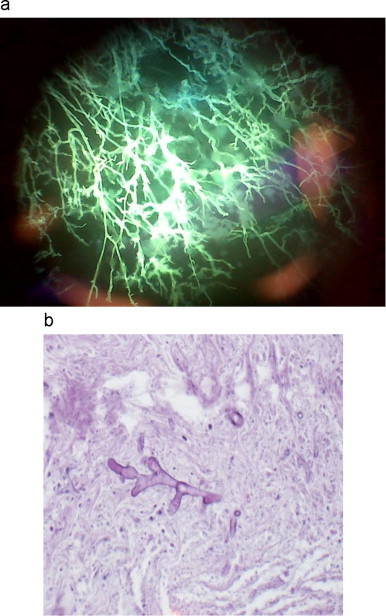
(a) Direct fluorescence microscopy on excised tissue. Microscopic image of Rhizopus arrhizus with broad hyphae and irregular branching with Calcofluor white stain. (b) Haematoxylin and eosin stain of Rhizopus arrhizus.
The patient was commenced on antimicrobial therapy on day +2, which consisted of AmBisome 300 mg (3–5 mg/kg/day) intravenously and posaconazole 420 mg orally, twice daily. After a total of 6 days of treatment with intravenous AmBisome and posaconazole, treatment was discontinued on day +8 due to derangement of renal function and liver function test.
Post-operatively, the patient continued to experience worsening headache, nausea and paraesthesia over the left infra-orbital region. Serial MRI and CT scans were performed which showed extension of the disease process which now involved the left orbit and frontal sinus.
The patient's case was re-discussed with the local microbiology team who recommended salvage treatment with deferasirox. The patient received an oral dose of deferasirox 15 mg/kg however due to significant and rapidly worsening renal function, treatment was stop within 48 h of the first dose.
On day +11 the patient underwent left eye exenteration (Fig. 6) with histological analysis of eye tissue consistent with rhizopus arrhizus infection and angioinvasion (Fig. 7).
Fig. 6.
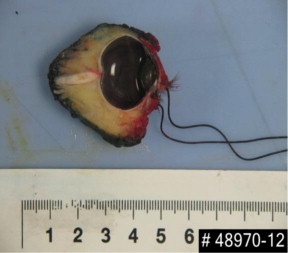
Image of enucleated left eye.
Fig. 7.
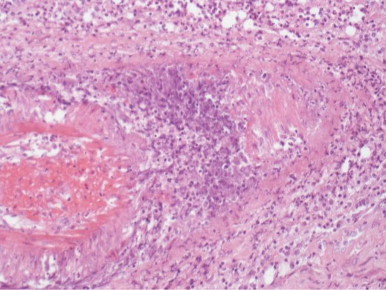
Vasculitis consistent with inflammatory response to mucor angio-invasion (magnification x100).
An MRI was performed on day +21 and did not show further deterioration or intra-cranial abscess. The patient was restarted on intravenous AmBisome and discharged home on day +21 with support from the ‘hospital at home’ team who managed his intravenous AmBisome which was continued for a total of 11 weeks.
Despite treatment he continued to experienced persistence of symptoms and on his second admission day +44, he was noted to have frontal lobe involvement and underwent endoscopic clearance of his left frontal recess in a tertiary specialist centre. He was also noted to have continued acute kidney injury and reactive depression. The patient on day +44 was restarted and managed on daily oral prophylactic posaconazole for a total period of 4 months. He has since remained stable and is currently recuperating in the community.
3. Discussion
Rhinocerebral mucormycosis is a rare opportunistic infection effecting the nasal passages, sinuses, oral cavity and brain. It is caused by saprophytic aerobic fungi, of the class phycomycetes and order mucorales and is also known as phycomycetes. Ubiquitous in nature, phycomycetes are also found colonizing the mucosal surfaces of the oral and nasal cavities as well as the paranasal sinuses with little pathogenic sequelae in immunocompetent patients.
Pathogenic activity is, however seen by the same organism in the immunocompromised due to the reduction in the phagocytic containment of the organism. This leads to germination and hyphae formation allowing invasion of blood vessels. It is believed that in most cases the fungi enter the body by inhalation of aerosolized spores (3–11 µm) through the sinuses with infiltration and spread along neurovascular structures. The infection can progress rapidly and erode through the boney walls of the sinuses into the orbit, retro-orbital area, skull base and extending into the brain. Direct fungal invasion causes a purulent arteritis, thrombosis of vessels, ischaemia and finally infarction of tissue. Thrombosis of the cavernous sinus, carotid arteries, and jugular vein indicate a very poor prognosis. In patients with intracranial involvement, fatality rates can excess 80% with death occurring in less than 2 weeks from initial onset if mucormycosis cannot be successfully treated. Prior to 1955 and the availability of amphotericin there were no surviving cases of mucormycosis reported [1].
Survival rates for patients with invasive sinus disease without intracranial involvement are far better with survival being estimated at 50–80%. Early identification and treatment of infection has a more favourable outcome, with a meta-analysis conducted by Yohai et al. indicating that survival rates decline if there is a delay of longer than 6 days from diagnosis of mucormycosis to treatment [5].
The true incidence of disease is unknown as mucormycosis is not a reportable disease. In 1992–1993 a population-based surveillance study conducted in San Francisco, California, revealed that the annual incidence of mucormycosis was 1.7 cases per 1 million individuals, equivalent to 500 cases per year [4]. The incidence of disease is believed to be rising, Bitar D et al. French study demonstrated an increased incidence of disseminated mucormycosis cases from 0.7/million in 1997 to 1.2/million in 2006 (P<.001) in immunocompromised patients suffering from haematologic malignancies or bone marrow transplants [3]. The finding was echoed by Saegeman et al., attributing the rise in incidence of disease to the growing immunosuppressed population [2]. Over the last 10 years there has been a surge in predisposing factors for mucormycosis, with diabetes mellitus being the most common underlying disorder in an estimated 70% of cases of mucor and renal disease being the second most common risk factor [7].
Patients with diabetic acidosis are particularly susceptible to rhinocerebral mucormycosis as acidic sera enhances angioinvasion of fungus, as iron is more freely available and is required for mucor to spread. In this case report, the hyperglycaemia (blood glucose (BM) 14.3–23.5) and concurrent acidosis prevented an appropriate innate immune response, with the hyperglycaemia and acidosis causing impairment of neutrophil chemotaxis, oxidative and non-oxidative fungicidal mechanisms.
The management of rhinocerebral mucormycosis has centred on early recognition of disease, aggressive surgical intervention with the use of concurrent adjuvant antifungal therapy. The patient in this case underwent immediate surgical intervention consisting of extensive debridement and subsequent left eye exenteration. Institution of dual therapy, liposomal amphotericin B and posaconazole was required for successful treatment of this life threatening infection.
In vitro and in vivo evidence support rapid institution of liposomal amphotericin B (AmBisome) as the main efficacious pharmacotherapeutic intervention [6].
To effect successful management of the disease radical surgical debridement is also required as pharmacological agents are only fungostatic. The preferred fungostatic agent is liposomal amphotericin B (AmBisome) due to better tolerance and safety record compared to amphotericin B, which is a highly toxic agent. Amphotericin B commonly causes toxic renal and systemic side effects due to the need for high doses and prolonged treatment [6]. Unfortunately our patient was unable to tolerate initial treatment with liposomal amphotericin B (AmBisome) and developed worsening of pre-existing renal failure. However, reinstatement of treatment was possible with careful monitoring of renal function by hospital at home team, undertaken over an 11-week period.
Mucormycosis is resistant to most azoles, apart from posaconazole, which has been shown to have a 70% success rate as salvage therapy in one study. Thus, posaconazole was reinitiated when disease spread was observed on MRI to involve the frontal intracranial region. Like liposomal amphotericin B (AmBisome), posaconazole was also discontinued initially due to the development of abnormal blood test (liver function). But was later reinstated as a prophylactic agent once disease clearance was evidenced on imaging and the patient's symptoms had stabilized. Posaconazole has been suggested for secondary prophylaxis in patients at high risk of relapse. The utility of posaconazole is limited by the need for a high fat diet and side effects like hepatotoxicity, as initially experienced by our case. Management of rhinocerebral mucormycosis is often difficult especially in this case where use of fungistatic and triazole was limited by the impaired renal function.
An alternative treatment option that has been used in cases where disease has spread intracranially despite surgical and medical management has been iron-chelating agents like Deferasirox. The use of iron chelators as a salvage agent has been an area of controversy as studies, which used iron chelators to treat iron and aluminium overload in renal dialysis patients reported an increase rate of mucormycosis and therefore cited iron chelators as a risk factor for angioinvasive mucormycosis [9]. The rise in risk of developing mucormycosis appears to be specific to the use of Deferoxamine, which acts as an iron siderophore for mucorales. Iron is required by virtually all microbial pathogens for growth. Deferoxamine enables the supply of previously unavailable iron to the fungi enhancing virulence [10]. In contrast, other iron chelators, Deferiprone and Deferasirox, do not supply iron to the fungus and were shown to be fungicidal in vitro. Animal models have shown similar fungicidal activity of both iron chelators with markedly improved survival of treatment of mucor-infected mice or guinea pigs [9,10]. However, human studies on deferasirox therapy have shown mixed results. Spellberg et al. randomized controlled trial on the use of deferasirox-AmBisome therapy for mucormycosis, reported higher mortality rates of patients treated with deferasirox and AmBisome at 90 days compared with placebo and AmBisome [11]. There are several possibilities, which may explain the discrepancy in results. The imbalance in baseline characteristics of disease and health status of the two groups and the small number of patients enroled in the trial meaning that multifactorial analysis could not be performed, make accurate interpretation of the results difficult. As other phase II human trials found deferasirox was safe in patients with proven mucormycosis, the recommendation from the European Society of Clinical Microbiology and Infectious Diseases, (ESCMID) is divided [12,13]. For haematological malignancies the use of deferasirox is not recommended whilst use of deferasirox is marginally supported for other patient groups. In the case of our patient, deferasirox was used as salvage therapy only after initial treatment with AmBisome and posaconazole was discontinued due to toxicity experience by the patient.
Even with medical and surgical intervention rhino-cerebral-orbital mucormycosis still carries immense morbidity if the patient survives. As treatment involves wide spread surgical debridement of infected areas and toxic medical treatments. The case described required significant levels of multidisciplinary input and support. The patient continued to be monitored in the community for 7 months post initial presentation for a positive outcome to be achieved.Nevertheless, despite the difficulties experienced with medical management in this case due the patient's underlying medical problem, there is evidence to suggest that iron chelating agents and combined or even triple therapy with fungistatic and triazole may be beneficial in improving survival.
Conflict of interest
There are no financial or personal conflicts of interests.
Acknowledgements
We would like to thank Dr Nichola Chaston, Consultant Pathologist, Dr Mark Baker and the Department of Laboratory Medicine at the William Harvey Hospital for their analysis and interpretation of the cultures. We would also like to thank Moira Talpaert for critical reading of the manuscript
References
- 1.M.T. Yen, W.P. Baugh, Rhinocerebral Mucormycosis [Internet], [Place unknown]: Medline, 2011. Avaliable from: 〈http://emedicine.medscape.com/article/227586-overview〉 (Updated 08.12.11).
- 2.Saegeman V., Maertens J., Meersseman W., Spriet I., Verbeken E., Lagrou K. Vol. 16. University Hospital, Belgium; 2010. Increasing Incidence of Mucormycosis. pp. 1456–1458 (dispatch) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bitar D., Van Cauteren D., Lanternier F., Dannaoui E., Che D., Dromer F. Increasing incidence of zygomycosis (mucormycosis), France, 1997–2006. Emerg. Infect. Dis. 2009;15:1395–1401. doi: 10.3201/eid1509.090334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petrikkos G., Skiada A., Lortholary O., Roilides E., Walsh J.T., Kontoyiannis D.P. Epidemiology and clinical manifestations of mucormycosis. Clin. Infect. Dis. 2012;54(1):23–34. doi: 10.1093/cid/cir866. [DOI] [PubMed] [Google Scholar]
- 5.Yohai R.A., Bullock J.D., Aziz A.A., Markert R.J. Survival factors in rhino-orbital-cerebral mucormycosis. Surv. Ophthalmol. 1994;39(1):3–22. doi: 10.1016/s0039-6257(05)80041-4. [DOI] [PubMed] [Google Scholar]
- 6.Goldstein E.J.C., Spellberg B., Walsh T.J., Kontoyiannis D.P., Edwards J., Jr, Ibrahim A. Recent advances in the management of mucormycosis: from bench to bedside. Clin. Infect. Dis. 2009;48(12):1743–1751. doi: 10.1086/599105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adler D.E., Milhorat T.H., Miller J.I. Treatment of rhinocerebral mucormycosis with intravenous interstitial, and cerebrospinal fluid administration of amphotericin B: case report. Neurosurgery. 1998;42(3):644–648. doi: 10.1097/00006123-199803000-00037. [DOI] [PubMed] [Google Scholar]
- 8.Sheth S.M., Talwalkar N.C., Desai A.P. Rhinocerebral mucormycosis in a case of renal failure. J. Postgrad. Med. 1981;27(3):190b–193b. [PubMed] [Google Scholar]
- 9.Boelaert R., de Locht M., Van Cutsem J., Kerrels V., Cantinieaux B., Verdonck A. Mucormycosis during deferoxamine therapy is a siderophore-mediated infection. In vitro and in vivo animal studies. J. Clin. Investig. 1993;91(5):1979–1986. doi: 10.1172/JCI116419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reed C., Ibrahim A., Edwards J.E., Jr. Deferasirox, an iron-chelating agent, as salvage therapy for rhinocerebral mucormycosis. Antimicrob. Agents Chemother. 2006;50(11):3968–3969. doi: 10.1128/AAC.01065-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spellberg B., Ibrahim A.S., Chin-Hong P.V., Kontoyiannis D.P., Morris M.I., Perfect J.R. The Deferasirox-Am-Bisome therapy for mucormycosis (DEFEAT Mucor) study: a randomized, double-blinded, placebo-controlled trial. J. Antimicrob. Chemother. 2012;67:715–722. doi: 10.1093/jac/dkr375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spellberg B., Andes D., Perez M., Anglim A., Bonilla H., Mathisen G.E. Safety and outcomes of open-label deferasirox iron chelation therapy for mucormycosis. Antimicrob. Agents Chemother. 2009;53:3122–3125. doi: 10.1128/AAC.00361-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cornely O.A., Arikan-Akdagli S., Dannaoui E., Groll A.H., Lagrou K., Chakrabarti A. ESCMID and ECMM joint clinical guidelines for the diagnosis and management of mucormycosis. Clin. Microbiol. Infect. 2014;20(3):5–26. doi: 10.1111/1469-0691.12371. [DOI] [PubMed] [Google Scholar]


