Abstract
Cellular senescence is a state of irreversible cell cycle arrest that has been involved in many gastrointestinal diseases, including human cholestatic liver disorders. Senescence may play a role in biliary atresia, primary sclerosing cholangitis, cellular rejection, and primary biliary cirrhosis, four liver diseases affecting cholangiocytes and the biliary system. In this review, we examine proposed mechanisms of senescence-related biliary diseases, including hypotheses associated with the senescence-associated phenotype, induction of senescence in nearby cells, and the depletion of stem cell subpopulations. Current evidence for the molecular mechanisms of senescence in the previously mentioned diseases is discussed in detail, with attention to recent advances on the role of pathways associated with senescence-associated phenotype, stress-induced senescence, telomere dysfunction, and autophagy.
Cellular senescence is a state of irreversible growth arrest in the G1 phase of the cell cycle.1,2 Telomere shortening, double-stranded DNA damage, inflammation, and other forms of cell stress all function as stimuli for cell senescence. Although traditionally cellular senescence has been viewed as a protective response to cellular injury or disruption, the study of the senescence-associated secretory phenotype (SASP) has become increasingly implicated in the pathogenesis of hepatobiliary disease. Specifically, senescence has been hypothesized to play a prominent role in cholangiopathies, including primary sclerosing cholangitis (PSC), primary biliary cirrhosis (PBC), cellular rejection (CR), and biliary atresia (BA). Over time, the SASP may provide proinflammatory or other adverse stimulus to hepatobiliary stem/progenitor cells, leading to the observed disease phenotypes.3–5 This review summarizes current evidence for the molecular mechanisms of cellular senescence in the pathogenesis of select biliary diseases.
Cellular Senescence
First observed in 1965, human fibroblasts were noted to have a limited ability to replicate in culture.6 Senescent cells are metabolically active cells that often remain in situ but no longer maintain proliferative activity. This development is associated with morphological changes causing the cells to appear large and flat with characteristic nuclear changes, including vacuolation.7–10 Two main tumor-suppressor pathways, p53 and p16INK4a/pRB, have been shown to regulate senescence responses.11 The expression of associated cell cycle inhibitors, including p15INK4B, p16INK4a, p21WAF1/Cip1, p53, and increased senescence-associated β-galactosidase (SA-β-GAL) activity, is commonly observed. In addition, the production of senescence-associated heterochromatin foci and DNA damage response proteins have all been used to aid in the identification of senescent cells in association with the absence of replicative markers (Ki-67 or thymidine analogue bromodeoxyuridine in vitro).12 Overall, senescence has been shown to cause different effects in different tissues and cell types (Table 1). Correlating expression of these markers with disease states has demonstrated the widespread prevalence of the senescent states in hepatobiliary diseases.
Table 1.
Summary of Effects of the Consequences on Various Cell Types
| Cell type | Consequences of senescence∗ |
|---|---|
| Hepatocytes | Unclear, but senescence correlated with fibrosis stage13 |
| Cholangiocytes | Unclear, but senescence correlated with Banff grade in acute rejection14–16 |
| Hepatic stellate cells | Ameliorated fibrosis during acute liver damage via secretion of metalloproteinases17 |
| Retinal pigment epithelial cells | Disruption of division and migration and atrophy of retina in adult macular degeneration18 |
| Fibroblasts | Disrupted tissue structure locally via secretion of VEGF1 |
| Melanocytes | Reinforcing senescence via secretion of IL-6, IL-8, and PAI-119 |
PAI, plasminogen activator inhibitor; VEGF, vascular endothelial growth factor.
Senescence causes different effects in different tissues and cell types.
Replicative Senescence
Multiple effectors have been identified leading to the development of senescence. Classically, replicative senescence develops from telomere erosion associated with the accumulation of replication cycles (aging) in the absence of corrective telomerase (Figure 1). The process of telomere erosion elicits a DNA damage response, which acts to induce the expression of γ-H2Ax (a phosphorylated histone variant of H2Ax) and the DNA damage response proteins p53-binding protein 1, nibrin, and mediator of DNA damage checkpoint protein 1. DNA damage kinases ataxia telangiectasia mutated and ataxia telangiectasia and Rad3-related protein are subsequently activated and, in turn, activate checkpoint kinases 1 and 2.20,21 The resultant signal cascade leads to differential expression of p53 isoforms, and has been linked directly to senescent phenotypes, although the process, including breakpoints between apoptosis and senescence, remains incompletely understood, with some role of NF-κB in its regulation.22,23 In cholestatic liver disease, the role of age-related telomere erosion does not seem prominent. In a study of telomeres using quantitative fluorescent in situ hybridization (Q-FISH) and confirmed by Southern blot analysis from 73 normal liver samples, cholangiocytes and hepatocytes were shown to maintain their telomere lengths independent of age with only Kupffer and stellate cells, demonstrating age-related attrition.24
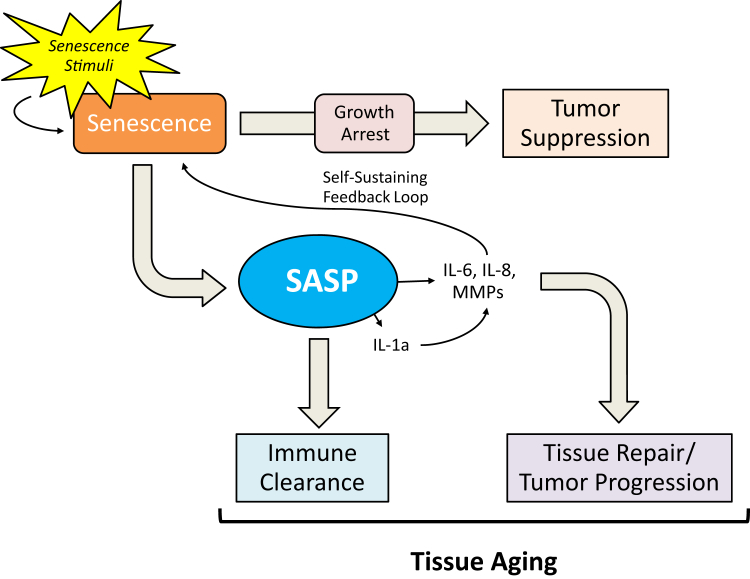
Figure 1.
The senescence-associated secretory phenotype (SASP) during aging, cancer development, and progression. Cellular senescence has dual effects on human health during the progression of aging and various human disorders. The beneficial effects include tumor suppression and tissue repair after injury; the deleterious effects include tumor and aging promotion that involve several key SASP molecules, including IL-6, IL-8, matrix metalloproteinases (MMPs), and specific miRNAs.
Premature Senescence
Numerous other mechanisms independent of replicative senescence have been identified as pathways of so-called premature senescence, including chromatin instability, DNA damage, dysfunctional telomeres, oncogenic mutations, overexpression of cell cycle inhibitors, and stress signals (Figure 2). Oncogenic-induced senescence has been commonly characterized in vitro using mutant H-RasV12, an oncogene, to induce cell cycle arrest signals by activating p53 and p16INK4A/pRB pathways.25 p53 contributes to cellular senescence by transactivating genes that block cellular proliferation, including the p21/Cip1/WAF1 cyclin-dependent kinase inhibitor and miR-34 class of miRNAs.26 Similarly, loss of tumor suppressors (phosphatase and tensin homolog and neurofibromin 1) has been shown to induce premature senescent pathways.27,28 Although our understanding of DNA damage–related senescence provides new insights into these molecular processes, stress-induced senescence is currently more a focal point for research on the pathobiology of cholangiopathies. In vitro, stress-induced senescence has been modeled with alterations in culture medium, growth factors, and oxidative stress in various human cell lines.29–32 In mouse cholangiocytes, proinflammatory cytokines, such as tumor necrosis factor α, interferons β and γ, have been used to generate reactive oxygen species and were shown to induce senescence via the p53/p21WAF1/Cip1 pathway.33 The evidence linking autoinflammatory cholangiopathies to this pathway will be discussed later.
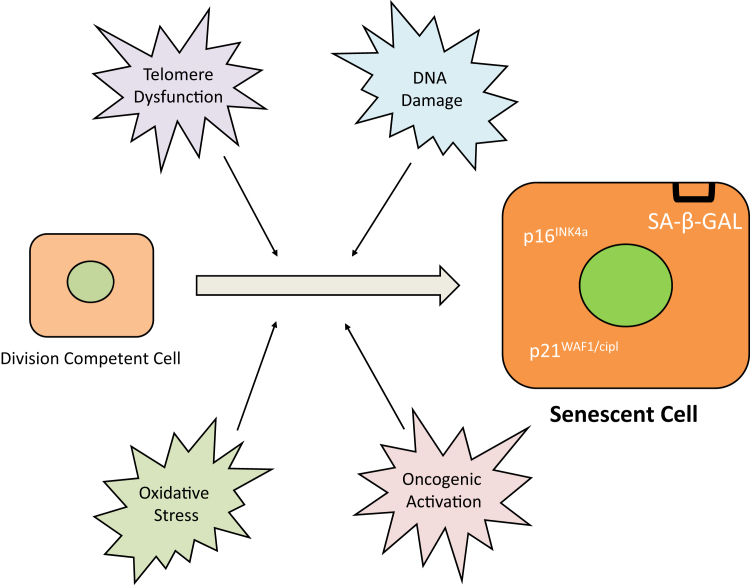
Figure 2.
Overview of senescence. Various factors, such as telomeric dysfunction, DNA damage, oncogenic activation, and oxidative stress, induce senescence in division competent cells. On becoming senescent, the cell and its nucleus become enlarged and begin to express p16INK4a, p21WAF1/Cip1, and senescence-associated β-galactosidase (SA-β-GAL). Senescent cells present a large flattened morphology and build up a SA-β-GAL activity that distinguishes them from most quiescent cells.
Potential Mechanisms of Senescence-Related Human Diseases
Senescence-Associated Secretory Phenotype
Senescent cells exhibit altered gene expression and prominently secrete cytokines (eg, IL-1 and IL-6), chemokines (eg, IL-8, CXCL-1, and CXCL-2), insulin-like growth factor–binding proteins (IGFBPs), and several other factors, including matrix metalloproteinases, serine proteases, and proinflammatory colony-stimulating factors. These soluble signaling factors are referred to as the SASP.34 Acting via paracrine and autocrine mechanisms, these proteins alter the microenvironment of the cells, which may lead to a disruption of tissue structure and function (Figure 3). Although SASP has attracted interest for its potential pathological role in many human diseases, the biological function of SASP as a regulatory mechanism in tumor suppression and other areas remains incompletely understood. Evidence has emerged related to the antifibrotic effects of SASP in the setting of acute liver injury. As hepatic stellate cells proliferate and secrete extracellular matrix (ECM) components and undergo senescence to develop SASP (Figure 4), they secrete metalloproteinases and other proinflammatory molecules that digest the ECM proteins counterbalancing the initial fibrotic response.35 Because senescence has also been reported in chronic bile duct inflammation in the setting of primary biliary cirrhosis and primary sclerosing cholangitis,36 and cholangiocytes in PSC liver exhibit increased expression of SASP components,15 the senescence program may also limit the fibrogenic response to cholestatic liver injury through the similar hepatic stellate cell—related mechanisms.
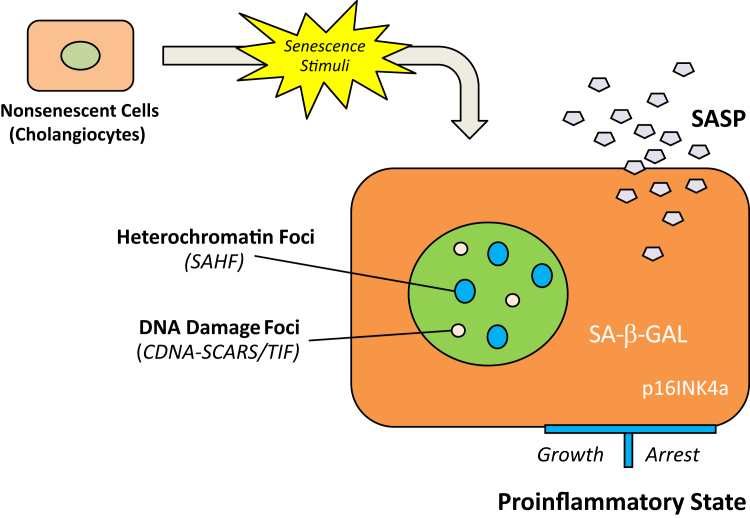
Figure 3.
Formation of senescence-associated secretory phenotype (SASP). Senescent cells/cholangiocytes display different phenotypes from quiescent or terminally differentiated cells (nondividing), whereas the definite feature of the senescent phenotype is notably defined. The significant markers of senescent cells/cholangiocytes include an essentially irreversible growth arrest; expression of senescence-associated β-galactosidase (SA-β-GAL) and p16INK4a; nuclear foci containing DNA damage response proteins (DNA-SCARS/TIF) or senescence-associated heterochromatin foci (SAHF); and robust secretion of various growth factors, cytokines (proinflammatory), proteases, and other proteins, such as SASP. Proinflammatory SASP mediators (C-X-C chemokine receptor 2, IL-6, and IL-6 receptor) can reinforce cellular senescence in an autocrine or paracrine manner. The secretion of SASP is the most significant of these effects because it turns senescent cells/cholangiocytes into a proinflammatory state that has the ability to promote disease progression. SCARS, segments with chromatin alterations reinforcing senescence; TIF, telomere dysfunction-induced foci.
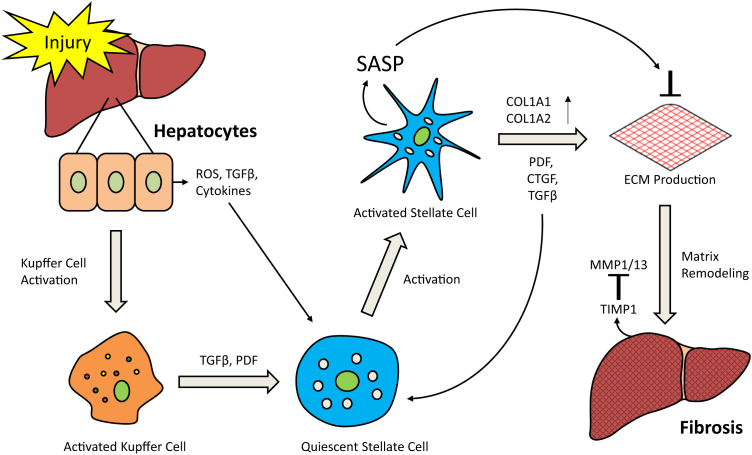
Figure 4.
Recovery effect of hepatic stellate cells drives the senescence-associated secretory phenotype (SASP) during liver fibrosis. The secretion of SASP by activated stellate cells can prevent further proliferation of extracellular matrix (ECM)-producing cells, promote ECM degradation, and accelerate clearance of activated hepatic stellate cells from the site of liver injury/fibrosis. COL1A1, type I collagen; COL1A2, collagen 1 chain type II; CTGF, connective tissue growth factor; MMP, matrix metalloproteinases; PDF, pigment-dispersing factor; ROS, reactive oxygen species; TGF, transforming growth factor; TIMP, tissue inhibitor of metalloproteinases.
Induction of Senescence in Nearby Cells
The ability of SASP to propagate senescence through positive feedback has been hypothesized to potentially be a pathological mechanism in cholangiopathies. To date, the evidence for this effect remains limited. The observation of clustered expression of p16INK4a in nevi has led to the hypothesis that senescent cells secrete senescence-inducing agents that act on other cells locally.37 Subsequently, in human melanoma cell lines, high levels of IGFBP P-rP1 (IGFBP-rP1, previously known as IGFBP7 and mac25) inhibit BRAF-MEK-ERK oncogene signaling and activate senescence or apoptotic pathways.38 As discussed later, overexpression of IGFBP-rP1 has also been observed in BA. However, its exact function in the setting of the disease remains unclear. IGFBP-5 has also been implicated as a protein, which may act locally to induce senescence. Human umbilical endothelial cells treated with exogenous recombinant human IGFBP-5 were found to increase expression of p53 and p21 and decrease proliferation compared to untreated controls.39
Depletion of Progenitor Cell Populations
The potential pathological link between senescence and the depletion of progenitor cells is perhaps theoretically more obvious. The antiproliferative properties of senescence can be detrimental to essential functions of progenitor cell populations, which are crucial to tissue repair and regeneration in numerous organs, particularly the liver. It is possible that as injury or other stimuli deplete these populations, it interferes with proper tissue function. Some of the most direct evidence for this pathological mechanism comes from research on cardiac anthracycline toxicity using a rat model. Treatment of mice with doxorubicin led to expansion of a p16INK4a-positive cardiac stem cell population exhibiting irreversible growth arrest, and this population was significantly associated with left ventricular dysfunction. By using intramyocardial injections of syngeneic cardiac stem cells, researchers were able to rescue the cardiac function of the treated animals, which led to significantly improved survival compared to untreated controls.40 The functional role of a strong cellular senescence inducer, the homeobox transcription factor prospero homeobox protein 1 (Prox1), has been recently clarified in liver progenitors.41 Prox1 depletion in bipotent hepatoblasts significantly decreased the expression of multiple hepatocyte genes and led to defective hepatocyte morphogenesis. Consequently, abnormal epithelial structures expressing hepatocyte and cholangiocyte markers or resembling ectopic bile ducts developed in the Prox1-deficient liver parenchyma. Nevertheless, excessive commitment of hepatoblasts into cholangiocytes, premature intrahepatic bile duct morphogenesis, and biliary hyperplasia occurred in periportal areas of Prox1-deficient livers.41 Certainly, the crucial role of liver progenitor cells could suggest that there may be some contribution of this model in hepatobiliary disease as well.
Cholangiopathies
Biliary Atresia
BA is a progressive, fibro-obliterative disease of the extrahepatic biliary tree that represents the most common cause of pediatric liver transplantation. Even with early Kasai hepatoportoenterostomy, liver transplantation remains necessary for 60% to 80% of patients, and without transplant, the 5-year survival is an unfortunate 60%.42–44 The pathophysiology of this disease remains unknown, although a combination of viral, toxic, genetic, and immunological etiologies have been generally considered.
Interestingly, senescence markers have been observed in affected pediatric patients. In two patients with cirrhosis secondary to BA, p53 and SA-β-GAL were seen expressed in nodular hepatocytes, canals of Hering, and cholangioles with an absent expression of p16 or p21.45 These observations correlate with the results of a genome-wide gene expression analysis of normal, diseased control, and end-stage BA livers, which found strong expression of IGFBP-rP1 compared to the normal and non-BA diseased controls. Previously, IGFBP-rP1 has been shown to be up-regulated in various cell lines with senescent phenotypes and has shown to be a p53-responsive gene.46,47 Although the data are limited, taken together, these findings may suggest a possible mechanism for senescence of cholangiocytes in BA.
Although previously there have been little data involving telomere dysfunction as a mechanism leading to BA, a study by Sanada et al48 measured hepatocyte telomeres using Q-FISH and showed preserved telomere length, but normalized telomere/centromere ratios, being markedly reduced compared to healthy controls. Whether these findings represent a true cause or consequence of the BA remains unclear. Previously, estimations of hepatocyte telomere lengths using Q-FISH in patients with cirrhosis from chronic viral hepatitis, autoimmune hepatitis, primary sclerosing cholangitis, primary biliary cirrhosis, and alcoholic liver disease have found that telomere length correlates to Child-Pugh score independent of associated disease.49 Although the role of telomere dysfunction in BA remains unclear, these studies suggest that hepatocyte telomere length may have some potential role as an adjunct biomarker to clinical scoring models of cirrhosis, such as pediatric end-stage liver disease, model for end-stage liver disease, and Child-Pugh in certain diseases.
Primary Sclerosing Cholangitis
PSC is an idiopathic, autoinflammatory disorder characterized by fibrosis, stricturing, and obliteration of medium and large ducts throughout the biliary epithelium. This incurable disease has a prominent association with inflammatory bowel disease in 70% of patients, particularly ulcerative colitis, and its progressive nature leads to complications, including cholestasis, hepatic failure, and cholangiocarcinoma.50 Median survival in the absence of liver transplant is between 10 and 12 years.51,52
The role of cell senescence in PSC remains an emerging field of research. Cholangiocytes in PSC have been shown to have increased expression of SA-β-GAL, p16INK4a, and p21WAF1.53 We have demonstrated that two cellular senescence markers, plasminogen activator inhibitor-1 and p57, are significantly increased in isolated cholangiocytes from MDR2 knockout mice, which develop periportal fibrosis similar to human PSC (unpublished data). Plasminogen activator inhibitor-1 is an essential mediator of replicative senescence in vitro and is one of the biochemical fingerprints of senescence in vivo.54 The p57 overexpression induced cellular senescence through cell cycle arrest in the G1 phase.55 It has been demonstrated that p57 is involved in hepatocyte growth arrest at two distinct points during liver development: the perinatal period and the postnatal transition to a quiescent adult hepatocyte phenotype.56 Recently, Tabibian et al15 demonstrated increased expression of γ-H2Ax, a marker of DNA damage and up-regulation of SASP markers IL-6, IL-8, chemokine ligand 2, and plasminogen activator inhibitor-1 in diseased samples. Corollary to these findings, by using the lipopolysaccharide inflammatory stress model, they reproduced cholangiocyte senescence-inducing expression of SASP markers and bystander cholangiocyte senescence. Furthermore, their study showed significantly increased expression of N-Ras protein colocalization with activated RAS in PSC, which was absent in PBC, hepatitis C, or control samples. The data build on their previous work suggesting that N-Ras protein mediates lipopolysaccharide-induced inflammation, further supporting a potential role for N-Ras signaling in the pathogenesis of PSC, adding a molecular mechanism to our understanding of PSC as an inflammatory disease.57
One recent study has characterized and identified phenotypic and signaling features of isolated PSC patient-derived cholangiocytes.58 The cholangiocytes from stage 4 PSC patient liver explants were isolated by dissection, differential filtration, and immune-magnetic bead separation. The proportion of cholangiocytes staining positive for senescence-associated β-galactosidase was found to be much higher in PSC cholangiocytes compared with cultured normal human cholangiocytes (48% versus 5%; P < 0.01).58 Interestingly, by using Q-FISH, telomere length in the cholangiocytes of nine PSC samples was found to be maintained compared to normal controls.15 Adjacent hepatocyte telomere length was not reported. If the data from Wiemann et al49 on shortened hepatocyte telomeres in PSCs are substantiated, a complex interaction of senescent pathways could be involved in the pathogenesis of this disease. Certainly, differences in stages of liver disease may play a role, but further clarification of the data on the telomere length of hepatocytes and cholangiocytes in PSCs may be helpful.
Cellular Rejection
Acute CR has historically been associated with a progressive, obliterative cholangiopathy. Histopathology shows a nonsuppurative cholangitis of the interlobular bile ducts with cholangiocytes exhibiting cellular and nuclear enlargement, multinucleation with uneven nuclear spacing, and thickening of the basement membrane.59 In recent years with more aggressive immunosuppression, the rate of CR has decreased from 60% to 70% to 15% to 20% in graft recipients, with ductopenia becoming even rarer.60 Despite therapeutic advances, CR remains a significant cause of morbidity and cost in liver transplant.
Even in early stages of CR, senescence has been observed with absent proliferation of cholangiocytes despite their stereotypical proliferative response to injury.61 The number of p21WAF1/Cip1-positive cholangiocytes has been shown to correlate with the Banff grade of acute rejection and declines with treatment.62,63 Previously, it has been observed that patients with failed allografts who had received cyclosporine had more ductopenia than patients who had received tacrolimus.64 Interestingly in other epithelial tissues, cyclosporine, but not tacrolimus, has been shown to augment transforming growth factor-β expression, which is known to induce p21WAF1/Cip1 expression.65 Building on these findings, Brain et al14 presented observational data on S100A4 expression in CR and in an in vitro model that described dedifferentiation driven by transforming growth factor-β2. Given the diverse expression of S100A4, the precise implications of this work are uncertain, yet intriguing.
Primary Biliary Cirrhosis
PBC is a progressive, autoimmune cholangiopathy involving the small intrahepatic bile ducts. The disease tends to affect women >40 years who may present with symptoms of cholestasis, fibrotic biliary lesions, and hepatomegaly.66,67 Over time if untreated, the sustained loss of bile duct epithelial cells in PBC leads to cirrhosis and liver failure. Serum antimitochondrial antibodies can be detected in the 90% of cases. These antibodies target the E2 and E3BP subunits of the pyruvate dehydrogenase complex (PDC-E2 and PDC-E3BP).68,69 However, their involvement in the pathogenesis of PBC has been uncertain.
Sasaki et al53 have led research on the role of cellular senescence in pathogenesis of PBC. The authors have shown that PBC cholangiocytes exhibit senescence markers SA-β-GAL, p16INK4, and p21WAF1/Cip far more frequently than normal and viral hepatitis controls and observed the increased presence of infiltrating myeloperoxidase-positive inflammatory cells in PBC.53 The role of the inflammatory cells remains unclear, but it suggests that oxidative stress–induced senescence may contribute to the pathogenesis of PBC. Examining telomere length using Q-FISH, they demonstrated that diseased small bile ducts and ductules in PBC compared with normal-appearing bile ducts in PBC, chronic viral hepatitis, and normal livers had significantly shorter telomeres, although the role of telomere length in PBC is unknown.
More interestingly, autophagy, the lysosomal pathway involving the catabolism of cellular components, has been identified as a necessary component to the activation of cell senescence.70 Sasaki et al71 showed that senescent PBC cholangiocytes accumulated markers of autophagy, including microtubule-associated proteins-light chain 3β, cathepsin D, and lysosome-associated membrane protein-1. They further showed aggregation of p62 sequestosome-1, a specific marker for autophagy, in diseased small bile ducts, suggesting some impairment of autophagy in PBC.2,70–72 Subsequently, the group has shown colocalization of mitochondrial antigen PDC-E2 with autophagy marker light chain 3β and increased in vitro cell surface expression of PDC-E2 in cultured biliary epithelial cells on exposure to various stresses.73 Taken together, these findings provide insight into the relationship between autophagy, the expression of mitochondrial antigens, and autoimmunity in PBC. The exact mechanisms behind this pathological interaction remain unclear, but certainly this work suggests further understanding of the pathways related to senescence, particularly autophagy, may yield new insights in the development of biliary disease.
Conclusion and Perspectives
Cellular senescence is linked to the development of various human diseases, and its role in biliary disease remains to be completely elucidated. Even as recent data suggest new questions related to the mechanisms of senescence in the development of cholangiopathies, the role cellular senescence plays in the regulation of biliary growth, injury, and response to injury continues to expand our understanding of biliary disease and hopefully, in the future, yield new opportunities for targeted therapies.
Footnotes
Supported in part by NIH R01 grants DK062975, DK054811, and DK076898 (G.A., S.G., and F.M.), the Department of Veteran's Affairs Merit Review Awards (G.A., S.G., and F.M.), the VA CD-2 Award (H.F.), and the PSC Foundation grant (H.F.).
The views expressed herein are those of the authors and do not necessarily reflect the views of the National Institutes of Health or the Department of Veterans Affairs.
L.M., M.Q., and P.L. contributed equally to this work.
Disclosures: None declared.
References
- 1.Rodier F., Campisi J. Four faces of cellular senescence. J Cell Biol. 2011;192:547–556. doi: 10.1083/jcb.201009094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sasaki M., Nakanuma Y. Novel approach to bile duct damage in primary biliary cirrhosis: participation of cellular senescence and autophagy. Int J Hepatol. 2012;2012:452143. doi: 10.1155/2012/452143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yildiz G., Arslan-Ergul A., Bagislar S., Konu O., Yuzugullu H., Gursoy-Yuzugullu O., Ozturk N., Ozen C., Ozdag H., Erdal E., Karademir S., Sagol O., Mizrak D., Bozkaya H., Ilk H.G., Ilk O., Bilen B., Cetin-Atalay R., Akar N., Ozturk M. Genome-wide transcriptional reorganization associated with senescence-to-immortality switch during human hepatocellular carcinogenesis. PLoS One. 2013;8:e64016. doi: 10.1371/journal.pone.0064016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krizhanovsky V., Xue W., Zender L., Yon M., Hernando E., Lowe S.W. Implications of cellular senescence in tissue damage response, tumor suppression, and stem cell biology. Cold Spring Harb Symp Quant Biol. 2008;73:513–522. doi: 10.1101/sqb.2008.73.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ozturk M., Arslan-Ergul A., Bagislar S., Senturk S., Yuzugullu H. Senescence and immortality in hepatocellular carcinoma. Cancer Lett. 2009;286:103–113. doi: 10.1016/j.canlet.2008.10.048. [DOI] [PubMed] [Google Scholar]
- 6.Hayflick L. The limited in vitro lifetime of human diploid cell strains. Exp Cell Res. 1965;37:614–636. doi: 10.1016/0014-4827(65)90211-9. [DOI] [PubMed] [Google Scholar]
- 7.Serrano M., Lin A.W., McCurrach M.E., Beach D., Lowe S.W. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88:593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- 8.Chen Q., Ames B.N. Senescence-like growth arrest induced by hydrogen peroxide in human diploid fibroblast F65 cells. Proc Natl Acad Sci U S A. 1994;91:4130–4134. doi: 10.1073/pnas.91.10.4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parrinello S., Samper E., Krtolica A., Goldstein J., Melov S., Campisi J. Oxygen sensitivity severely limits the replicative lifespan of murine fibroblasts. Nat Cell Biol. 2003;5:741–747. doi: 10.1038/ncb1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aravinthan A., Verma S., Coleman N., Davies S., Allison M., Alexander G. Vacuolation in hepatocyte nuclei is a marker of senescence. J Clin Pathol. 2012;65:557–560. doi: 10.1136/jclinpath-2011-200641. [DOI] [PubMed] [Google Scholar]
- 11.Lowe S.W., Cepero E., Evan G. Intrinsic tumour suppression. Nature. 2004;432:307–315. doi: 10.1038/nature03098. [DOI] [PubMed] [Google Scholar]
- 12.Collado M., Gil J., Efeyan A., Guerra C., Schuhmacher A.J., Barradas M., Benguría A., Zaballos A., Flores J.M., Barbacid M. Tumour biology: senescence in premalignant tumours. Nature. 2005;436:642. doi: 10.1038/436642a. [DOI] [PubMed] [Google Scholar]
- 13.Hoare M., Das T., Alexander G. Ageing, telomeres, senescence, and liver injury. J Hepatol. 2010;53:950–961. doi: 10.1016/j.jhep.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 14.Brain J.G., Robertson H., Thompson E., Humphreys E.H., Gardner A., Booth T.A., Jones D.E., Afford S.C., von Zglinicki T., Burt A.D., Kirby J.A. Biliary epithelial senescence and plasticity in acute cellular rejection. Am J Transplant. 2013;13:1688–1702. doi: 10.1111/ajt.12271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tabibian J.H., O'Hara S.P., Splinter P.L., Trussoni C.E., LaRusso N.F. Cholangiocyte senescence by way of N-ras activation is a characteristic of primary sclerosing cholangitis. Hepatology. 2014;59:2263–2275. doi: 10.1002/hep.26993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taguchi K., Hirano I., Itoh T., Tanaka M., Miyajima A., Suzuki A., Motohashi H., Yamamoto M. Nrf2 enhances cholangiocyte expansion in Pten-deficient livers. Mol Cell Biol. 2014;34:900–913. doi: 10.1128/MCB.01384-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pellicoro A., Ramachandran P., Iredale J.P., Fallowfield J.A. Liver fibrosis and repair: immune regulation of wound healing in a solid organ. Nat Rev Immunol. 2014;14:181–194. doi: 10.1038/nri3623. [DOI] [PubMed] [Google Scholar]
- 18.Ambati J., Atkinson J.P., Gelfand B.D. Immunology of age-related macular degeneration. Nat Rev Immunol. 2013;13:438–451. doi: 10.1038/nri3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chinta S.J., Lieu C.A., Demaria M., Laberge R.M., Campisi J., Andersen J.K. Environmental stress, ageing and glial cell senescence: a novel mechanistic link to Parkinson's disease? J Intern Med. 2013;273:429–436. doi: 10.1111/joim.12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.di Fagagna FdA., Reaper P.M., Clay-Farrace L., Fiegler H., Carr P., von Zglinicki T., Saretzki G., Carter N.P., Jackson S.P. A DNA damage checkpoint response in telomere-initiated senescence. Nature. 2003;426:194–198. doi: 10.1038/nature02118. [DOI] [PubMed] [Google Scholar]
- 21.Ward I.M., Chen J. Histone H2AX is phosphorylated in an ATR-dependent manner in response to replicational stress. J Biol Chem. 2001;276:47759–47762. doi: 10.1074/jbc.C100569200. [DOI] [PubMed] [Google Scholar]
- 22.Fujita K., Mondal A.M., Horikawa I., Nguyen G.H., Kumamoto K., Sohn J.J., Bowman E.D., Mathe E.A., Schetter A.J., Pine S.R. p53 isoforms Δ133p53 and p53β are endogenous regulators of replicative cellular senescence. Nat Cell Biol. 2009;11:1135–1142. doi: 10.1038/ncb1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crescenzi E., Pacifico F., Lavorgna A., De Palma R., D'Aiuto E., Palumbo G., Formisano S., Leonardi A. NF-κB-dependent cytokine secretion controls Fas expression on chemotherapy-induced premature senescent tumor cells. Oncogene. 2011;30:2707–2717. doi: 10.1038/onc.2011.1. [DOI] [PubMed] [Google Scholar]
- 24.Verma S., Tachtatzis P., Penrhyn-Lowe S., Scarpini C., Jurk D., Von Zglinicki T., Coleman N., Alexander G.J. Sustained telomere length in hepatocytes and cholangiocytes with increasing age in normal liver. Hepatology. 2012;56:1510–1520. doi: 10.1002/hep.25787. [DOI] [PubMed] [Google Scholar]
- 25.Ben-Porath I., Weinberg R.A. The signals and pathways activating cellular senescence. Int J Biochem Cell Biol. 2005;37:961–976. doi: 10.1016/j.biocel.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 26.He L., He X., Lowe S.W., Hannon G.J. microRNAs join the p53 network–another piece in the tumour-suppression puzzle. Nat Rev Cancer. 2007;7:819–822. doi: 10.1038/nrc2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen Z., Trotman L.C., Shaffer D., Lin H.-K., Dotan Z.A., Niki M., Koutcher J.A., Scher H.I., Ludwig T., Gerald W. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature. 2005;436:725–730. doi: 10.1038/nature03918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Courtois-Cox S., Genther Williams S.M., Reczek E.E., Johnson B.W., McGillicuddy L.T., Johannessen C.M., Hollstein P.E., MacCollin M., Cichowski K. A negative feedback signaling network underlies oncogene-induced senescence. Cancer Cell. 2006;10:459–472. doi: 10.1016/j.ccr.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen Q., Fischer A., Reagan J.D., Yan L.-J., Ames B.N. Oxidative DNA damage and senescence of human diploid fibroblast cells. Proc Natl Acad Sci U S A. 1995;92:4337–4341. doi: 10.1073/pnas.92.10.4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yuan H., Kaneko T., Matsuo M. Relevance of oxidative stress to the limited replicative capacity of cultured human diploid cells: the limit of cumulative population doublings increases under low concentrations of oxygen and decreases in response to aminotriazole. Mech Ageing Dev. 1995;81:159–168. doi: 10.1016/0047-6374(95)01584-m. [DOI] [PubMed] [Google Scholar]
- 31.Bennett D.C., Medrano E.E. Molecular regulation of melanocyte senescence. Pigment Cell Res. 2002;15:242–250. doi: 10.1034/j.1600-0749.2002.02036.x. [DOI] [PubMed] [Google Scholar]
- 32.Wright W.E., Shay J.W. Historical claims and current interpretations of replicative aging. Nat Biotechnol. 2002;20:682–688. doi: 10.1038/nbt0702-682. [DOI] [PubMed] [Google Scholar]
- 33.Sasaki M., Ikeda H., Sato Y., Nakanuma Y. Proinflammatory cytokine-induced cellular senescence of biliary epithelial cells is mediated via oxidative stress and activation of ATM pathway: a culture study. Free Radic Res. 2008;42:625–632. doi: 10.1080/10715760802244768. [DOI] [PubMed] [Google Scholar]
- 34.Coppé J.-P., Patil C.K., Rodier F., Sun Y., Muñoz D.P., Goldstein J., Nelson P.S., Desprez P.-Y., Campisi J. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6:e301. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krizhanovsky V., Yon M., Dickins R.A., Hearn S., Simon J., Miething C., Yee H., Zender L., Lowe S.W. Senescence of activated stellate cells limits liver fibrosis. Cell. 2008;134:657–667. doi: 10.1016/j.cell.2008.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sasaki M., Ikeda H., Yamaguchi J., Nakada S., Nakanuma Y. Telomere shortening in the damaged small bile ducts in primary biliary cirrhosis reflects ongoing cellular senescence. Hepatology. 2008;48:186–195. doi: 10.1002/hep.22348. [DOI] [PubMed] [Google Scholar]
- 37.Gray-Schopfer V., Wellbrock C., Marais R. Melanoma biology and new targeted therapy. Nature. 2007;445:851–857. doi: 10.1038/nature05661. [DOI] [PubMed] [Google Scholar]
- 38.Wajapeyee N., Serra R.W., Zhu X., Mahalingam M., Green M.R. Oncogenic BRAF induces senescence and apoptosis through pathways mediated by the secreted protein IGFBP7. Cell. 2008;132:363–374. doi: 10.1016/j.cell.2007.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim K.S., Seu Y.B., Baek S.-H., Kim M.J., Kim K.J., Kim J.H., Kim J.-R. Induction of cellular senescence by insulin-like growth factor binding protein-5 through a p53-dependent mechanism. Mol Biol Cell. 2007;18:4543–4552. doi: 10.1091/mbc.E07-03-0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Angelis A., Piegari E., Cappetta D., Marino L., Filippelli A., Berrino L., Ferreira-Martins J., Zheng H., Hosoda T., Rota M. Anthracycline cardiomyopathy is mediated by depletion of the cardiac stem cell pool and is rescued by restoration of progenitor cell function. Circulation. 2010;121:276–292. doi: 10.1161/CIRCULATIONAHA.109.895771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seth A., Ye J., Yu N., Guez F., Bedford D.C., Neale G.A., Cordi S., Brindle P.K., Lemaigre F.P., Kaestner K.H., Sosa-Pineda B. Prox1 ablation in hepatic progenitors causes defective hepatocyte specification and increases biliary cell commitment. Development. 2014;141:538–547. doi: 10.1242/dev.099481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chardot C., Carton M., Spire-Bendelac N., Le Pommelet C., Golmard J.L., Auvert B. Prognosis of biliary atresia in the era of liver transplantation: French national study from 1986 to 1996. Hepatology. 1999;30:606–611. doi: 10.1002/hep.510300330. [DOI] [PubMed] [Google Scholar]
- 43.Serinet M.-O., Wildhaber B.E., Broué P., Lachaux A., Sarles J., Jacquemin E., Gauthier F., Chardot C. Impact of age at Kasai operation on its results in late childhood and adolescence: a rational basis for biliary atresia screening. Pediatrics. 2009;123:1280–1286. doi: 10.1542/peds.2008-1949. [DOI] [PubMed] [Google Scholar]
- 44.Hadžic N., Davenport M., Tizzard S., Singer J., Howard E.R., Mieli-Vergani G. Long-term survival following Kasai portoenterostomy: is chronic liver disease inevitable? J Pediatr Gastroenterol Nutr. 2003;37:430–433. doi: 10.1097/00005176-200310000-00006. [DOI] [PubMed] [Google Scholar]
- 45.Gutierrez-Reyes G., de Leon MdCG., Varela-Fascinetto G., Valencia P., Tamayo R.P., Rosado C.G., Labonne B.F., Rochilin N.M., Garcia R.M., Valadez J.A. Cellular senescence in livers from children with end stage liver disease. PLoS One. 2010;5:e10231. doi: 10.1371/journal.pone.0010231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Swisshelm K., Ryan K., Tsuchiya K., Sager R. Enhanced expression of an insulin growth factor-like binding protein (mac25) in senescent human mammary epithelial cells and induced expression with retinoic acid. Proc Natl Acad Sci U S A. 1995;92:4472–4476. doi: 10.1073/pnas.92.10.4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Suzuki H., Igarashi S., Nojima M., Maruyama R., Yamamoto E., Kai M., Akashi H., Watanabe Y., Yamamoto H., Sasaki Y. IGFBP7 is a p53-responsive gene specifically silenced in colorectal cancer with CpG island methylator phenotype. Carcinogenesis. 2010;31:342–349. doi: 10.1093/carcin/bgp179. [DOI] [PubMed] [Google Scholar]
- 48.Sanada Y., Kawano Y., Miki A., Aida J., Nakamura K., Shimomura N., Ishikawa N., Arai T., Hirata Y., Yamada N., Okada N., Wakiya T., Ihara Y., Urahashi T., Yasuda Y., Takubo K., Mizuta K. Maternal grafts protect daughter recipients from acute cellular rejection after pediatric living donor liver transplantation for biliary atresia. Transpl Int. 2014;27:383–390. doi: 10.1111/tri.12273. [DOI] [PubMed] [Google Scholar]
- 49.Wiemann S.U., Satyanarayana A., Tsahuridu M., Tillman H.L., Zender L., Klempnaur J., Flemming P., Franco S., Blasco M.A., Manns M.P. Hepatocyte telomere shortening and senescence are general markers of human liver cirrhosis. FASEB J. 2002;16:935–942. doi: 10.1096/fj.01-0977com. [DOI] [PubMed] [Google Scholar]
- 50.Boonstra K., Beuers U., Ponsioen C.Y. Epidemiology of primary sclerosing cholangitis and primary biliary cirrhosis: a systematic review. J Hepatol. 2012;56:1181–1188. doi: 10.1016/j.jhep.2011.10.025. [DOI] [PubMed] [Google Scholar]
- 51.Wiesner R.H., Grambsch P.M., Dickson E.R., Ludwig J., Maccarty R.L., Hunter E.B., Fleming T.R., Fisher L.D., Beaver S.J., Larusso N.F. Primary sclerosing cholangitis: natural history, prognostic factors and survival analysis. Hepatology. 1989;10:430–436. doi: 10.1002/hep.1840100406. [DOI] [PubMed] [Google Scholar]
- 52.Broome U., Olsson R., Lööf L., Bodemar G., Hultcrantz R., Danielsson A., Prytz H., Sandberg-Gertzen H., Wallerstedt S., Lindberg G. Natural history and prognostic factors in 305 Swedish patients with primary sclerosing cholangitis. Gut. 1996;38:610–615. doi: 10.1136/gut.38.4.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sasaki M., Ikeda H., Haga H., Manabe T., Nakanuma Y. Frequent cellular senescence in small bile ducts in primary biliary cirrhosis: a possible role in bile duct loss. J Pathol. 2005;205:451–459. doi: 10.1002/path.1729. [DOI] [PubMed] [Google Scholar]
- 54.Eren M., Boe A.E., Murphy S.B., Place A.T., Nagpal V., Morales-Nebreda L., Urich D., Quaggin S.E., Budinger G.R., Mutlu G.M., Miyata T., Vaughan D.E. PAI-1-regulated extracellular proteolysis governs senescence and survival in Klotho mice. Proc Natl Acad Sci U S A. 2014;111:7090–7095. doi: 10.1073/pnas.1321942111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tsugu A., Sakai K., Dirks P.B., Jung S., Weksberg R., Fei Y.L., Mondal S., Ivanchuk S., Ackerley C., Hamel P.A., Rutka J.T. Expression of p57(KIP2) potently blocks the growth of human astrocytomas and induces cell senescence. Am J Pathol. 2000;157:919–932. doi: 10.1016/S0002-9440(10)64605-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Awad M.M., Sanders J.A., Gruppuso P.A. A potential role for p15(Ink4b) and p57(Kip2) in liver development. FEBS Lett. 2000;483:160–164. doi: 10.1016/s0014-5793(00)02108-6. [DOI] [PubMed] [Google Scholar]
- 57.O'Hara S.P., Splinter P.L., Trussoni C.E., Gajdos G.B., Lineswala P.N., LaRusso N.F. Cholangiocyte N-Ras protein mediates lipopolysaccharide-induced interleukin 6 secretion and proliferation. J Biol Chem. 2011;286:30352–30360. doi: 10.1074/jbc.M111.269464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tabibian J.H., Trussoni C.E., O'Hara S.P., Splinter P.L., Heimbach J.K., LaRusso N.F. Characterization of cultured cholangiocytes isolated from livers of patients with primary sclerosing cholangitis. Lab Invest. 2014;94:1126–1133. doi: 10.1038/labinvest.2014.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vierling J.M., Fennell R.H. Histopathology of early and late human hepatic allograft rejection: evidence of progressive destruction of interlobular bile ducts. Hepatology. 1985;5:1076–1082. doi: 10.1002/hep.1840050603. [DOI] [PubMed] [Google Scholar]
- 60.Maluf D.G., Stravitz R.T., Cotterell A.H., Posner M.P., Nakatsuka M., Sterling R.K., Luketic V.A., Shiffman M.L., Ham J.M., Marcos A. Adult living donor versus deceased donor liver transplantation: a 6-year single center experience. Am J Transplant. 2005;5:149–156. doi: 10.1111/j.1600-6143.2004.00654.x. [DOI] [PubMed] [Google Scholar]
- 61.Koukoulis G.K., Shen J., Karademir S., Jensen D., Williams J. Cholangiocytic apoptosis in chronic ductopenic rejection. Hum Pathol. 2001;32:823–827. doi: 10.1053/hupa.2001.26465. [DOI] [PubMed] [Google Scholar]
- 62.Ray M.B., Schroeder T., Michaels S.E., Hanto D.W. Increased expression of proliferating cell nuclear antigen in liver allograft rejection. Liver Transpl Surg. 1996;2:337–342. doi: 10.1002/lt.500020502. [DOI] [PubMed] [Google Scholar]
- 63.Lunz J.G., III, Contrucci S., Ruppert K., Murase N., Fung J.J., Starzl T.E., Demetris A.J. Replicative senescence of biliary epithelial cells precedes bile duct loss in chronic liver allograft rejection: increased expression of p21WAF1/Cip1 as a disease marker and the influence of immunosuppressive drugs. Am J Pathol. 2001;158:1379–1390. doi: 10.1016/S0002-9440(10)64089-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Blakolmer K., Seaberg E.C., Batts K., Ferrell L., Markin R., Wiesner R., Detre K., Demetris A. Analysis of the reversibility of chronic liver allograft rejection implications for a staging schema. Am J Surg Pathol. 1999;23:1328–1339. doi: 10.1097/00000478-199911000-00003. [DOI] [PubMed] [Google Scholar]
- 65.Zhang J.G., Walmsley M., Moy J., Cunningham A., Talbot D., Dark J., Kirby J. Differential effects of cyclosporin A and tacrolimus on the production of TGF-β: implications for the development of obliterative bronchiolitis after lung transplantation. Transpl Int. 1998;11:S325–S327. doi: 10.1007/s001470050489. [DOI] [PubMed] [Google Scholar]
- 66.Jones D.E. Pathogenesis of primary biliary cirrhosis. Clin Liver Dis. 2008;12:305–321. doi: 10.1016/j.cld.2008.02.004. viii. [DOI] [PubMed] [Google Scholar]
- 67.Invernizzi P., Gershwin M.E. Primary biliary cirrhosis: bad genes, bad luck. Dig Dis Sci. 2012;57:599–601. doi: 10.1007/s10620-011-1993-3. [DOI] [PubMed] [Google Scholar]
- 68.Fussey S.P., Guest J.R., James O.F., Bassendine M.F., Yeaman S.J. Identification and analysis of the major M2 autoantigens in primary biliary cirrhosis. Proc Natl Acad Sci U S A. 1988;85:8654–8658. doi: 10.1073/pnas.85.22.8654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shimoda S., Van de Water J., Ansari A., Nakamura M., Ishibashi H., Coppel R.L., Lake J., Keeffe E.B., Roche T.E., Gershwin M.E. Identification and precursor frequency analysis of a common T cell epitope motif in mitochondrial autoantigens in primary biliary cirrhosis. J Clin Invest. 1998;102:1831–1840. doi: 10.1172/JCI4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Young A.R., Narita M., Ferreira M., Kirschner K., Sadaie M., Darot J.F., Tavaré S., Arakawa S., Shimizu S., Watt F.M. Autophagy mediates the mitotic senescence transition. Genes Dev. 2009;23:798–803. doi: 10.1101/gad.519709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sasaki M., Miyakoshi M., Sato Y., Nakanuma Y. A possible involvement of p62/sequestosome-1 in the process of biliary epithelial autophagy and senescence in primary biliary cirrhosis. Liver Int. 2012;32:487–499. doi: 10.1111/j.1478-3231.2011.02656.x. [DOI] [PubMed] [Google Scholar]
- 72.Sasaki M., Miyakoshi M., Sato Y., Nakanuma Y. Autophagy mediates the process of cellular senescence characterizing bile duct damages in primary biliary cirrhosis. Lab Invest. 2010;90:835–843. doi: 10.1038/labinvest.2010.56. [DOI] [PubMed] [Google Scholar]
- 73.Sasaki M., Miyakoshi M., Sato Y., Nakanuma Y. Increased expression of mitochondrial proteins associated with autophagy in biliary epithelial lesions in primary biliary cirrhosis. Liver Int. 2013;33:312–320. doi: 10.1111/liv.12049. [DOI] [PubMed] [Google Scholar]