Graphical abstract
Keywords: Hydrogel, Preparation, Processing, Optimization, Innovation
Abstract
Hydrogel products constitute a group of polymeric materials, the hydrophilic structure of which renders them capable of holding large amounts of water in their three-dimensional networks. Extensive employment of these products in a number of industrial and environmental areas of application is considered to be of prime importance. As expected, natural hydrogels were gradually replaced by synthetic types due to their higher water absorption capacity, long service life, and wide varieties of raw chemical resources. Literature on this subject was found to be expanding, especially in the scientific areas of research. However, a number of publications and technical reports dealing with hydrogel products from the engineering points of view were examined to overview technological aspects covering this growing multidisciplinary field of research. The primary objective of this article is to review the literature concerning classification of hydrogels on different bases, physical and chemical characteristics of these products, and technical feasibility of their utilization. It also involved technologies adopted for hydrogel production together with process design implications, block diagrams, and optimized conditions of the preparation process. An innovated category of recent generations of hydrogel materials was also presented in some details.
Introduction
The materials of interest in this brief review are primarily hydrogels, which are polymer networks extensively swollen with water. Hydrophilic gels that are usually referred to as hydrogels are networks of polymer chains that are sometimes found as colloidal gels in which water is the dispersion medium [1].
Researchers, over the years, have defined hydrogels in many different ways. The most common of these is that hydrogel is a water-swollen, and cross-linked polymeric network produced by the simple reaction of one or more monomers. Another definition is that it is a polymeric material that exhibits the ability to swell and retain a significant fraction of water within its structure, but will not dissolve in water. Hydrogels have received considerable attention in the past 50 years, due to their exceptional promise in wide range of applications [2–4]. They possess also a degree of flexibility very similar to natural tissue due to their large water content.
The ability of hydrogels to absorb water arises from hydrophilic functional groups attached to the polymeric backbone, while their resistance to dissolution arises from cross-links between network chains. Many materials, both naturally occurring and synthetic, fit the definition of hydrogels.
During last two decades, natural Hydrogels were gradually replaced by synthetic hydrogels which has long service life, high capacity of water absorption, and high gel strength. Fortunately, synthetic polymers usually have well-defined structures that can be modified to yield tailor able degradability and functionality. Hydrogels can be synthesized from purely synthetic components. Also, it is stable in the conditions of sharp and strong fluctuations of temperatures [5].
Recently, hydrogels have been defined as two- or multi-component systems consisting of a three-dimensional network of polymer chains and water that fills the space between macromolecules. Depending on the properties of the polymer (polymers) used, as well as on the nature and density of the network joints, such structures in an equilibrium can contain various amounts of water; typically in the swollen state, the mass fraction of water in a hydrogel is much higher than the mass fraction of polymer. In practice, to achieve high degrees of swelling, it is common to use synthetic polymers that are water-soluble when in non-cross-linked form.
Hydrogels may be synthesized in a number of “classical” chemical ways. These include one-step procedures like polymerization and parallel cross-linking of multifunctional monomers, as well as multiple step procedures involving synthesis of polymer molecules having reactive groups and their subsequent cross-linking, possibly also by reacting polymers with suitable cross-linking agents. The polymer engineer can design and synthesize polymer networks with molecular-scale control over structure such as cross-linking density and with tailored properties, such as biodegradation, mechanical strength, and chemical and biological response to stimuli [6].
Classification of hydrogel products
The hydrogel products can be classified on different bases as detailed below:
Classification based on source
Hydrogels can be classified into two groups based on their natural or synthetic origins [7].
Classification according to polymeric composition
The method of preparation leads to formations of some important classes of hydrogels. These can be exemplified by the following:
-
(a)
Homopolymeric hydrogels are referred to polymer network derived from a single species of monomer, which is a basic structural unit comprising of any polymer network [8]. Homopolymers may have cross-linked skeletal structure depending on the nature of the monomer and polymerization technique.
-
(b)
Copolymeric hydrogels are comprised of two or more different monomer species with at least one hydrophilic component, arranged in a random, block or alternating configuration along the chain of the polymer network [9].
-
(c)
Multipolymer Interpenetrating polymeric hydrogel (IPN), an important class of hydrogels, is made of two independent cross-linked synthetic and/or natural polymer component, contained in a network form. In semi-IPN hydrogel, one component is a cross-linked polymer and other component is a non-cross-linked polymer [10,11].
Classification based on configuration
The classification of hydrogels depends on their physical structure and chemical composition can be classified as follows:
-
(a)
Amorphous (non-crystalline).
-
(b)
Semicrystalline: A complex mixture of amorphous and crystalline phases.
-
(c)
Crystalline.
Classification based on type of cross-linking
Hydrogels can be divided into two categories based on the chemical or physical nature of the cross-link junctions. Chemically cross-linked networks have permanent junctions, while physical networks have transient junctions that arise from either polymer chain entanglements or physical interactions such as ionic interactions, hydrogen bonds, or hydrophobic interactions [11].
Classification based on physical appearance
Hydrogels appearance as matrix, film, or microsphere depends on the technique of polymerization involved in the preparation process.
Classification according to network electrical charge
Hydrogels may be categorized into four groups on the basis of presence or absence of electrical charge located on the cross-linked chains:
-
(a)
Nonionic (neutral).
-
(b)
Ionic (including anionic or cationic).
-
(c)
Amphoteric electrolyte (ampholytic) containing both acidic and basic groups.
-
(d)
Zwitterionic (polybetaines) containing both anionic and cationic groups in each structural repeating unit.
Hydrogel-forming natural polymers include proteins such as collagen and gelatine and polysaccharides such as starch, alginate, and agarose. Synthetic polymers that form hydrogels are traditionally prepared using chemical polymerization methods.
Hydrogel product sensitive to environmental conditions
As mentioned above, hydrogels as three-dimensional cross-linked hydrophilic polymer networks are capable of swelling or de-swelling reversibly in water and retaining large volume of liquid in swollen state. Hydrogels can be designed with controllable responses as to shrink or expand with changes in external environmental conditions.
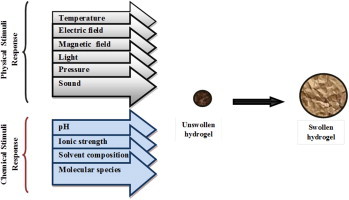
They may perform dramatic volume transition in response to a variety of physical and chemical stimuli, where the physical stimuli include temperature, electric or magnetic field, light, pressure, and sound, while the chemical stimuli include pH, solvent composition, ionic strength, and molecular species (Fig. 1).
Fig. 1.
Stimuli response swelling hydrogel.
The extent of swelling or de-swelling in response to the changes in the external environment of the hydrogel could be so drastic that the phenomenon is referred to as volume collapse or phase transition [12]. Synthetic hydrogels have been a field of extensive research for the past four decades, and it still remains a very active area of research today.
Utilization of hydrogel products
With the establishment of the first synthetic hydrogels by Wichterle and Lim in 1954 [13], the hydrogel technologies may be applied to hygienic products [14], agriculture [15], drug delivery systems [14,16], sealing [14], coal dewatering [17], artificial snow [14], food additives [18], pharmaceuticals [19], biomedical applications [20,21] tissue engineering and regenerative medicines [22,23], diagnostics [24], wound dressing [25], separation of biomolecules or cells [26] and barrier materials to regulate biological adhesions [27], and Biosensor [28].
In addition, the ever growing spectrum of functional monomers and macromeres widen their applicability. They were used in early agricultural water absorbents based on biopolymers through grafting of hydrophilic monomers onto starch and other polysaccharides [29,30]. Hydrogel products for hygienic applications are mainly based on acrylic acid and its salts. Acrylamide is a main component employed for preparation of agricultural hydrogel products [14].
Various publications on this subject have discussed in detail synthetic methods and applications of hydrogels. For example, a comprehensive review of the chemistry and various synthetic schemes employed for hydrogel preparation can be found in various chapters of a compilation edited by Peppas [31]. More recently, hydrogels produced by radiation polymerization and grafting have been published by Khoylou [32]. Mi-Ran Park [33] described the preparation and chemical properties of hydrogels employed in agricultural applications. Vijayalakshmi and Kenichi have reviewed the potential of hydrogels in sensor utilizations [34]. Dimitrios et al. [21] discussed the tailoring of hydrogels for various applications of medical interest.
Technologies adopted in hydrogel preparation
By definition, hydrogels are polymer networks having hydrophilic properties. While hydrogels are generally prepared based on hydrophilic monomers, hydrophobic monomers are sometimes used in hydrogel preparation to regulate the properties for specific applications.
In general, hydrogels can be prepared from either synthetic polymers or natural polymers. The synthetic polymers are hydrophobic in nature and chemically stronger compared to natural polymers. Their mechanical strength results in slow degradation rate, but on the other hand, mechanical strength provides the durability as well. These two opposite properties should be balanced through optimal design [35]. Also, it can be applied to preparation of hydrogels based on natural polymers provided that these polymers have suitable functional groups or have been functionalized with radically polymerizable groups [36].
In the most succinct sense, a hydrogel is simply a hydrophilic polymeric network cross-linked in some fashion to produce an elastic structure. Thus, any technique which can be used to create a cross-linked polymer can be used to produce a hydrogel. Copolymerization/cross-linking free-radical polymerizations are commonly used to produce hydrogels by reacting hydrophilic monomers with multifunctional cross-linkers. Water-soluble linear polymers of both natural and synthetic origin are cross-linked to form hydrogels in a number of ways:
-
1.
Linking polymer chains via chemical reaction.
-
2.
Using ionizing radiation to generate main-chain free radicals which can recombine as cross-link junctions.
-
3.
Physical interactions such as entanglements, electrostatics, and crystallite formation.
Any of the various polymerization techniques can be used to form gels, including bulk, solution, and suspension polymerization.
In general, the three integral parts of the hydrogels preparation are monomer, initiator, and cross-linker. To control the heat of polymerization and the final hydrogels properties, diluents can be used, such as water or other aqueous solutions. Then, the hydrogel mass needs to be washed to remove impurities left from the preparation process. These include non-reacted monomer, initiators, cross-linkers, and unwanted products produced via side reactions (Fig. 2).
Fig. 2.
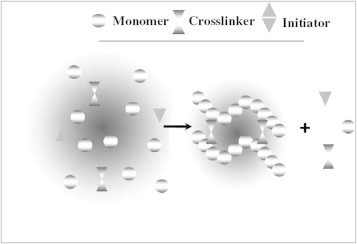
Schematic diagram of hydrogel preparation.
Preparation of hydrogel based on acrylamide, acrylic acid, and its salts by inverse-suspension polymerization [37] and diluted solution polymerization have been investigated elsewhere. Fewer studies have been done on highly concentrated solution polymerization of acrylic monomers, which are mostly patented [38]. Chen [39] produced acrylic acid-sodium acrylate superabsorbent through concentrated (43.6 wt%) solution polymerization using potassium persulphate as a thermal initiator.
Hydrogels are usually prepared from polar monomers. According to their starting materials, they can be divided into natural polymer hydrogels, synthetic polymer hydrogels, and combinations of the two classes.
From a preparative point of view, they can be obtained by graft polymerization, cross-linking polymerization, networks formation of water-soluble polymer, and radiation cross-linking, etc. There are many types of hydrogels; mostly, they are lightly cross-linked copolymers of acrylate and acrylic acid, and grafted starch-acrylic acid polymers prepared by inverse-suspension, emulsion polymerization, and solution polymerization. The polymerization techniques have been described below.
Bulk polymerization
Many vinyl monomers can potentially be used for the productions of hydrogels. Bulk hydrogels can be formed with one or more types of monomers. The wide variety of monomers enables one to prepare the hydrogel with desired physical properties for a given application. Usually, a small amount of cross-linking agent is added in any hydrogel formulation. The polymerization reaction is normally initiated with radiation, ultraviolet, or chemical catalysts.
The choice of a suitable initiator depends upon the type of monomers and solvents being used. The polymerized hydrogel may be produced in a wide variety of forms including films and membranes, rods, particles, and emulsions.
Bulk polymerization is the simplest technique which involves only monomer and monomer-soluble initiators. High rate of polymerization and degree of polymerization occur because of the high concentration of monomer. However, the viscosity of reaction increases markedly with the conversion which generates the heat during polymerization. These problems can be avoided by controlling the reaction at low conversions [40]. The bulk polymerization of monomers to make a homogeneous hydrogel produces a glassy, transparent polymer matrix which is very hard. When immersed in water, the glassy matrix swells to become soft and flexible.
Solution polymerization/cross-linking
In solution copolymerization/cross-linking reactions, the ionic or neutral monomers are mixed with the multifunctional cross-linking agent. The polymerization is initiated thermally by UV-irradiation or by a redox initiator system. The presence of solvent serving as a heat sink is the major advantage of the solution polymerization over the bulk polymerization. The prepared hydrogels need to be washed with distilled water to remove the monomers, oligomers, cross-linking agent, the initiator, the soluble and extractable polymer, and other impurities. Phase separation occurs and the heterogeneous hydrogel is formed when the amount of water during polymerization is more than the water content corresponding to the equilibrium swelling.
Typical solvents used for solution polymerization of hydrogels include water, ethanol, water–ethanol mixtures, and benzyl alcohol. The synthesis solvent may then be removed after formation of the gel by swelling the hydrogels in water.
Suspension polymerization or inverse-suspension polymerization
Dispersion polymerization is an advantageous method since the products are obtained as powder or microspheres (beads), and thus, grinding is not required. Since water-in-oil (W/O) process is chosen instead of the more common oil-in-water (O/W), the polymerization is referred to as “inverse-suspension”.
In this technique, the monomers and initiator are dispersed in the hydrocarbon phase as a homogenous mixture. The viscosity of the monomer solution, agitation speed, rotor design, and dispersant type mainly governs the resin particle size and shape [41]. Some detailed discussions on hetero-phase polymerizations have already been published [42,43]. The dispersion is thermodynamically unstable and requires both continuous agitation and addition of a low hydrophilic–lipophilic-balance (HLB) suspending agent.
Grafting to a support
Generally, hydrogels prepared by bulk polymerization have inherent weak structure. To improve the mechanical properties of a hydrogel, it can be grafted on surface coated onto a stronger support. This technique that involves the generation of free radicals onto a stronger support surface and then polymerizing monomers directly onto it as a result a chain of monomers are covalently bonded to the support. A variety of polymeric supports have been used for the synthesis of hydrogel by grafting techniques [44,45].
Polymerization by irradiation
Ionizing high energy radiation, like gamma rays [46] and electron beams [47], has been used as an initiator to prepare the hydrogels of unsaturated compounds. The irradiation of aqueous polymer solution results in the formation of radicals on the polymer chains. Also, radiolysis of water molecules results in the formation of hydroxyl radicals, which also attack the polymer chains, resulting in the formation of macro-radicals.
Recombination of the macro-radicals on different chains results in the formation of covalent bonds, so finally, a cross-linked structure is formed. Examples of polymers cross-linked by the radiation method are poly (vinyl alcohol), poly(ethylene glycol), and poly(acrylic acid). The major advantage of the radiation initiation over the chemical initiation is the production of relatively pure and initiator-free hydrogels.
Hydrogel technical features
The functional features of an ideal hydrogel material can be listed as follows [48]:
-
•
The highest absorption capacity (maximum equilibrium swelling) in saline.
-
•
Desired rate of absorption (preferred particle size and porosity) depending on the application requirement.
-
•
The highest absorbency under load (AUL).
-
•
The lowest soluble content and residual monomer.
-
•
The lowest price.
-
•
The highest durability and stability in the swelling environment and during the storage.
-
•
The highest biodegradability without formation of toxic species following the degradation.
-
•
pH-neutrality after swelling in water.
-
•
Colorlessness, odorlessness, and absolute non-toxic.
-
•
Photo stability.
-
•
Re-wetting capability (if required) the hydrogel has to be able to give back the imbibed solution or to maintain it; depending on the application requirement (e.g., in agricultural or hygienic applications).
Obviously, it is impossible that a hydrogel sample would simultaneously fulfill all the above mentioned required features. In fact, the synthetic components for achieving the maximum level of some of these features will lead to inefficiency of the rest. Therefore, in practice, the production reaction variables must be optimized such that an appropriate balance between the properties is achieved. For example, a hygienic products of hydrogels must possess the highest absorption rate, the lowest re-wetting, and the lowest residual monomer, and the hydrogels used in drug delivery must be porous and response to either pH or temperature.
Process design implications
The production of polymeric hydrogels is typically accomplished by one of two well-established schemes: (a) polymerization of hydrophilic monomers and (b) modification or functionalization of existing polymers (natural or artificial). The technology of hydrogel production will be discussed in the following sections with an emphasis on recent methods and techniques.
The original sources of hydrogels are often divided into two main classes; i.e., artificial (petrochemical-based) and natural. The latter can be divided into two main groups, i.e., the hydrogels based on polysaccharides and others based on polypeptides (proteins). The natural-based hydrogels are usually prepared through addition of some synthetic parts onto the natural substrates, e.g., graft copolymerization of vinyl monomers on polysaccharides.
It should be pointed out when the term “hydrogel” is used without specifying its type; it actually implies the most conventional type of hydrogels, i.e., the anionic acrylic that comprises a copolymeric network based on the partially neutralized acrylic acid (AA) or acrylamide (AM) [49].
The greatest volume of hydrogels comprises full artificial or of petrochemical origin. They are produced from the acrylic monomers. Acrylic acid (AA) and its sodium or potassium salts, and acrylamide (AM) are most frequently used in the hydrogel industrial production. The two general pathways to prepare acrylic hydrogel networks are simultaneous polymerization and cross-linking by a polyvinyl cross-linker and cross-linking of a water-soluble prepolymer by a polyfunctional cross-linker.
The most common and most versatile technique for the production of synthetic hydrogels is the free-radical multifunctional vinyl monomers.
Each of these monomers contains a carbon double bond through which an active center may propagate to produce polymer chains. The method for generating active centers depends on the particular monomers, solvents, and the reaction conditions to be employed, but may be based on heat (thermal initiators), light (photoinitiators), γ-radiation, or electron beams [49].
Preparation of poly(acrylic acid) hydrogel
Variety of monomers, mostly acrylics, is employed to prepare hydrogels. Acrylic acid (AA) and its sodium or potassium salts are most often used in the industrial production of hydrogels. AA, a colorless liquid with vinegar odor, however, has an ability to convert into its dimer (DAA). In this regard, the DAA level must be minimized to prevent the final product deficiencies, e.g., yield reduction, loss of soluble fraction, residual monomers, etc. Due to the potential problems originating from the inherent nature of AA to dimerize over time, manufacturers work properly with AA, such as timely order placement, just-in-time delivery, moisture exclusion, and temperature-controlled storage (typically 17–18 °C) [49].
As mentioned before, the hydrogel materials are often synthesized through free-radically-initiated polymerization of acrylic monomers. The resins are prepared either in aqueous medium using solution polymerization or in a hydrocarbon medium where the monomers are well-dispersed. These different methods are briefly discussed in the following sections.
Preparation and process optimization of hydrogel by solution polymerization technique
Free-radical initiated polymerization of acrylic acid (AA) and its salts, with a cross-linker, is frequently used for hydrogel preparation. The carboxylic acid groups of the product are partially neutralized before or after the polymerization step. Initiation is most often carried out chemically with free-radical azo or peroxide thermal dissociative species or by reaction of a reducing agent with an oxidizing agent (redox system) [50].
The solution polymerization of AA and/or its salts with a water-soluble cross-linker, e.g., methylene bis-acrylamide (MBA) in an aqueous solution is a straight forward process. The reactants are dissolved in water at desired concentrations, usually about 10–70%.
A fast exothermic reaction yields a gel-like elastic product which is dried, and the macro-porous mass is pulverized and sieved to obtain the required particle size. This preparative method usually suffers from the necessity to handle a rubbery/solid reaction product, lack of a sufficient reaction control, non-exact particle size distribution, and increasing the sol content mainly due to undesired effects of hydrolytic and thermal cleavage. However, for a general production of a hydrogel with acceptable swelling properties, the less expensive and faster technique, i.e., solution method may often be preferred by the manufacturers [49].
The AA monomer is inhibited by methoxyhydroquinone (MHC) to prevent spontaneous polymerization during storage. In industrial production, the inhibitor is not usually removed due to some technical reasons [51]. Meanwhile, AA is converted to an undesired dimer that must be removed or minimized. The minimization of acrylic acid dimer (DAA) in the monomer is important due to its indirect adverse effects on the final product specifications, typically soluble fraction and the residual monomer. As soon as AA is produced, diacrylic acid is formed spontaneously in the bulk of AA reaction. Since temperature, water content, and pH have impact on the rate of DAA formation, the rate can be minimized by controlling the temperature of stored monomer and excluding the moisture [52].
Increasing water concentration has a relatively small impact on the DAA formation rate. Nevertheless, the rate roughly doubles for every 5 °C increase in temperature. For example, in an AA sample having 0.5% water, the dimerization rate is 76 and 1672 ppm/day at 20 °C and 40 °C, respectively. DAA, however, can be hydrolyzed in alkaline media to produce AA and diacrylic acid. Since the latter is unable to be polymerized, it remains as part of the hydrogel soluble fraction.
Javad Alaei et al. [53] stated that production of hydrogels in industry consists of solution and reversed suspension and reversed emulsion polymerizations. Fig. 3 represents a block diagram of a generic solution polymerization process. This figure provides the major procedures for hydrogel manufacturing in the semi-pilot and industrial scales.
Fig. 3.
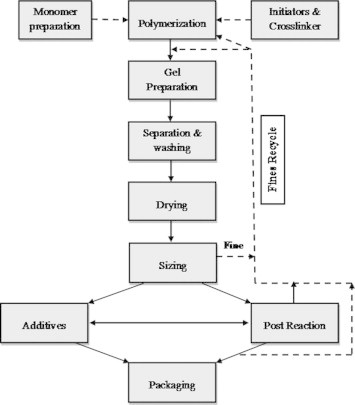
Hydrogel preparation block diagram (solution polymerization/cross-linking procedure).
The flow sheet captures many of the elements of actual free-radical copolymerization reactor installations [54–58]. As shown in Fig. 4, monomers A and B are continuously added with initiator, solvent, and chain transfer agent. In addition, an inhibitor may enter with the fresh feeds as an impurity. These feed streams are combined (stream 1) with the recycle (stream 2) and flow to the reactor (stream 3), which is assumed to be a jacketed well-mixed tank. A coolant flows through the jacket to remove the heat of polymerization. Polymer, solvent, unreacted monomers, initiator, and chain transfer agent flow out of the reactor to the separator (stream 4) where polymer, residual initiator, and chain transfer agent are removed. Unreacted monomers and solvent (stream 7) then continue onto a purge point (stream 8), which represents venting and other losses and is required to prevent accumulation of inerts in the system. After the purge, the monomers and solvent (stream 9) are stored in the recycle hold tank, which acts as a surge capacity to smooth out variations in the recycle flow and composition. The effluent (stream 2) recycle is then added to the fresh feeds.
Fig. 4.
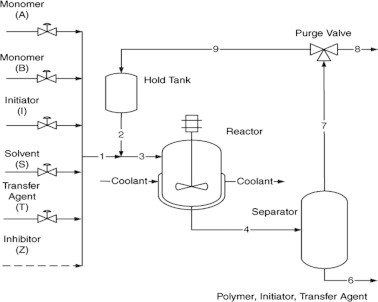
Solution polymerization with recycle loop.
Preparation and process optimization of hydrogel beads using a suspension polymerization technique
The inverse-suspension is a highly flexible and versatile technique to produce hydrogels with high swelling ability and fast absorption kinetics [59]. A water-soluble initiator shows a better efficiency than the oil-soluble type. When the initiator dissolves in the dispersed (aqueous) phase, each particle contains all the reactive species and therefore behaves like an isolated micro-batch polymerization reactor [60].
The resulting microspherical particles are easily removed by filtration or centrifugation from the continuous organic phase. Upon drying, these particles or beads will directly provide a free flowing powder. In addition to the unique flowing properties of these beads, the inverse-suspension process displays additional advantages compared to the solution method. These include a better control of the reaction heat removal, regulation of particle size distribution, and further possibilities for adjusting particle structure or morphology alteration [61].
This method is employed to prepare spherical hydrogels microparticles with size range of 1 μm to 1 mm. In suspension polymerization, the monomer solution is dispersed in the non-solvent forming fine monomer droplets, which are stabilized by the addition of stabilizer. The polymerization is initiated by radicals from thermal decomposition of an initiator. The newly formed microparticles are then washed to remove monomers, cross-linking agent, and initiator.
Recently, the inverse-suspension technique has been widely used for polyacrylamide-based hydrogels because of its easy removal and management of the hazardous, residual acrylamide monomer in the polymer. Fig. 5 represented the block diagram of suspension polymerization process for hydrogel production. Parameters critical to the preparation of hydrogel beads by suspension polymerization remain mostly proprietary or unclear in the literature.
Fig. 5.
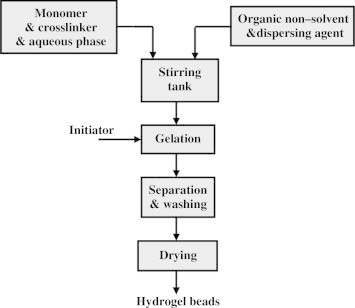
Block diagram of suspension polymerization process.
Furthermore, Lee [61] studied the ranges of process parameters critical to the suspension polymerization of hydrogel beads based on poly-2-hydroxyethyl methacrylate (PHEMA). The PHEMA beads were prepared by free-radical suspension polymerization of 2-hydroxyethyl methacrylate (HEMA) lightly cross-linked with ethylene glycol dimethacrylate (EGDMA) using magnesium hydroxide as the suspension stabilizer.
The Suspension polymerization process flow sheet of Fig. 6 is very similar to the solution polymerization process of Fig. 4, with the exception that water replaces the solvent and the reactor operates adiabatically.
Fig. 6.
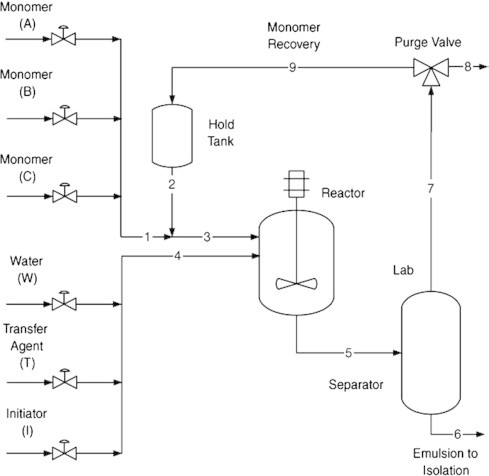
Suspension terpolymerization process with recycle loop.
Optimization of parameters affecting the polymerization process was carried out to maximize bead yield, smoothness, sphericity, and clarity and to achieve a narrow size distribution while reducing the amount of non-bead material. Suspension polymerization inherently produces size-dispersed beads, but their particle size distribution can be controlled by stirring rpm. Parameters found to influence polymer properties in a decreasing order of importance are as follows: initiator type and purity, salt concentration, temperature of polymerization, suspending agent type and concentration, rate and type of stirring, and ratio of dispersed to continuous phase.
Conditions that resulted in a good yield of quality PHEMA beads were found to consist of 0.85–1.7% suspending agent, 18–20% dissolved salt, 3.5–5.25 continuous phase to monomer ratio, 0.2–0.4% initiator, and a stirring speed of 80–120 rpm. Suspension polymerization using a typical setup yielded PHEMA beads of a diameter range between 75 μm and 1000 μm, but largely (>50% by wt.) between 500 and 850 μm, depending on stirring rate. These beads have equilibrium water swelling of 38–41% (w/w). The optimization of preparing conditions of PHEMA hydrogel [61] can be summarized in Table 1.
Table 1.
The optimized conditions for PHEMA hydrogel preparation.
| Parameter | Range |
|---|---|
| Suspending agent | 0.85–1.7% |
| Dissolved salt | 18–20% |
| Continuous phase/monomer ratio | 3.5–5.25 |
| Initiator | 0.2–0.4% |
| Stirring speed | 80–120 rpm |
Preparation and process optimization of hydrogel based on grafted starch
Hydrogels may be based on natural polymers, including macromolecules extracted from animal collagen, plants, and seaweed. These natural macromolecules are typically polysaccharides and proteins comprised of glycosidic and amino acid repeating units, respectively.
Hydrogels of natural polymers, especially polysaccharides, are in general, non-toxic and biodegradable. Considerable research and technical work have been reported. The chemical modification of starch or modified starch via vinyl graft copolymerization constitutes the most important fields for improving the properties of starch and enlarging the range of its utilization. The starch graft-copolymer such as starch-g-polystyrene, starch-g-polyvinyl alcohol, starch-g-methacrylonitrile, and starch-g-acrylonitrile have been produced by generating free radicals on the surface of the starch granules followed by copolymerization of these free radicals with the respective vinyl monomers. These copolymers have also limited biodegradability because of the presence of a non-biodegradable part of the polymer [44].
It has been reported that the synthesis of hydrogels by modification of natural polymers (for example, biocatalytic) has been used for preparation of sugar-containing poly(acrylate) hydrogels. These authors found that by the introduction of small quantities of agar, they were able to eliminate the relative brittleness of the polyacrylamide hydrogels and reduce the formation of undesirable fine particles during wet milling. Raju et al. [37] grafted acrylonitrile onto cassava starch by polymerization initiated by ceric ions. These authors investigated the effects of the reactant concentrations and duration of the polymerization.
The grafting copolymers of many hydrophilic monomers such as acrylamide (AM), acrylic acid (AA), and acrylonitrile (AN) onto starch have been utilized to prepare superabsorbent hydrogels. Among the hydrogels, starch-based hydrogels prepared by hydrolyzing starch graft-polyacrylonitrile have been studied in detail.
Talaat et al. [44] thoroughly investigated the preparation of starch-g-acrylonitrile hydrogel. The main processes of this procedure are mixing of starch and water, grafting with acrylonitrile, separation and drying followed by saponification with alkali at 95 °C for an hour, precipitation with methanol, washing with water free ethanol, and drying under vacuum at 60 °C for 3 h. A redox system (Fe2+/H2O2) has been employed as a source of [OH•] free radicals.
Fig. 7 represents a block diagram of the design process for hydrogel preparation via grafting onto a polysaccharide (starch). The main process parameters concluded in this study may be outlined as follows [44]:
Fig. 7.
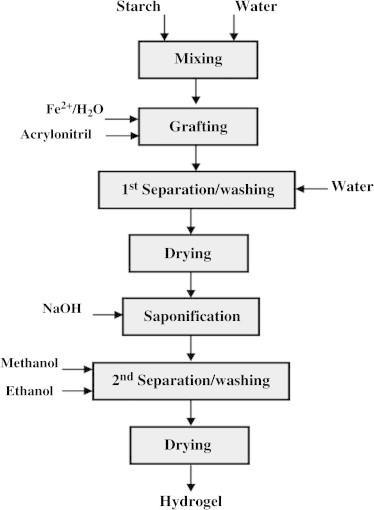
Block diagram for the preparation of the high swelling hydrogel.
AN/starch, 1.4; H2O2 dose, 1.2; and 1.5 g/g corn and potato starches, respectively, H2O2/FeSO4·7H2O = 6 (w/w); Liquor to solid ratio, 10:1; grafting temperature, 30 °C; grafting time 90 min.; saponification time, 90 min; 9 ml NaOH (0.7 N)/g of grafted starch; saponification temperature, 95 °C; methanol used in precipitation and washing (20 ml/g grafted starch); water; drying temperature, 60 °C and drying time, 3 h. Thus, the total duration of hydrogel preparation was about 5 h.
The work done by Qunyi and Ganwei [45] that superabsorbents comprising the graft polymer of acrylonitrile and 2-acrylamido-2-methylpropanesulfonic acid (AMPS) onto starch were prepared using a manganese pyrophosphate redox initiating system. The addition of AMPS resulted in a gradual decrease in saponification time for the graft polymer. Accordingly, the total duration of superabsorbents production also decreases. The effect of potassium hydroxide dose and saponification temperature on the water absorbency of superabsorbent was investigated. Fig. 8 represents the block diagram of a hydrogel prepared by Qunyi and Ganwei [45].
Fig. 8.
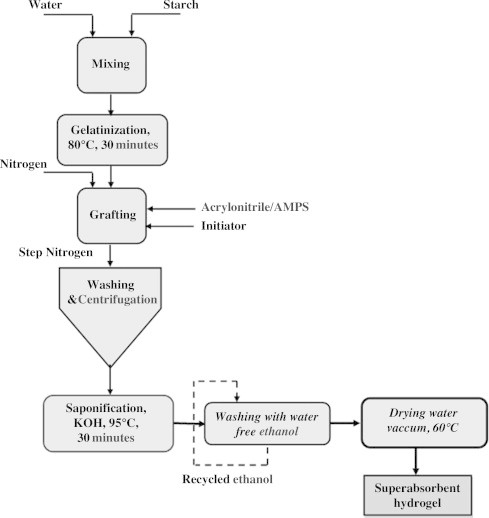
Block diagram of the rapid preparation process of superabsorbent hydrogel.
The maximum response at the optimal saponification conditions can be obtained. The water absorbency was 1345 g/g dry superabsorbent, using the following saponification conditions: KOH volume 203.7 ml, KOH concentration 0.51 mol/l, and saponification temperature 92.6 °C. The shortest saponification time is 17 min, and then, the total synthesis time of superabsorbents is 2.5 h.
The biodegradable superabsorbent polymers [62] were prepared by the graft copolymerization between the gelatinized starch and acrylamide/itaconic acid via foamed solution polymerization using ammonium persulphate (APS) and tetramethylethylene diamine (TEMED) as an oxidation–reduction initiator and co-intiator, respectively, while methylene bisacrylamide (MBA) as a cross-linking agent.
It was found that the presence of both acrylamide and itaconic acid is essential for the grafting reaction on the gelatinized cassava starch to obtain high absorbency such as the water absorption of 379 ± 10 g/g prepared from the optimum mole ratio of AM-to-IA of 90:10 and the optimum weight ratio of starch to the monomer of 1:2 to give the highest percentage of grafting efficiency and the highest water absorption. A preparative scheme outlining the main process for production of starch graft copolymers and side reaction products is demonstrated by the flowchart presented in Fig. 9.
Fig. 9.
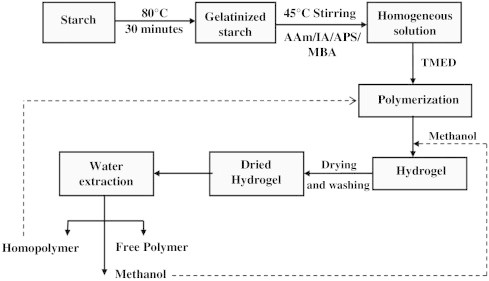
Preparative flowchart for grafted starch and P(AM-co-IA) hydrogel.
A higher amount of the monomers provided the higher grafting opportunity to starch grafting substrate in the other phase. The concentration of the redox initiator APS: TEMED of 1:2 wt% of monomers gave the optimum result to achieve the highest water absorption. Increasing the cross-linking agent concentration in the graft copolymerization enhanced the percentages of grafting efficiency, add-on, and grafting ratio. The optimum condition of the cross-linking agent MBA of 2.0 wt% gave the highest water absorption. The optimum conditions of graft copolymerization of acrylamide and itaconic acid onto cassava starch to prepare a superabsorbent hydrogel [62] can be summarized as shown in Table 2.
Table 2.
Optimum conditions of graft copolymerization of cassava starch and acrylamide/itaconic acid.
| Composition | Ratio (w/w, g/g) |
|---|---|
| Weight ratio of the monomer, AAm/IA | 90:10 |
| Weight ratio of starch to monomer | 1:2 |
| APS (wt%) of monomer | 0.5–2.0 |
| APS/TEMED | 1:2 |
| MBA (wt%) | 2 |
| Temperature | 45 °C |
| Stirring rate | 250 rpm |
Preparation of acrylamide hydrogel by irradiation
Preparation of acrylamide hydrogel from aqueous solutions using γ-ray irradiation has been investigated, and the effects of solution concentration, γ-ray dose, pH, and time have been observed in the characterization of the produced gels. Gel fraction increases with doses for all concentrations, and nearly 100% conversion of gel is attained at 5 KGy for homogeneous solutions in the range of 20–50% concentration. On the one hand, total gel fraction not greater than 86% is obtained even at higher doses (30 KGy) for the solution of 10% concentration.
On the other hand, the solution of 60% concentration is not homogeneous though it gives about 100% gel fraction. Thus, there is a limiting concentration above which the solution is not homogeneous and below which higher doses are needed for the preparation of expected gel. Swelling varies with both the doses and the concentrations due to the change in cross-linking density in the hydrogels. The maximum volume change in hydrogels during swelling occurs within 24 h [63,64].
Design and optimization of efficient, safe, and economically sound radiation-based technologies of hydrogel formation requires the knowledge of the underlying radiation chemistry. This need has been since long one of the main factors stimulating the investigations on radiolysis of polymers in aqueous solutions. Hydrogels can be obtained by radiation technique in a few ways, including irradiation of solid polymer, monomer (in bulk or in solution), or aqueous solution of polymer.
The first method, i.e., irradiation of hydrophilic polymer in a dry form [64], has some drawbacks. It may require special sample preparation (like pressing or melting), and some difficulties may be encountered in obtaining homogeneous macroscopic hydrogels. Moreover, it requires usually much higher doses of ionizing radiation to obtain a gel compared to irradiation in solution, and furthermore, it may be difficult to remove fully the oxygen that can promote unwanted side reactions [65].
An Innovative category of hydrogel products
About three decades ago, superabsorbent polymers (SAPs) were introduced into the agriculture and diaper industries, and then, their applications were extended to other industries where an excellent water holding property was of prime importance.
In 1998, superporous hydrogels (SPHs) were considered as a different category of water-absorbent polymer systems. The original SPHs were developed into next generations of SPHs with more useful properties, such as high mechanical strength and elastic properties. In this review, evolution of SPHs is described in detail, and the differences between SPHs and superabsorbents (SAPs) are also explained. SAPs, just like SPHs, are structurally cross-linked hydrophilic polymers, which have the ability to absorb considerable amounts of water or aqueous fluids (10–1000 times of their original weight or volume) in relatively short periods of time [60].
Depending on the manufacturing process and the materials used during preparation, the swelling rate of SAPs ranges from fraction of a minute to hours. The fast swelling, however, is mainly based on the small size of the SAP samples. On the other hand, the swelling kinetics of SPHs is always fast regardless of the size of the final product. The porous hydrogels are prepared using several techniques, such as freeze-drying [66], microemulsion formation, and phase separation [67]. On the other hand, modern SAPs and SPHs are normally prepared utilizing a gas blowing technique in which acid induced decomposition of a bicarbonate compound is exploited [68].
Although both SAPs and SPHs are porous in structure, they are different from each other as compared in Table 3. The SPHs swell immediately upon contact with water regardless of their size in the dried state [69].The same monomer solution can produce different types of water-absorbing polymer networks, such as nonporous, porous, and superporous structures depending on the presence of foaming agent, foaming aid and foam stabilizer, as shown in Table 4. The comparisons made in Table 4 are based on SAP and SPH prepared by using acrylamide and acrylic acid.
Table 3.
General features of superabsorbent (SAPs) and superporous (SPHs) hydrogels.
| Point of comparison | SAPs | SPHs |
|---|---|---|
| Commonly used monomer | Acrylamide, acrylic acid, salts of acrylic acid including sodium and potassium acrylates | Acrylamide, acrylic acid, salts of acrylic acid including sodium and sulfopropyl acrylates, 2-hydroxyethyl methacrylate |
| Method of synthesis | Bulk, solution, inverse-suspension | Mostly aqueous solution |
| Initiating system | Thermal, redox | Mostly redox |
| Porous structure | Random closed to semi open cells | Interconnected open cells |
| Final product | Particles | Any shape including particles, sheet, film, rod. |
| Applications | Where high swelling, fast-medium rate of swelling is required | Where size-independent high and very fast swelling is required |
Table 4.
Typical formulations of aqueous solution polymerization for SAPs and SPHs preparation.
| Starting material | Role | Nonporous SAP | Porous SAP | Superporous SPH |
|---|---|---|---|---|
| Acrylamide, acrylic acid | Monomer | |||
| Bisacrylamide | Cross-linker | |||
| Deionized water | Solvent | |||
| Ammonium persulphate | Oxidant | |||
| Tetramethyl ethylenediamine | Reductant | |||
| Glacial acetic acid | Foaming aid | |||
| Sodium bicarbonate | Foaming agent | |||
| PEO-PPO-PEO block copolymer | Foam stabilizer | |||
| Starting reaction temperature (°C) | 25 | 25 | 25 | |
| Reaction temperature | Within 30 s (after 15 s of inhibition period) the reaction temperature rises from 25 to about 75 °C with the rate of about 2 °C/s | Within 66 s (after 80 s of inhibition period) the reaction temperature rises from 25 to about 65 °C with the rate of about 1 °C/s | Within 78 s (after 80 s of inhibition period) the reaction temperature rises from 25 to about 55 °C with the rate of about 0.7 °C/s | |
| Reaction product after synthesis | Solid rigid hydrogel | Solid flexible unstable foam | Solid flexible Stable foam |
Preparation of super-absorbent and super-porous hydrogels
In the preparation process of SAPs described by the steps displayed in Fig. 10, the following general procedure is applied regardless of the type of materials used. Since hydrophilic monomers have a very high heat of polymerization, their bulk polymerization is normally associated with a violent exothermic reaction that results in a heterogeneous structure, so-called popcorn product with no water-absorbing properties.
Fig. 10.
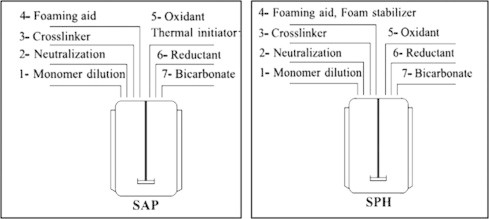
Preparative steps in the production of SAPs and SPHs.
Because of the phenomenon described above, the monomer is first diluted with certain amount of water to reach a desired monomer concentration (Step 1). Dilution with water also makes it easy to handle the monomers. For instance, the water-diluted glacial acrylic acid possesses superior handling properties as compared with acrylic acid because of its lower freezing temperature [69].
Normally, the monomer is mixed with water at room temperature under gentle mixing. To produce ionic superabsorbent, monomers, such as acrylic acid, may be neutralized to some degree, normally to 75 mol% (Step 2), followed by addition of a cross-linker (Step 3). Since neutralization can be accompanied by the sudden release of significant amounts of heat, a double-surfaced reactor equipped with external or internal cooling jackets or coils may be employed.
All modern superabsorbent polymers are produced to possess large amounts of pores necessary to acquire fast water absorption property [70]. This property can normally be achieved by generating gas bubbles. To produce foam during polymerization, foaming aid such as glacial acetic acid is added to the monomer solution (Step 4). For promoted polymerization, thermal and redox initiators, such as ammonium persulphate or potassium persulphate, are normally used. Oxidant and reductant are added to the monomer solution under gentle mixing (Steps 5–6). Gas bubbles are generated by addition of acid-dependent foaming agent, such as sodium bicarbonate (Step 7). Fig. 13 represents a schematic diagram of the post-preparation steps of both SAPs polymer and SPHs hydrogel.
Fig. 13.
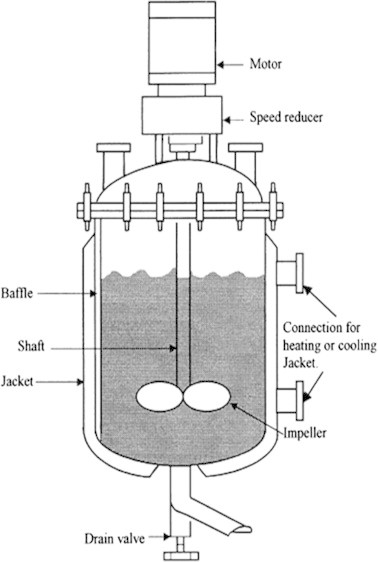
Schematic diagram of batch reactor.
Superporous hydrogels are produced via adding of a foam stabilizer during the process (Step 4). Since the foam stability is essential for producing homogeneous SPHs, surfactants, such as PEO–PPO–PEO triblock copolymers, are used during the preparation process. The aqueous surfactant solution is added to the monomer solution and mixed under gentle mixing. Another unique step produce SPHs is using redox couple initiators such as ammonium persulphate/sodium metabisulphite or potassium persulphate/sodium metabisulphite (Steps 5–6). Almost all SPHs are produced using an oxidant/reductant couple, while SAPs are produced via both thermal and redox systems.
The reactions involved in the preparation of SAPs and SPHs are cross-linking polymerization (which is also known as gelation) and foaming. Dispersion and dissolution of the bicarbonate (Step 7) increases the pH of the reaction medium to a level at which the initiator decomposes faster. As the formation of initiator radicals reaches a certain level, the polymerization reaction proceeds rapidly and the reacting mixture becomes viscous. Concurrently, bicarbonate interacts with the acid component of the system to produce CO2 gases required for the blowing process. The two processes, i.e., gelation and foaming processes need to be conducted in such a way to enable harmonized foaming and gelation.
Since no foam stabilizer is normally used in the preparation of SAPs, the foam spontaneously collapses under its weight and shrinks into a smaller volume. Therefore, pore structures are not preserved in a controlled manner. Consistency of the hydrogel after its formation can affect the foam stabilization. For instance, polymerization of highly concentrated monomer solutions results in sudden gelation of the reacting mixture to a brittle and solid product. Thus, mobility of the polymer chains is prevented, and hence, the pores could be preserved to some extent. The foamed product is then dried and mechanically ground [69].
In case of SPHs, the prepared foamed product is soaked into non-solvents, usually ethanol, to be dehydrated. Dehydration using ethanol helps to stabilize the foamed product and prevent it from shrinking. Complete dehydration results in a solid, brittle porous product, which is white in color because of heterogeneous combination of polymer and pores. Fig. 11 represents a comparative post-preparative scheme between SAPs and SPHs and its reflection on their technical features [69].
Fig. 11.
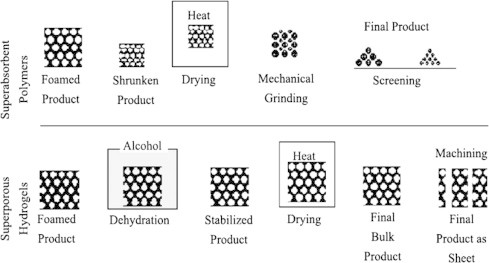
Post-preparation steps of SAPs and SPHs.
The first generation SPHs (conventional SPHs, CSPHs)
In 1999, Chen et al. [71] prepared SPHs with fast swelling kinetics and superabsorbent properties for the first time. In conventional SPHs, the most commonly used monomers for preparation of the first generation of SPHs are highly hydrophilic acrylamide and salts of acrylic acid. The dried SPHs are hard and brittle, but the hydrophilic nature of the polymer results in moisture-induced plasticization of the rigid structures into soft and flexible structures. The dried SPHs swell fast to a large size, larger than a few hundred times of their own volume in the dried state.
Due to very small fraction of the polymer in the swollen state, the swollen SPHs are sometimes difficult to handle without breaking. When the SPHs are dried, the porous structure becomes collapsed or shrunken due to the surface tension of water pulling the polymer chains together during the drying process. To avoid this problem, water inside SPHs is replaced with alcohol (e.g., ethanol). The low surface tension of alcohol prevents the porous structure from collapsing during drying.
The second generation SPHs (SPH composite, SPHCs)
Park et al. [72], for the first time, introduced SPH composites by modifying the conventional SPHs. SPH composites are a matrix of a continuous phase having an incorporated dispersed phase. Composite structures are generally made to attain certain properties, which cannot otherwise be achieved by each matrix alone.
For making SPH composites, a matrix-swelling, additive, or a composite agent is utilized. A composite agent used in SPH composites is a cross-linked water-absorbent hydrophilic polymer that can absorb the solution of monomer, cross-linker, initiator, and remaining components of the SPH preparation. Upon polymerization, the composite agent serves as the local point of physical cross-linking of the formed polymer chains.
During the polymerization process, each composite agent particle acts as an isolated individual reactor in which cross-linking polymerization occurs. As the cross-linking polymerization proceeds throughout the solution, individual swollen composite agent particles are connected together through polymer chains. The presence of composite agent in SPH composites results in improved mechanical properties over conventional (i.e., the first generation) SPH, but the SPH composites are still brittle and thus break into pieces upon application of stresses.
This modification over conventional SPHs resembles modification of superabsorbent polymers through surface cross-linking. Overall, this type of modification results in a higher modulus polymer network in the swollen state, which is susceptible to failure under the brittle fracture mechanism. For many years, this second generation of SPHs has been an attractive research tool for peroral and intestinal drug delivery applications [73–75].
The third generation SPHs: SPH hybrids
To produce SPHs with very high mechanical or elastic properties, the third generation of SPHs was developed based on SPH hybrids [76,77]. Unlike SPH composites wherein a pre-cross-linked matrix-swelling additive is added, SPH hybrids are prepared by adding a hybrid agent that can be cross-linked after SPH is formed. The hybrid agent is a water-soluble or water dispersible polymer that can form cross-linked structure through chemical or physical cross-linking.
Examples of hybrid agents are polysaccharides including sodium alginate, pectin, Chitosan, or synthetic water-soluble hydrophilic polymers such as poly(vinyl alcohol). Once the second network is formed, the whole system becomes similar to interpenetrating polymer networks.
An example of SPH hybrids is the production of acrylamide-based SPH in the presence of sodium alginate, followed by the cross-linking of alginate chains by calcium ions. One of the unique properties of SPH hybrids is that the gels are highly elastic in the swollen state. As compared with conventional SPHs and SPH composites, SPH hybrids are not easily breakable when stretched. The elastic and rubbery properties make SPH hybrids a choice for various applications where resilient gels are preferred. The resiliency of the fully water-swollen SPHs has never previously been observed. Elastic water-swollen SPH hybrids can resist various types of stresses, including tension, compression, bending, and twisting. General structural, swelling, and mechanical properties of different generations of SPHs are shown in Fig. 12.
Fig. 12.

Typical swelling and mechanical properties of: first (A and B), second (C and D) and third (E and F) SPH generations.
Aspects of designing batch polymerization reactor
Most industrial reactors for the manufacture of commodity polymers operate under non-isothermal, batch, or semi-batch reactor conditions. Initially, the temperature of the reaction mass is increased from the ambient temperature to the set point as fast as possible, and then, either an isothermal or a non-isothermal temperature history is imposed. There are three main challenges in this area, viz., the exothermic nature of the polymerization reaction, the viscosity of the reaction mass increasing with polymerization, and the nonavailability of online sensors for monitoring the state variables (monomer conversion xm and weight average molecular weight Mw) characterizing the system. The last of these has triggered the development of software sensors that can predict the properties of the reaction mass using secondary measurements, e.g., temperature, viscosity, and related variables (e.g., power input to a constant speed stirrer motor), heat removal from the reactor, etc. Chien and Penlidis [78] have presented an extensive review of online sensors for polymerization reactors.
The stirred tank batch reactor is still the most widely used reactor type both in the laboratory and in the industry. A batch reactor is one in which a feed material is treated as a whole for a fixed period of time (Fig. 13).
During some polymerization reactions, the viscosity varies in a large range and the level of liquid is not constant. If mixing is not well effected at all times, and at every point in the batch reactor, the reaction is difficult to carry out, and the quality of products can be greatly affected.
Le Cardinal et al., [79] compared, in laboratory stole tanks, the performance of three Impellers known to be effective in high viscosity ranges.
-
–
Ribbon mixer with a screw around the axis.
-
–
Screw mixer with four baffles.
-
–
Double ribbon mixer.
The three propellers (Fig. 14) known to be effective in high viscous Newtonian solutions were Batch reactors that may be preferred for small-scale production of high priced products, particularly if many sequential operations are carried out to obtain high product yields. Batch reactors may also be justified when multiple, low volume products are produced in the same equipment or when continuous flow is difficult, as it is with highly viscous or sticky solids-laden liquids. Because residence time can be more uniform in batch reactors, better yields and higher selectivity may be obtained than with continuous reactors.
Fig. 14.
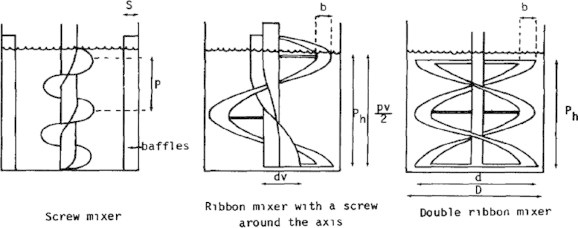
Schematic diagram of impellers used in high viscosity range.
Conclusions
Recently, many hydrogel based networks have been designed and tailored to meet the needs of different applications. The favorable property of these hydrogels is either ability to swell when put in contact with an aqueous solution. The presented review demonstrates the literature concerning classification of hydrogels on different bases, physical and chemical characteristics of these products and technical feasibility of their utilization.
It also involved technologies adopted for hydrogel production together with process design implications, block diagrams and optimized conditions of the preparation process. An innovated category of recent generations of hydrogel materials was also presented in some details. Super-porous hydrogels are new materials that, regardless of their original size, rapidly swell to a large size. Different generations of SPHs evolved to address the needs for certain applications. Based on the literature survey, it can be concluded that batch or semi-batch reactors are suitable reactors for polymerization processes. The variables for batch reactors include temperature, pressure, batch cycle time, the amount of reactants, and the feed addition strategy. Optimization variables such as batch cycle time and amount of reactant are continuous variables with fixed values for a certain batch reactor system depends mainly upon material and energy balance. Ribbon mixer with a screw around the axis, screw mixer with four baffles, and double ribbon mixer are three Impellers known to be effective in high viscosity ranges.
Conflict of interest
The author has declared no conflict of interest.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Biography

Enas M. Ahmed obtained her PhD (Chemical Engineering) from Cairo University, Egypt, in 2005. She is currently an Assistant Professor in Chemical Engineering and Pilot Plant Department, National Research Center, Cairo, Egypt. She is a principal investigator for ongoing research project entitled “Towards Improved Application of Super Absorbent Polymers in Agriculture Fields”. Her era of interest, include Chemical modification of synthetic Polymers; Polymer gels Nanoparticles; and Waste water treatment.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Ahmed Enas M., Aggor Fatma S., Awad Ahmed M., El-Aref Ahmed T. An innovative method for preparation of nanometal hydroxide superabsorbent hydrogel. Carbohydr Polym. 2013;91:693–698. doi: 10.1016/j.carbpol.2012.08.056. [DOI] [PubMed] [Google Scholar]
- 2.Buchholz F.L., Graham A.T. Wiley- VCH; New York: 1998. Modern superabsorbent polymer technology. [chapters 1–7] [Google Scholar]
- 3.Brannon-Peppas L., Harland R.S. Absorbent polymer technology. J Controlled Release. 1991;17(3):297–298. [Google Scholar]
- 4.Li Yuhui, Huang Guoyou, Zhang Xiaohui, Li Baoqiang, Chen Yongmei, Lu Tingli, Lu Tian Jian, Xu Feng. Magnetic hydrogels and their potential biomedical applications. Adv Funct Mater. 2013;23(6):660–672. [Google Scholar]
- 5.http://vikno.eu/eng/health/health/scientists-develop-synthetic-hydrogel.html.
- 6.Burkert Sina, Schmidt Thomas, Gohs Uwe, Dorschner Helmut. Karl-Friedrich Arndt cross-linking of poly(N-vinyl pyrrolidone) films by electron beam irradiation. Radiat Phys Chem. 2007;76(8–9):1324–1328. [Google Scholar]
- 7.Wen Zhao, Xing Jin, Yang Cong, Yuying Liu, Jun Fu. Degradable natural polymer hydrogels for articular cartilage tissue engineering. J Chem Technol Biotechnol. 2013;88(3):327–339. [Google Scholar]
- 8.Takashi L., Hatsumi T., Makoto M., Takashi I., Takehiko G., Shuji S. Synthesis of porous poly(N-isopropylacrylamide) gel beads by sedimentation polymerization and their morphology. J Appl Polym Sci. 2007;104(2):842. [Google Scholar]
- 9.Yang L., Chu J.S., Fix J.A. Colon-specific drug delivery: new approaches and in vitro/in vivo evaluation. Int J Pharm. 2002;235:1–15. doi: 10.1016/s0378-5173(02)00004-2. [DOI] [PubMed] [Google Scholar]
- 10.Maolin Z., Jun L., Min Y., Hongfei H. The swelling behaviour of radiation prepared semi-interpenetrating polymer networks composed of polyNIPAAm and hydrophilic polymers. Radiat Phys Chem. 2000;58:397–400. [Google Scholar]
- 11.Hacker MC, Mikos AG. Synthetic polymers, principles of regenerative medicine. 2nd ed.; 2011. p. 587–622.
- 12.Shin Jinsub., Braun Paul.V., Lee Wonmok. Fast response photonic crystal pH sensor based on templated photo-polymerized hydrogel inverse opal. Sens Actuat B: Chem. 2010;150(1):183–190. [Google Scholar]
- 13.Wichterle O. Hydrophilic gels for biological use. Nature. 1960;185:117. [Google Scholar]
- 14.Singh Anisha, Sharma Pramod Kumar, Garg Vipin Kumar, Garg Garima. Hydrogels: a review. 2010;4(2):Article 016. ISSN: 0976-044X [September–October].
- 15.Amulya K. Saxena synthetic biodegradable hydrogel (Pleura Seal) sealant for sealing of lung tissue after thoracoscopic resection. J Thoracic Cardiovasc Surg. 2010;139(2):496–497. doi: 10.1016/j.jtcvs.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 16.Hamidi Mehrdad, Azadi Amir, Rafiei Pedram. Hydrogel nanoparticles in drug delivery. Adv Drug Deliv Rev. 2009;60(15):1638–1649. doi: 10.1016/j.addr.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 17.Sun X., Zhang G., Shi Q., Tang B., Wu Z.J. Preparation and characterization of water-swellable natural rubbers. J Appl Polym Sci. 2002;86:3212–3717. [Google Scholar]
- 18.Chen X., Martin B.D., Neubauer T.K., Linhardt R.J., Dordick J.S., Rethwisch D.G. Enzymatic and chemoenzymatic approaches to synthesis of sugar based polymer and hydrogels. Carbohydr Polym. 1995;28:15–21. [Google Scholar]
- 19.Kashyap N., Kumar N., Kumar M. Hydrogels for pharmaceutical and biomedical applications. Crit Rev Ther Drug Carr Syst. 2005;22:107–149. doi: 10.1615/critrevtherdrugcarriersyst.v22.i2.10. [DOI] [PubMed] [Google Scholar]
- 20.Kaihara Sachiko., Matsumura Shuichi., Fisher John.P. Synthesis and characterization of cyclic acetal based degradable hydrogels. Eur J Pharm Biopharm. 2008;68(1):67–73. doi: 10.1016/j.ejpb.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 21.Stamatialis Dimitrios F., Papenburg Bernke J., Gironés Miriam, Saiful Saiful, Bettahalli Srivatsa N.M., Schmitmeier Stephanie, Wessling Matthias. Medical applications of membranes: drug delivery, artificial organs and tissue engineering. J Membr Sci. 2008;308(1–2):1–34. [Google Scholar]
- 22.Zhang Ling, Li Kuifeng, Xiao Wenqian, Zheng Li, Xiao Yumei, Fan Hongsong. Preparation of collagen–chondroitin sulfate–hyaluronic acid hybrid hydrogel scaffolds and cell compatibility in vitro. Carbohydr Polym. 2011;84(1):118–125. [Google Scholar]
- 23.Saul Justin M, Williams David F. Hydrogels in regenerative medicine, principles of regenerative medicine. 2nd ed.; 2011. p. 637–61.
- 24.Van der Linden H.J., Herber S., Olthuis W., Bergveld P. Patterned dual pH responsive core shell hydrogels with controllable swelling kinetics and volume. Analyst. 2003;128:325–331. [Google Scholar]
- 25.Sikareepaisan Panprung, Ruktanonchai Uracha, Supaphol Pitt. Preparation and characterization of asiaticoside-loaded alginate films and their potential for use as effectual wound dressings. Carbohydr Polym. 2011;83(4):1457–1469. [Google Scholar]
- 26.Wang Feng, Li Zhenqing, Khan Mahmood, Tamama Kenichi, Kuppusamy Periannan. Injectable, rapid gelling and highly flexible hydrogel composites as growth factor and cell carriers. Acta Biomater. 2010;6(6):1978–1991. doi: 10.1016/j.actbio.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 27.Roy Debashish, Cambre Jennifer N., Brent S. Sumerlin future perspectives and recent advances in stimuli-responsive materials. Prog Polym Sci. 2010;35(12):278–301. [Google Scholar]
- 28.Krsko Peter, McCann Thomas E., Thach Thu-Trang, Laabs Tracy L., Geller Herbert M., Libera Matthew R. Length-scale mediated adhesion and directed growth of neural cells by surface-patterned poly(ethylene glycol) hydrogels Original Research Article. Biomaterials. 2009;30(5):721–729. doi: 10.1016/j.biomaterials.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park J.H., Kim D. Study on foaming water-swellable EPDM rubber. J Appl Polym Sci. 2001;80:115–121. [Google Scholar]
- 30.Pourjavadi A., Harzandi A.M., Hosseinzadeh H. Modified carrageenan 3. Synthesis of a novel polysaccharide-based superabsorbent hydrogel via graft copolymerization of acrylic acid onto kappa-carrageenan in air. Eur Polym J. 2004;40(7):1363–1370. [Google Scholar]
- 31.Peppas NA, Mikos AG. In: Peppas NA, editor. Hydrogels in medicine and pharmacy – fundamentals, vol. I. Florida: CRC Press, Inc.; 1986. p. 1–25.
- 32.Khoylou F., Naimian F. Radiation synthesis of superabsorbent polyethylene oxide/tragacanth hydrogel. Radiat Phys Chem. 2009;78(3):195–198. [Google Scholar]
- 33.Park Mi-Ran, Chun Chang Ju, Ahn Sung-Won, Ki Min-Hyo, Cho Chong-Su, Song Soo-Chang. Sustained delivery of human growth hormone using a polyelectrolyte complex-loaded thermosensitive polyphosphazene hydrogel. J Controlled Release. 2010;147(3):359–367. doi: 10.1016/j.jconrel.2010.07.126. [DOI] [PubMed] [Google Scholar]
- 34.Vijayalakshmi Sridhar, Kenichi Takahata. A hydrogel-based passive wireless sensor using a flex-circuit inductive transducer. Sens Actuat A: Phys. 2009;155(1):58–65. [Google Scholar]
- 35.Tabata Y. Biomaterial technology for tissue engineering applications. J R Soc Interf. 2009;6:S311–S324. doi: 10.1098/rsif.2008.0448.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shantha K.L., Harding D.R.K. Synthesis and evaluation of sucrose-containing polymeric hydrogels for oral drug delivery. J Appl Polym Sci. 2002;84:2597. [Google Scholar]
- 37.Raju K.M., Raju M.P. Synthesis of novel superabsorbing copolymers for agricultural and horticultural applications. Polym Int. 2001;50:946–951. [Google Scholar]
- 38.Takeda H, Taniguchi Y. Production process for highly water absorbable polymer. US Patent 1985; 4,525,527.
- 39.Chen J., Zhao Y. Relation between water absorbency and reaction conditions in aqueous solution polymerization of polyacrylate superabsorbent polymers. J Appl Polym Sci. 2000;75:808–814. [Google Scholar]
- 40.Kiatkamjornwong Suda. Superabsorbent polymers and superabsorbent polymer composites. Science Asia 2007;33(Suppl):1.39–43.
- 41.Ogata Tomonari., Nagayoshi Kana., Nagasako Tadashi., Kurihara Seiji., Nonaka Takamasa. Synthesis of hydrogel beads having phosphinic acid groups and its adsorption ability for lanthanide ions. React Funct Polym. 2006;66(6):625–633. [Google Scholar]
- 42.Hunkeler D. Synthesis and characterization of high molecular weight water-soluble polymers. Polym Int. 1992;27:23–33. [Google Scholar]
- 43.Watanabe N., Hosoya Y., Tamura A., Kosuge H. Characteristics of water-absorbent polymer emulsions. Polym Int. 1993;30:525–531. [Google Scholar]
- 44.Talaat H.A., Sorour M.H., Aboulnour A.G., Shaalan H.F., Ahmed Enas M., Awad A.M., Ahmed M.A. Development of a multi-component fertilizing hydrogel with relevant techno-economic indicators. Am-Euras J Agric Environ Sci. 2008;3(5):764–770. [Google Scholar]
- 45.Qunyi Tong., Ganwei Zhang. Rapid synthesis of a superabsorbent from a saponified starch and acrylonitrile/AMPS graft copolymers. Carbohydr Polym. 2005;62:74–79. [Google Scholar]
- 46.Karadað E., Saraydin D., Gûven O. Radiation induced superabsorbent hydrogels. acrylamide/itaconic acid copolymers. Macromol Mater Eng. 2001;286:34–42. [Google Scholar]
- 47.Ajji Z., Mirjalili G., Alkhatab A., Dada H. Use of electron beam for the production of hydrogel dressings. Radiat Phys Chem. 2008;77(2):200–202. [Google Scholar]
- 48.Zohuriaan-Mehr M.J. Iran Polymer Society; Tehran: 2006. Super-absorbents. p. 2–4 [in Persian] [Google Scholar]
- 49.Zohuriaan-Mehr Mohammad J., Kabiri Kourosh. Superabsorbent polymer materials: a review. Iran Polym J. 2008;17(6):451–477. [Google Scholar]
- 50.Don Trong-Ming, Huang Mei-Lien, Chiu Ai-Chien, Kuo Kuo-Huai, Chiu Wen-Yen, Chiu Lien-Hua. Preparation of thermo-responsive acrylic hydrogels useful for the application in transdermal drug delivery systems. Mater Chem Phys. 2008;107(2–3):266–273. [Google Scholar]
- 51.Pourjavadi A., Kurdtabar M., Mahdavinia G.R., Hosseinzadeh H. Synthesis and super-swelling behavior of a novel protein-based superabsorbent hydrogel. Polym Bull. 2006;57:813–824. [Google Scholar]
- 52.Wampler F.M. Formation of diacrylic acid during acrylic acid storage. Plant/Operation Prog. 1988;7(3):183–189. [Google Scholar]
- 53.Alaei Javad, Boroojerdi Saeid Hasan, Rabiei Zahra. Application of hydrogels in drying operation. Petrol Coal. 2005;47(3):32–37. [Google Scholar]
- 54.BenAmor S., Doyle F.J., McFarlane R. Polymer grade transition control using advanced real-time optimization software. J Process Control. 2004;14:349. [Google Scholar]
- 55.Bindlish R., Rawlings J.B. Target linearization and model predictive control of polymerization processes. AIChE J. 2003;49:2885. [Google Scholar]
- 56.Harris K.R., Palazŏglu A. Control of nonlinear processes using functional expansion models. Comput Chem Eng. 2003;27:1061. [Google Scholar]
- 57.Özkan L., Kothare M.V., Georgakis C. Control of a solution copolymerization reactor using multi-model predictive control. Chem Eng Sci. 2003;58:1207. [Google Scholar]
- 58.Park M.-J., Hur S.-M., Rhee H.-K. Online estimation and control of polymer quality in a copolymerization reactor. AIChE J. 2002;48:10. [Google Scholar]
- 59.Hong Jinho, Lee Jeongwoo, Rhym Young-Mok, Kim Doo-Hyun, Shim Sang Eun. Polyelectrolyte-assisted synthesis of polystyrene microspheres by dispersion polymerization and the subsequent formation of silica shell. J Colloid Interface Sci. 2010;344(2):410–416. doi: 10.1016/j.jcis.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 60.Turakhiya Jignesh M, Savani Hitesh D, Patel Jainish M, Akbari Bhavesh V, Prajapati Neha G, Shah Vyoma S. A review superporous hydrogel (SPH) – an approach for controlled drug delivery. Univ J Pharm 2013:2(1):47–58.
- 61.Ma Y., Lee P. Investigation of suspension polymerization of hydrogel beads for drug delivery. Iran Polym J. 2009;18(4):307–313. [Google Scholar]
- 62.Lanthong P., Nuisin R., Kiatkamjornwong S. Graft copolymerization characterization and degradation of cassava starch-g-acrylamide/itaconic acid superabsorbents. Carbohydr Polym. 2006;66:229–245. [Google Scholar]
- 63.Chowdhury M.A., Alam M.M., Mina M.F., Akhtar F., Kabir S.E. Optimization of the synthetic of acrylamide hydrogel by ɣ-ray irradiation. Chin J Polym Sci. 2004;22(3):253–258. [Google Scholar]
- 64.Francis S., Mitra D., Dhanawade B.R., Varshney L., Sabharwal S. Gamma radiation synthesis of rapid swelling superporous polyacrylamide hydrogels. Radiat Phys Chem. 2009;78(11):951–953. [Google Scholar]
- 65.Varshney Lalit. Role of natural polysaccharides in radiation formation of PVA–hydrogel wound dressing. Nucl Instrum Methods Phys Res, Sect B. 2007;255(2):343–349. [Google Scholar]
- 66.Elbert Donald.L. Liquid–liquid two-phase systems for the production of porous hydrogels and hydrogel microspheres for biomedical applications: a tutorial review. Acta Biomater. 2011;7(1):31–56. doi: 10.1016/j.actbio.2010.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chirila T.V., Constable I.J., Crawford G.J., Vijayasekaran S., Thompson D.E., Chen Y.C., Fletcher W.A. Poly (2-hydroxyethyl methacrylate) sponges as implant materials: in vivo and in vitro evaluation of cellular invasion. Biomaterials. 1993;14:26–38. doi: 10.1016/0142-9612(93)90072-a. [DOI] [PubMed] [Google Scholar]
- 68.Kabiri K., Omidian H., Hashemi S.A., Zohuriaan-Mehr M.J. Concise synthesis of fast-swelling superabsorbent hydrogels: effect of initiator concentration on porosity and absorption rate. J Polym Mater. 2003;(20):17–22. [Google Scholar]
- 69.Omidian Hossein, Rocca Jose G., Park Kinam. Advances in superporous hydrogels. J Pharm Pharmacol. 2007;59:317–327. doi: 10.1211/jpp.59.3.0001. [DOI] [PubMed] [Google Scholar]
- 70.Nochos Argyrios, Douroumis Dionysios, Bouropoulos Nikolaos. In vitro release of bovine serum albumin from alginate/HPMC hydrogel beads. Carbohydr Polym. 2008;74(3):451–457. [Google Scholar]
- 71.Chen J., Park H., Park K. Synthesis of superporous hydrogels: hydrogels with fast swelling and superabsorbent properties. J Biomed Mater Res. 1999;44:53–62. doi: 10.1002/(sici)1097-4636(199901)44:1<53::aid-jbm6>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 72.Park K, Chen J, Park H. Hydrogel composites and superporous hydrogel composites having fast swelling, high mechanical strength, and superabsorbent properties. US patent no. 6271278; 2001.
- 73.Polnok A., Verhoef J.C., Borchard G., Sarisuta N., Junginger H.E. In vitro evaluation of intestinal absorption of desmopressin using drug-delivery systems based on super porous hydrogels. Int J Pharm. 2004;269(2):303–310. doi: 10.1016/j.ijpharm.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 74.Dorkoosh F.A., Verhoef J.C., Borchard G., Rafiee-Tehrani M., Verheijden J.H.M., Junginger H.E. Intestinal absorption of human insulin in pigs using delivery systems based on superporous hydrogel polymers. Int J Pharm. 2002;247(1–2):47–55. doi: 10.1016/s0378-5173(02)00361-7. [DOI] [PubMed] [Google Scholar]
- 75.Dorkoosh F.A., Verhoef J.C., Verheijden J.H.M., Rafiee-Tehrani M., Borchard G., Junginger H.E. Peroral absorption of octreotide in pigs formulated in delivery systems on the basis of superporous hydrogel polymers. Pharm Res. 2002;19(10):1532–1536. doi: 10.1023/a:1020416918624. [DOI] [PubMed] [Google Scholar]
- 76.Omidian H, Qiu Y, Yang S, Kim D, Park H, Park K. Hydrogels having enhanced elasticity and mechanical strength properties. US patent 6960617; 2005.
- 77.Omidian H., Rocca J.G., Park K. Elastic superporous hydrogel hybrid of polyacrylamide and sodium alginate. Macromol Biosci. 2006;6:703–710. doi: 10.1002/mabi.200600062. [DOI] [PubMed] [Google Scholar]
- 78.Chien D.C.H., Penlidis A. On-line sensors for polymerization reactors. J Macromol Sci, Rev Macromol Chem Phys. 1990;30:1–42. [Google Scholar]
- 79.Le Cardinal G., Germain E., Gelus M., Guillon B. The design of stirred batch polymerization reactor. Chem Eng Sci. 1980;35:499–505. [Google Scholar]



