Abstract
Visceral fat necrosis has been associated with severe acute pancreatitis (SAP) for over 100 years; however, its pathogenesis and role in SAP outcomes are poorly understood. Based on recent work suggesting that pancreatic fat lipolysis plays an important role in SAP, we evaluated the role of pancreatic lipases in SAP-associated visceral fat necrosis, the inflammatory response, local injury, and outcomes of acute pancreatitis (AP). For this, cerulein pancreatitis was induced in lean and obese mice, alone or with the lipase inhibitor orlistat and parameters of AP induction (serum amylase and lipase), fat necrosis, pancreatic necrosis, and multisystem organ failure, and inflammatory response were assessed. Pancreatic lipases were measured in fat necrosis and were overexpressed in 3T3-L1 cells. We noted obesity to convert mild cerulein AP to SAP with greater cytokines, unsaturated fatty acids (UFAs), and multisystem organ failure, and 100% mortality without affecting AP induction or pancreatic necrosis. Increased pancreatic lipase amounts and activity were noted in the extensive visceral fat necrosis of dying obese mice. Lipase inhibition reduced fat necrosis, UFAs, organ failure, and mortality but not the parameters of AP induction. Pancreatic lipase expression increased lipolysis in 3T3-L1 cells. We conclude that UFAs generated via lipolysis of visceral fat by pancreatic lipases convert mild AP to SAP independent of pancreatic necrosis and the inflammatory response.
Visceral fat necrosis has been noted to occur with pancreatitis for over 100 years.1,2 This fat is typically located in or around the pancreas3,4 and is a major component of necrotizing pancreatitis and peripancreatic necrosis.5,6 Despite being a part of the criteria for staging the severity of acute pancreatitis (AP) in humans7–9 and being included as a separate entity in the recently revised Atlanta criteria,6 the pathogenesis and role of fat necrosis, that is, whether it is a marker or mediator of severe AP (SAP), remains unclear.
Clinical correlates of the relevance of fat necrosis include the several epidemiological studies that show individuals with increased intra-abdominal fat10–13 or obese patients being at an increased risk for SAP.11,14–20 Peripancreatic fat necrosis may occur independent of pancreatic necrosis5 and is associated with an increased risk for severe attacks.21,22 Although there is basolateral leakage of pancreatic enzymes during pancreatitis,23–26 and lipases have been noted in fat necrosis in human disease,27,28 it is unclear whether these lipases cause fat necrosis or are a remnant of pancreatic damage.
Triglycerides compose 80% to 90% of the volume of adipocytes29–31 and can be hydrolyzed by lipases released basolaterally during pancreatitis.23–26 Although in vitro studies done more than 2 decades ago showed lipase inhibition to reduce pancreatic acinar injury on co-incubation with pancreatic homogenates and triglycerides,32 in vivo lipase inhibition in SAP models showed no benefit33 until recently, when this was studied in the context of increased visceral fat.34,35
Previous studies have shown that long-chain unsaturated fatty acids (UFAs) form the majority of the nonesterified fatty acids in necrotic collections36,37 and are more toxic than are saturated fatty acids.32,34,35,38 Since obese (ob/ob) mice and obese humans may have a similar visceral fat composition,3,35 we chose to study whether a classically mild model of pancreatitis, that is, cerulein pancreatitis, would result in different outcomes in these mice versus lean mice and explore the role of fat necrosis in these outcomes. Our previous work has shown a benefit of pharmacological inhibition of pancreatic lipases in biliary, IL-12–induced, and IL-18–induced pancreatitis34,35; we therefore used this as a tool to influence outcomes. Since obesity is also known to be associated with an exaggerated baseline inflammatory state, we went on to study whether the inflammatory response was different between the groups that had different outcomes. Additionally, since SAP may occur along with severe pancreatic necrosis or with multisystem organ failure (MSOF) with insignificant necrosis,39–41 we also compared pancreatic necrosis between groups with different outcomes.
Reasons for preferring a pharmacological approach34,35 over a genetic one include: i) previous validation in mechanistically distinct models,34,35 ii) the redundant roles of pancreatic lipases42 (which are supported by this study), iii) the deletion of the two lipases of interest being embryonically lethal,43 and iv) the inability to make mice with a genetic deletion of lipases obese on a high-fat diet (unpublished data). Our results, in concert with those from previous studies,34,35 show that pancreatic lipase–dependent visceral fat lipolysis worsens outcomes of AP, unrelated to the initiator of the disease, and suggest alternate ways to interpret the parameters of severity in AP, along with suggesting a potential approach to improving SAP outcomes.
Materials and Methods
Animal Studies
Male ob/ob (B6.V-lepob/J) mice or C57bl6 (lean) mice (8 to 10 weeks) were purchased from Jackson Laboratories (Bar Harbor, ME) and were acclimatized for at least 2 days before use. All animals were housed with a 12-hour light/dark cycle at temperatures from 21°C to 25°C, fed standard laboratory chow, and allowed to drink ad libitum. AP was induced in lean (C57bl6) and ob/ob mice by hourly i.p. injections of cerulein (50 μg/kg) × 12 doses on 2 consecutive days (CR group). Obese mice were administered orlistat [50 mg/kg b.i.d. in the CRO2 group (sacrificed at the end of 2 days) and in the CRO5 group (sacrificed at the end of 5 days)] as described previously35 or its vehicle [10% triolein in phosphate-buffered saline, 100 μL per 30 g body weight in the CR + vehicle (CRV) group] i.p. for 2 days. The first injection was administered 2 hours after the induction of pancreatitis. The mice were followed up over a course of 5 days or until they were moribund or died overnight. Lean mice were also electively sacrificed at the end of the first 24 hours (CR1) or 48 hours after the first injection (CR2). Similarly, mice given orlistat (CRO2 group) were also electively sacrificed at 48 hours for comparison to the CR and CRV groups since 50% to 100% of the mortality occurred in these groups at 48 hours. All of the experiments were approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh (Pittsburgh, PA) and at the Mayo Clinic (Scottsdale, AZ).
Cytokine Assays
Cytokine assays were done as previously described,35,38 with the fluorescence-based capture sandwich immunoassay based on the Luminex FlowMetrix system (Life Technologies, Carlsbad, CA). The Milliplex mouse adipokine panel (7-plex) 1 kit (Millipore, Billerica, MA) was used for serum analysis. A Bio-Plex suspension array system, including a fluorescent reader and Bio-Plex Manager analytical software version 200 (Bio-Rad Laboratories, Hercules, CA), was used for analyzing samples at the University of Pittsburgh Cancer Institute.
Evaluation of Pancreatic Necrosis and Special Stains
Whole pancreas section slides stained by hematoxylin and eosin were examined by a trained morphologist (K.P.) blinded to the sample as described previously.35,44 Briefly, all contiguous areas were imaged using a 4× objective and photographed. Total fat area, necrotic fat area, perifat acinar necrosis, and total acinar necrotic area were measured in pixels as a percentage of total acinar area calculated for each pancreas. Von Kossa staining was done as described previously.35,38 Staining with terminal deoxynucleotidyl transferase dUTP nick end labeling was done on paraffin sections of the lungs and kidneys as described previously.35 Serial sections of gonadal fat pads were stained with hematoxylin and eosin and von Kossa staining. Von Kossa staining was done to detect calcium salts following the manufacturer's instructions (von Kossa stain kit, American MasterTech, Lodi, CA).
Nonesterified Fatty Acid Analysis
Long-chain fatty acid was analyzed using gas chromatography as described previously.35,38 UFA amounts were calculated by adding individual C16:1, C18:1, C18:2, and C20:4 fatty acids. Saturated fatty acid amounts were calculated by adding individual C12:0, C14:0, C16:0, and C18:0 fatty acids.
Biochemical Assays
Amylase, lipase, blood urea nitrogen, calcium, and glycerol were measured following the manufacturer's instructions (Pointe Scientific, Inc., Canton, MI). Tests were performed on a ChemWell-T Chemistry Analyzer (Awareness Technology, Palm City, FL).
Nuclear Magnetic Resonance Assessment of Visceral Fat
The mice were weighed, and their whole-body fat and lean tissue masses were determined using the MiniSpec Body Composition Analyzer (Bruker Corporation, Billerica, MA) based on nuclear magnetic resonance. The analyzer acquires and analyzes time domain–nuclear magnetic resonance signals from all protons in the entire sample volume and can provide the three components of interest: fat, free body fluid, and lean tissue values.
Western Blot Analysis
Gonadal fat pad or 3T3-L1 cells were homogenized in lysis buffer containing a protease inhibitor cocktail (Complete, EDTA Free; Roche, Mannheim, Germany). Lysates were used for Western blot analysis after protein estimation with a Pierce protein assay kit (Thermo Fisher Scientific, Rockford, IL). Lysates from fat pads or 3T3-L1 were analyzed by Western blots that were incubated with primary antibody [anti-hPTL (1:10,000; a kind gift from Dr. Mark Lowe, University of Pittsburgh) and anti-hPLRP2 (1:400; SantaCruz Biotechnology, Dallas, TX)] and then probed with horseradish peroxidase-labeled goat anti-rabbit IgG and donkey anti-goat IgG (Millipore), respectively. Bands were visualized by chemiluminescence using electrogenerated chemiluminescence plus a Western Blot Detection Kit (Amersham GE Healthcare, Buckinghamshire, UK).
Immunohistochemistry Analysis
The horseradish peroxidase immunohistochemistry technique was used for detecting CD11b, CD68, and myeloperoxidase in paraffin-embedded sections of gonadal fat pads of obese mice. In brief, after deparaffinization and antigen epitope retrieval, tissues were incubated with a rat polyclonal antibody against CD11b (1:25, Developmental studies hybridoma bank, Iowa City, IA), a rabbit polyclonal antibody against CD68 (1:25; Abcam, Cambridge, MA), or a rabbit polyclonal antibody against myeloperoxidase (1:50; Abcam), followed by the application of horseradish peroxidase–conjugated (1:200; Millipore or Thermo Pierce Scientific, Rockford, IL) secondary antibody. Staining was completed with chromogen incubation with AEC substrate kit for Peroxidase and Hematoxylin QS nuclear counterstain (Vector Laboratories, Burlingame, CA).
Cell Culture of 3T3-L1 Adipocytes
3T3-L1 preadipocytes cells were obtained from American Type Culture Collection (Manassas, VA). 3T3-L1 preadipocytes were grown in 75 cm2 tissue culture flask in Dulbecco’s modified Eagle’s medium containing 10% bovine calf serum in a humidified incubator at 37°C with 5% CO2. For adenoviral transduction experiment, differentiation was induced using 3T3-L1 differentiation medium (ZenBio Inc., Research Triangle Park, NC). After cells reached confluence, cells were further maintained in the 3T3-L1 adipocyte medium (ZenBio) for 10 to 12 days before adenoviral transfection. The cDNA sequence corresponding to mouse pancreatic triglyceride lipase (PTL) (GenBank; http://www.ncbi.nlm.nih.gov/nuccore; Accession number NM_026925.3) and mouse pancreatic lipase related protein-2 (PLRP2) (GenBank; Accession number NM_011128.2) were cloned into pAdlox vector to generate pAdlox/PTL or pAdlox/PLRP2 constructs. Adenoviruses of these constructs were generated at the Vector Core Laboratory, University of Pittsburgh. Mature 3T3-L1 adipocytes were transfected with 4 × 108 plaque-forming units per milliliter of recombinant PTL or PLRP2 adenoviruses for their overexpression in the presence of 50 μmol/L adipose triglyceride lipase inhibitor Atglistatin (Cayman Chemical Company, Ann Arbor, MI). Eighteen hours after viral infection, media were collected for the determination of lipase activity and glycerol level while cells were processed for PTL or PLRP2 expression by Western blot analysis.
Immunofluorescence Studies
Immunofluorescence studies were done on 3T3-L1 adipocytes, cultured and adenovirally transfected as described in the Cell Culture of 3T3-L1 Adipocytes section. Briefly, cells were fixed with 2% paraformaldehyde, permeabilized, blocked with 5% normal goat serum, and exposed to an antibody against PTL (1:500) for 1 hour. After three washes, goat anti-rabbit Alexa Flour 594 (1:200; Invitrogen, Carlsbad, CA), and high-content screening LipidTOX Red Neutral Lipid Stain (1:200; Life Technologies) were added for 1 hour. After washing, slides were mounted (Fluormount; Sigma-Aldrich, St. Louis, MO) and imaged on a Zeiss Meta (LSM510) confocal microscope using a 63 × lens and 1-μm-thick optical sections. Images were processed and analyzed using Photoshop CS4 (Adobe Systems, San Jose, CA).
Peripheral Blood Mononuclear Cell Experiments
Rodent blood collected by cardiac puncture was anticoagulated with isotonic EDTA, and erythrocytes were removed by aggregation and sedimentation using 2% methyl cellulose. The leukocyte-rich plasma was layered over OptiPrep medium (Sigma-Aldrich) and centrifuged at 700 × g for 20 minutes to separate peripheral blood mononuclear cells as described by Boyum et al.45 Residual erythrocytes were lysed in isotonic ammonium chloride solution. peripheral blood mononuclear cells were washed and resuspended in serum-free RPMI 1640 media containing 25 mmol/L HEPES either alone or treated for 10 minutes with 10 μmol/L linoleic or oleic acid at 37°C, followed by labeling cells with using the fluorescein isothiocyanate–Annexin V Apoptosis detection kit (BD Pharmigen; BD Biosciences, San Jose, CA). Flow cytometry was done on the BD LSR Fortessa Analyzer using the BD FACSDiva software version 6.1.3 (BD Biosciences). Acquisition was done after adjusting for forward and side scatter voltages, selecting appropriate fluorochrome channels using a minimum of 50,000 gated events per experimental condition.
Graphical Depiction and Statistics
Summary statistics include means ± SEM or 95% CIs, and/or medians and interquartile ranges. Tests for proportionality between groups were made using the χ2 test. One-way analysis of variance was used for comparing differences within a group. Pairwise comparisons between two groups were made using Mann-Whitney U tests. All significance levels were evaluated at the two-tailed, P < 0.05 level.
Results
Cerulein Pancreatitis in ob/ob Mice with Increased Visceral Unsaturated Triglycerides Causes 100% Mortality and Increased Serum UFAs, Which Are Reduced by Inhibition of Lipolysis
Initial comparison showed the ob/ob mice to weigh more than the lean mice (42.7 ± 2.6 g versus 26.4 ± 0.5 g; P < 0.001) (Figure 1A) and to have higher total body fat (42.5% ± 3.8% versus 10.6% ± 0.6%; P = 0.002) (Figure 1B), consistent with previously published data.24 Although the fat pad triglyceride composition in lean mice had equal proportions of saturated and unsaturated fat (51.8% ± 3.9% and 48.1% ± 3.9%, respectively; P = 0.5) (Figure 1C), the fat pads of ob/ob mice had 72.7% ± 0.4% UFA, similar to the composition in humans who are obese or have increased pancreatic fat.3,35
Figure 1.
Pancreatic lipase–dependent release of UFAs from visceral fat necrosis causes CR pancreatitis to be lethal in ob/ob mice. Bar graphs of lean (C57bl6) and ob/ob mice showing body weight (A), percentage composition (lean and fat) (B), and percentage triglyceride (TG) composition of gonadal fat pads (C). ob/ob Mice with cerulein (CR) acute pancreatitis (AP) have elevated nonesterified fatty acids (NEFAs) (D) and unsaturated fatty acids (UFAs) (E), but not saturated fatty acids (SFAs) (G), versus obese control (Con) or lean mice with CR AP for 1 or 2 days (CR1 and CR2, respectively). UFA levels are reduced at day 2 (CRO2) and day 5 (CRO5) in ob/ob mice, and hypocalcemia is prevented (H), with orlistat use. Box plots show the means (dashed lines), medians (solid lines), 25th and 75th percentiles (boxes), and 10th and 90th percentiles (brackets). F: Kaplan-Meyer 5-day survival curve showing 100% mortality in ob/ob mice with CR with or without vehicle (CR and CRV, respectively) by day 3, which is improved to 80% survival (2/10 mortality) with orlistat treatment. n ≥ 8 in each group. ∗P < 0.05 versus controls in the respective lean and ob/ob mice; †P < 0.05 versus ob/ob CR; ‡P < 0.05 for lean CR1 versus ob/ob CR. Sr., serum.
After the induction of cerulein pancreatitis, mice were followed up for 5 days. Consistent with the lipolysis of the visceral fat, a significant increase in serum nonesterified fatty acids (Figure 1D), UFAs (Figure 1E), and hypocalcemia (Figure 1H), similar to human SAP46 were noted at the time of mortality in ob/ob mice ± vehicle versus control ob/ob or lean mice on day 1 of AP (P < 0.02 in each case). The increase in nonesterified fatty acids, UFAs, and hypocalcemia in ob/ob mice with AP were significantly prevented in the orlistat-treated group at day 2 and day 5 (P < 0.03 versus cerulein or vehicle treated groups in all cases). Nonesterified saturated fatty acids did increase in the sera of ob/ob mice compared with those in controls but were not significantly different from those in any other group (Figure 1G). There was 100% mortality by days 2 and 3, respectively, in the ob/ob mice with cerulein pancreatitis or with vehicle treatment (ob/ob CR or ob/ob CRV) (Figure 1F). In contrast, lean mice had no mortality and ob/ob mice treated with orlistat (ob/ob CRO) had significantly reduced (20%) mortality (P = 0.015). Calcium supplementation normalized serum calcium but did not prevent mortality.
Mortality in ob/ob Mice Is Associated with Pancreatic Lipase-Induced Fat Necrosis
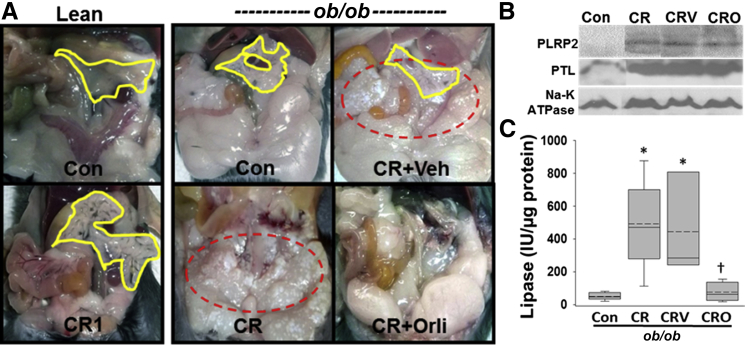
On necropsy, although the pancreata of the lean mice were edematous (Figure 2A); there was no fat necrosis. In contrast, ob/ob mice had gross evidence of visceral fat necrosis, appearing as cheesy white droplets. This fat necrosis was prevented in the mice that received orlistat.
Figure 2.
Fat necrosis is mediated by active pancreatic lipases. A: Gross appearance of the peritoneal cavities in ob/ob mice show chalky white dotted areas of saponification (dashed red outlines) in the visceral fat pads of mice with pancreatitis ± vehicle [cerulein (CR); CR + Veh]. Fat necrosis is undetectable in lean or orlistat-treated mice with pancreatitis (CR1 and CR + Orli, respectively) despite all pancreatitis groups having pancreatic edema (yellow lines). B: Western blots of the gonadal fat pads show increased, pancreatic lipase–related protein-2 (PLRP2) and pancreatic triglyceride lipase (PTL) in ob/ob mice with pancreatitis sacrificed at day 2 [CR, CR vehicle (CRV)], irrespective of orlistat treatment (CRO), which apparently reduces the activity of pancreatic lipase in these mice (C). Box plots show the means (dashed lines), medians (solid lines), 25th and 75th percentiles (boxes), and 10th and 90th percentiles (brackets). n ≥ 6 in each group. ∗P < 0.05 versus controls; †P < 0.05 versus ob/ob CR. Con, control.
Western blot analysis of necrosed fat pads revealed an increased amount of PTL and PLRP2 in the areas of fat necrosis despite orlistat treatment (CRO) (Figure 2B). This increase was associated with an increase in pancreatic lipase activity (Figure 2C) in the fat pads of ob/ob mice, which was prevented in the mice that received orlistat.
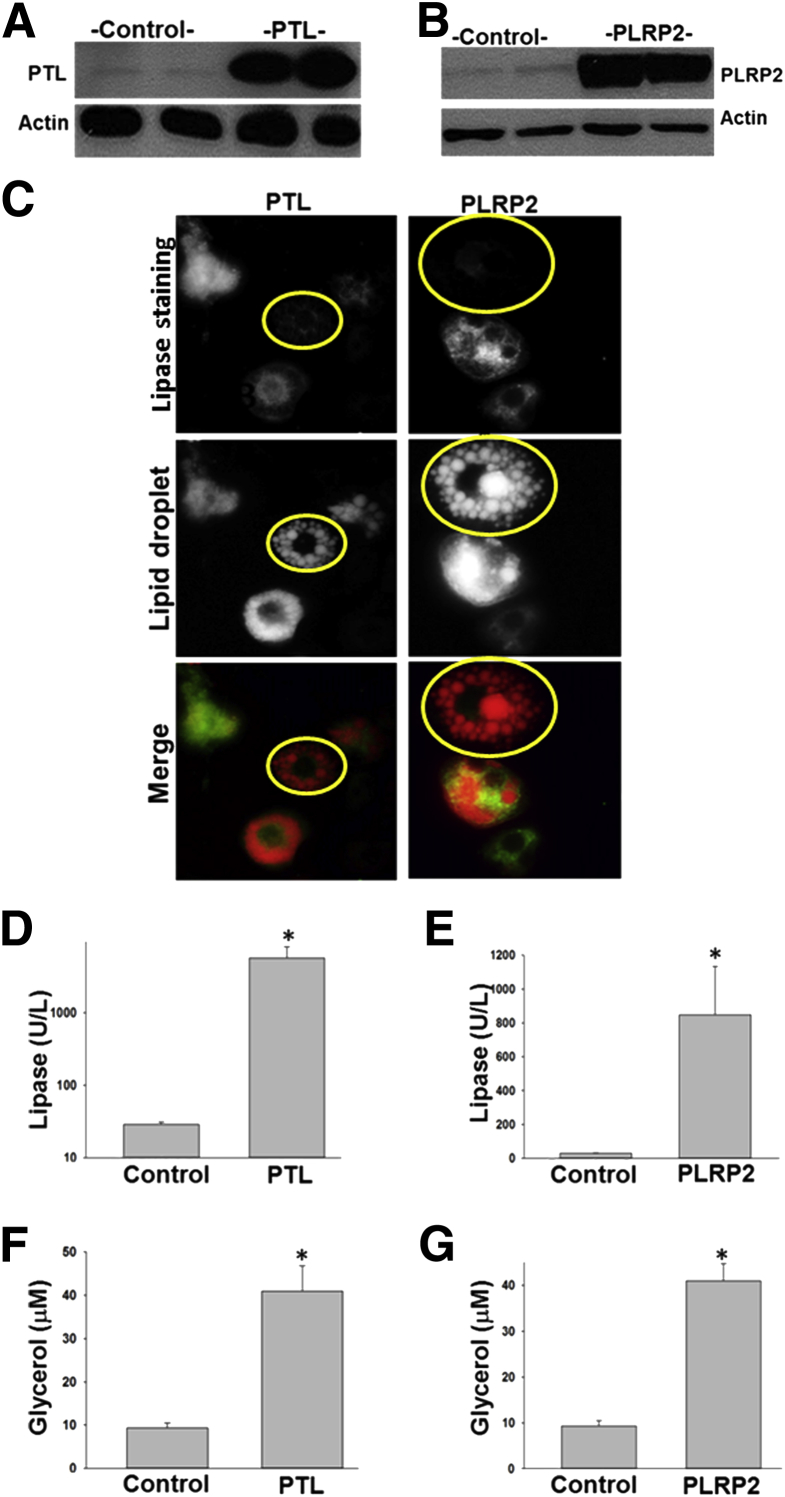
To understand this further, we adenovirally overexpressed PTL or PLRP2 in 3T3-L1 cells (Figure 3). The control 3T3-LI cells staining negative for pancreatic lipases had several lipid droplets, with no cytoplasmic staining of lipid. PTL or PLRP2 expression was associated with an increase in pancreatic lipase activity (Figure 3, A, B, D, and E) and lipolysis of the lipid droplets, evidenced by an increase in glycerol release into the medium (Figure 3, F and G), along with a progressive loss of lipid droplets (Figure 3C), with increased cytoplasmic lipid staining. This is mechanistically consistent with the in vivo observations of elevated lipolytic products (Figure 1, D, E, and G) and pancreatic lipase amounts in fat necrosis (Figure 2C) noted in ob/ob mice.
Figure 3.
Pancreatic lipases increase lipolysis in adipocytes. Adenoviral expression of pancreatic triglyceride lipase (PTL) and pancreatic lipase–related protein-2 (PLRP2) in 3T3-L1 increases their protein expression on Western blot analysis (A and B) associated with an increase in lipase activity (D and E) and glycerol release into the medium (F and G). C: Although control cells (yellow outlines), which do not express PTL or PLRP2, exhibit several discreet lipid droplets in all the images, the expression of PTL or PLRP2 causes a loss of this lipid-droplet morphology and an increase in lipid staining in the cytoplasm. n ≥ 4 in each group. *P < 0.05 versus controls. Original magnification, ×60 (C).
ob/ob Mice Develop Sustained MSOF Secondary to Lipotoxicity
Mortality from SAP in humans is associated with MSOF alone or with pancreatic or peripancreatic necrosis.6,14,21,22,35,38,41 We therefore assessed MSOF by examining evidence of kidney failure and apoptotic cells in the lungs, which have previously been associated with lipotoxic acute respiratory distress syndrome.35,47–50
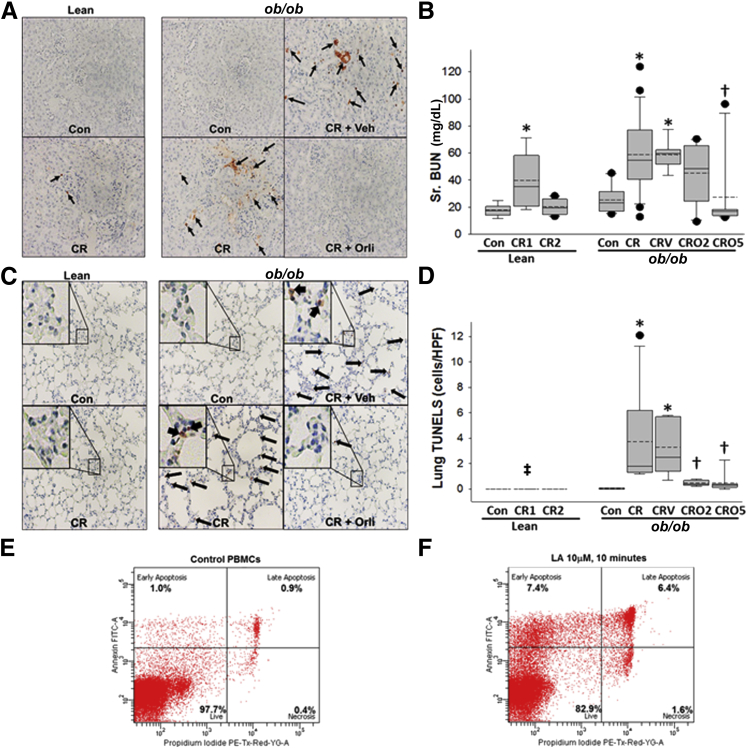
Obese mice with pancreatitis showed evidence of renal tubular damage with several terminal deoxynucleotidyl transferase dUTP nick end labeling–positive cells, which were dramatically reduced in the orlistat-treated group (Figure 4A). Serum blood urea nitrogen measurements in lean mice and in orlistat-treated ob/ob mice with pancreatitis increased transiently on day 1 and 2, respectively (Figure 4B), normalizing later. However, the blood urea nitrogen values in the obese mice with pancreatitis were elevated at the time of mortality and were apparently unaffected by the vehicle. Staining with terminal deoxynucleotidyl transferase dUTP nick end labeling of the lungs was dramatically increased in ob/ob mice dying with cerulein pancreatitis (consistent with lipotoxic acute respiratory distress syndrome35) and prevented by orlistat (Figure 4, C and D). The induction of apoptosis by linoleic acid (Figure 4F) and oleic acid (unpublished data) at low concentrations compared to controls (Figure 4E) supports the lipotoxic role of UFAs in these outcomes. Thus the overall picture suggests that mortality in ob/ob mice occurred from renal failure and lipotoxic acute respiratory distress syndrome, which after lipase inhibition were improved and similar to those in lean mice.
Figure 4.
Lipase inhibition prevents MSOF. Representative images show that terminal deoxynucleotidyl transferase dUTP nick end labeling–positive cells (arrows) increase in the kidney tubules of ob/ob mice with cerulein (CR) acute pancreatitis (AP) ± vehicle (Veh) (A), and serum (Sr.) blood urea nitrogen (BUN) (mg/dL) increases transiently in lean mice on day 1 and in ob/ob CR ± vehicle (CRV) mice at the time of mortality, which normalizes by day 5 of orlistat treatment (B). C and D: Concentrations of terminal deoxynucleotidyl transferase dUTP nick end labeling–positive cells in the lungs of ob/ob mice with CR AP (arrows) are reduced with orlistat use. Insets in C show the boxed area at higher magnification (×100). Box plots show the means (dashed lines), medians (solid lines), 25th and 75th percentiles (boxes), and 10th and 90th percentiles (brackets). The assessment of peripheral blood mononuclear cell (PBMC) apoptosis, indicated by annexin V staining (and necrosis by increased propidium iodide staining), show increased early and late apoptosis induced by 10 μmol/L linoleic acid (LA) within 10 minutes of exposure (F) compared with control PBMC (E). n ≥ 8 in each group. ∗P < 0.05 versus controls (Con) in the respective lean and ob/ob mice; †P < 0.05 versus ob/ob CR; ‡P < 0.05 for lean CR for 1 day versus ob/ob CR. Original magnification, ×10 (A and C). CR1, lean mice sacrificed at the end of day 1; CR2, lean mice sacrificed at the end of day 2; CRO2, mice given orlistat and sacrificed at the end of 2 days; CRO5, mice given orlistat and sacrificed at the end of 5 days; FITC-A, fluorescein isothiocyanate A; HPF, high-power field; PE-Tx-Red-YG-A, phytoerythrin-texas red-yellow green-A; Sr., serum.
ob/ob Mice Have Increased Intrapancreatic Fat, Fat Necrosis, and Associated Perifat Acinar Necrosis
Since obese humans have increased intrapancreatic fat and increased fat necrosis, which contributes to the damage of surrounding parenchyma, that is, perifat acinar necrosis,35,38 we studied whether similar phenomena occur in ob/ob mice.
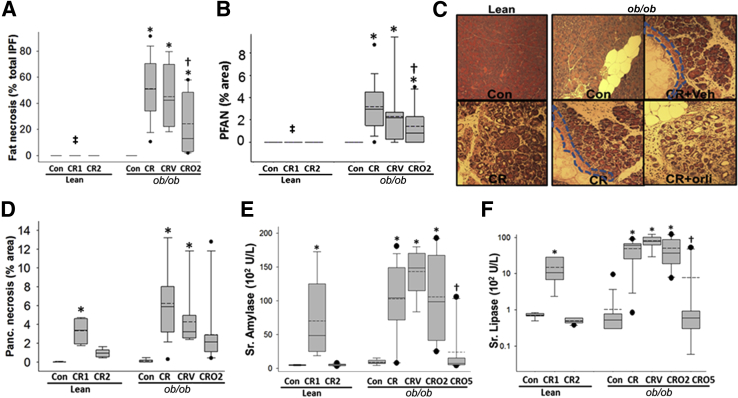
Intrapancreatic fat occupied 33.9% ± 15.9% of the pancreatic area in ob/ob mice, which was significantly higher compared with that in lean controls (3.6% ± 0.5%) (P < 0.001). This fat was necrosed in the ob/ob mice but not in lean mice (Figure 5A). A mean of 3.2% ± 2.1% of the parenchyma immediately contiguous with the necrosed fat also showed morphological evidence of necrosis (Figure 5, B and C) and contributed to about half of the total necrotic area (6.2 ± 3.8%) in the obese mice, but not in the lean mice, with pancreatitis (Figure 5, B and D). These results were not affected in the vehicle-treated group. Fat necrosis and perifat acinar necrosis were significantly reduced in the mice that received orlistat (Figure 5, A–C). Interestingly, however, the amount of total acinar necrosis was not significantly affected by orlistat (Figure 5D). Consistent with human data that serum amylase or lipase early in the disease are unrelated to severity, on day 2 these were similar in all groups with pancreatitis (Figure 5, E and F). However, by the 5th day, these parameters were significantly reduced by orlistat treatment and were no different from those in ob/ob controls, suggesting a significant reduction in ongoing pancreatic damage. These findings suggest that fat necrosis may play a more important role in determining the outcomes of pancreatitis than local pancreatic necrosis in this model.
Figure 5.
Lipase inhibition reduces pancreatic fat necrosis and perifat acinar necrosis but not pancreatic necrosis. The quantification and images of fat necrosis [amorphous deposits in necrotic intrapancreatic fat (IPF), in the ob/ob cerulein (CR) ± vehicle (V) group] (A) and perifat acinar necrosis (PFAN) (B). PFAN (dashed lines) is absent in lean mice and is reduced with orlistat (orli) use in ob/ob mice with AP (C). D: Pancreatic (Panc.) acinar necrosis is similar in all groups with AP. Serum amylase (E) and lipase (F) are increased similarly in lean mice on day 1 and in ob/ob mice irrespective of orlistat treatment on day 2 or at mortality. These concentrations are normalized by day 5 in surviving mice. ∗P < 0.05 versus controls in the respective lean and ob/ob mice; †P < 0.05 versus ob/ob CR; ‡P < 0.05 for lean CR for 1 day versus ob/ob CR. Box plots show the means (dashed lines), medians (solid lines), 25th and 75th percentiles (boxes), and 10th and 90th percentiles (brackets). n ≥ 8 in each group. Original magnification, ×4 (C). CR1, lean mice sacrificed at the end of day 1; CR2, lean mice sacrificed at the end of day 2; CRO2, mice given orlistat and sacrificed at the end of 2 days; CRO5, mice given orlistat and sacrificed at the end of 5 days; Veh, vehicle.
Inhibition of Lipotoxicity Reduces Serum Cytokines by Day 5 of Pancreatitis in ob/ob Mice
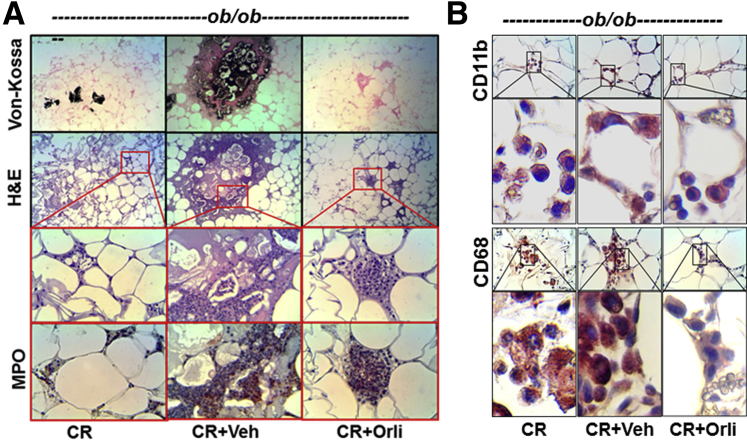
High serum IL-6, monocyte chemoattractant protein 1, resistin, and tumor necrosis factor α are associated with SAP in humans and rodents.51–58 In the current studies, these were significantly higher in ob/ob mice (± vehicle) versus lean mice with AP. IL-6 (Figure 6A), monocyte chemoattractant protein 1 (Figure 6B), and tumor necrosis factor α (Figure 6D), but not resistin (Figure 6C) were increased in lean and ob/ob mice versus controls and were unaffected by orlistat treatment at 2 days. These concentrations, however, were normalized on day 2 in lean mice and on the 5th day in orlistat-treated ob/ob AP mice. Tumor necrosis factor α showed a trend toward reduction but remained significantly elevated. Histologically, the necrosed fat pads showed an increase in von Kossa–positive staining, suggesting saponification, which was prevented in the mice that received orlistat (Figure 7A). However, like the serum cytokines, the accumulation of myeloperoxidase (Figure 7A) or CD11b (Figure 7B) positive cells on day 2 was unaffected with orlistat use, whereas CD68-positive macrophages were reduced, consistent with reduced fat necrosis (Figure 7B).
Figure 6.
Serum cytokine concentrations are greater in ob/ob mice and unaffected by lipase inhibition early in the disease. Box plots depicting serum IL-6 (A), monocyte chemoattractant protein 1 (MCP-1) (B), resistin (C), and tumor necrosis factor (TNF)-α (D) being significantly higher in ob/ob mice versus lean mice. These concentrations are unaffected with orlistat treatment on day 2 and are reduced by day 5. ∗P < 0.05 versus controls in the respective lean and ob/ob mice; †P < 0.05 versus ob/ob cerulein (CR); ‡P < 0.05 for the lean CR for 1 day versus ob/ob CR. Box plots show the means (dashed lines), medians (solid lines), 25th and 75th percentiles (boxes), and 10th and 90th percentiles (brackets). n ≥ 8 in each group. CR1, lean mice sacrificed at the end of day 1; CR2, lean mice sacrificed at the end of day 2; CRO2, mice given orlistat and sacrificed at the end of 2 days; CRO5, mice given orlistat and sacrificed at the end of 5 days; Veh, vehicle.
Figure 7.
Acute inflammatory cell infiltration of fat in ob/ob mice with AP is unaffected by lipase inhibition. A: Serial sections of ob/ob gonadal fat pads show fat necrosis as brown–black staining (Von-Kossa) and amorphous blue appearance (hematoxylin and eosin) corresponding to saponified fatty acids, which are reduced with orlistat (Orli) use. However, serial sections of inflammatory cell infiltrates (insets), positive for myeloperoxidase (MPO), are unaffected with orlistat use. B: Images and magnifications of immunohistochemistry analysis of CD11b-positive cells show these to be unaffected by lipase inhibition, whereas CD68 positivity is reduced in the orlistat-treated group (cerulein [CR] + Orli). Boxed areas shown at higher magnification below. Original magnification: ×10 (top two rows, A); ×40 (bottom two rows, A; first and third rows, B); ×100 (second and fourth rows, B).
Discussion
Here, we note that pancreatic lipase–dependent lipolysis of peripancreatic fat–containing unsaturated triglycerides causes fat necrosis, elevated UFAs, and MSOF independent of the inflammatory response or pancreatic necrosis. Using a classically mild model, we also learn that AP outcomes can be unrelated to the initiator, with lipotoxicity being an exacerbator, as may happen in hypertriglyceridemia or obese patients, who are more prone to SAP.14–16,18,19 These observations parallel and explain findings on human AP, including the mechanisms of fat necrosis and the varied outcomes in AP being unrelated to etiology, and they give direction in choosing targets for improving AP outcomes.
Pancreatic Lipase–Dependent Lipolysis of Visceral Fat Generates UFAs, Which Cause MSOF and Worsen AP
Studies on human tissue have shown evidence of pancreatic lipases in fat adjacent to pancreatic tissues in patients with AP.27,28 Basolateral leakage from acinar cells has also been noted by separate groups23–26 in models simulating AP. However it remains unclear whether this leakage of lipases has a role in fat necrosis or is a mere marker of enzymes leaked from a dying pancreas into the surrounding fat. Using an in vitro acinar-adipocyte coculture system, we previously showed an increase in lipase activity in the adipocyte compartment to be associated with an increase in nonesterified fatty acids in the acinar compartment, resulting in acinar cell death.38 Causality between the increase in nonesterified fatty acids and acinar cell death has been established in previous studies the showing prevention of such phenomena by inhibiting pancreatic lipases.32,35 Here we note induction of apoptosis soon after exposure of peripheral blood mononuclear cells to UFA. Mechanistically prolonged UFA exposure has been shown to inhibit mitochondrial complexes I and V.35 These findings would be consistent with a necroapoptotic mode of cell death induced by UFA.
Here, using ob/ob mice that have increased visceral fat enriched in unsaturated triglyceride, similar to obese humans,3,34,35 we note increased amounts of pancreatic lipases in AP-associated fat necrosis. This increase in pancreatic lipases likely occurs due to leakage from the inflamed pancreas since we could not detect an increase in the mRNA of pancreatic lipases in fat necrosis. These lipases cause lipolysis of the stored triglyceride in the fat, resulting in an increase in serum UFA. Using 3T3-L1 cells, we note that increasing pancreatic lipase amounts results in increased pancreatic lipase activity and lipolysis of the lipid droplets stored in these cells, similar to ob/ob mice that have an increase in serum UFA secondary to pancreatic lipase–mediated fat necrosis. The role of pancreatic lipases mediating UFA lipotoxicity via fat necrosis is supported by the following: i) the lack of lung injury, hypocalcemia, and sustained renal failure in lean mice and orlistat-treated ob/ob mice that have no fat necrosis, ii) clinical observations that SAP patients have elevated UFAs in the serum,59 iii) hypocalcemia is a part of the criteria predictive of SAP,60 iv) UFAs cause hypocalcemia46 (please note that preventing hypocalcaemia by supplementing calcium did not improve outcomes), v) i.v. oleic acid results in acute lung injury,47–50 vi) UFAs cause renal tubular toxicity,61,62 and vii) i.v. oleic acid results in renal failure.48 This spectrum of end points secondary to UFAs and our findings in Figure 4 support their role in MSOF and as a target of improving SAP outcomes.
Visceral Fat Necrosis May Worsen Inflammation and Outcomes Independent of Etiology or Pancreatic Necrosis
Although both lean and ob/ob mice used in this study fulfilled the clinical criteria for the diagnosis of AP,63 lean mice developed AP, mild hypercytokinemia, and transient single organ failure, with no mortality over the 3 to 5 days they were followed, thus behaving like the classically mild cerulein pancreatitis64 reported in the literature so far. In contrast, ob/ob mice, despite the similar increases in serum amylase and lipase, died from the complications described in the Results section. These findings are supported by previous studies showing lipotoxicity to worsen AP outcomes independent of etiology.34,35
Consistent with clinical literature on obese patients,51–58 serum cytokines were higher in ob/ob mice with SAP than in the lean mice with mild AP. Interestingly, serum cytokines were unaffected by orlistat treatment in the ob/ob mice electively sacrificed on day 2 of AP. Similarly, lipase inhibition, which decreased fat necrosis and improved outcomes, did not affect infiltration of fat by myeloperoxidase- and CD11b-positive cells. This finding may be due to the exaggerated pro-inflammatory state in obesity independent of UFA lipotoxicity. The improved survival with lipase inhibition suggests that the increased inflammatory response is a severity marker but not a severity mediator and is supported by the eventual reduction in serum cytokines in orlistat-treated mice electively sacrificed on the 5th day. Support for cytokines being SAP markers comes from the following: i) increases in serum IL-6 and tumor necrosis factor α secondary to UFAs,49 ii) mice administered IL-6 do not develop organ failure,65 iii) Il6 knockout mice develop SAP,65 iv) studies showing these cytokines have a protective role in AP and organ failure,66,67 and v) previous studies showing UFAs to up-regulate cytokines.35
Here we also note that the median necrosis was <10% in both the lean and ob/ob mice, similar to findings from previous reports,68 and is unaffected by lipase inhibition which prevents MSOF and mortality. These findings are is in agreement with those in humans, in whom mortality may occur with minimal or undetectable pancreatic necrosis39–41 and in contrast to animal models, in which severity has conventionally been graded by the extent of pancreatic necrosis,64 such as in the mouse cerulein model versus the rat model.69 Our current findings would therefore be consistent with the clinical observations that, during AP, failing organ systems portend a worse outcome even in the absence of necrosis.
In summary, pancreatic lipase–dependent generation of UFAs from fat necrosis causes MSOF, independent of pancreatic necrosis and inflammation, converting mild AP to SAP. Therefore, targeting UFA-mediated lipotoxicity, rather than the inflammatory response may be a better option for improving outcomes in SAP in obesity.
Acknowledgments
Anti-hPTL antibody was a kind gift from Mark Lowe (University of Pittsburgh, Pittsburgh, PA).
V.P.S. and S.N. designed and conceptualized the study; K.P., C.D., R.N.T., P.N., S.N., J.P.D., and R.A.C. acquired the data; K.P., C.D., P.N., S.N., J.P.D., R.A.C., and V.P.S. analyzed and interpreted the data; K.P., P.N., S.N., and V.P.S. drafted the manuscript; S.N. and V.P.S. performed critical revision of the manuscript for important intellectual content; P.N., K.P., R.N.T., and V.P.S. performed statistical analysis; S.N. and V.P.S. obtained funding; and V.P.S. supervised the study.
Footnotes
Supported by grants from the Clinical Translational Science Institute (RO1DK092460 to V.P.S.) and the NIH (UL1RR024153 and UL1TR000005; V.P.S., S.N.). This project used the UPCI Cancer Biomarkers Facility: Luminex Core Laboratory, which is supported in part by an award from NIH (P30CA047904). Funding was also provided by a startup package from the Department of Medicine, University of Pittsburgh (V.P.S.).
Disclosures: None declared.
References
- 1.Fitz R.H. Acute pancreatitis: a consideration of pancreatic hemorrhage, hemorrhagic, suppurative, and gangrenous pancreatitis, and of disseminated fat-necrosis. Boston Med Surg J. 1889;120:181–187. [Google Scholar]
- 2.Hotchkiss L.W. VIII. Acute Pancreatitis with Very Extensive Fat Necrosis. Ann Surg. 1912;56:111–117. doi: 10.1097/00000658-191207000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pinnick K.E., Collins S.C., Londos C., Gauguier D., Clark A., Fielding B.A. Pancreatic ectopic fat is characterized by adipocyte infiltration and altered lipid composition. Obesity (Silver Spring) 2008;16:522–530. doi: 10.1038/oby.2007.110. [DOI] [PubMed] [Google Scholar]
- 4.Saisho Y., Butler A.E., Meier J.J., Monchamp T., Allen-Auerbach M., Rizza R.A., Butler P.C. Pancreas volumes in humans from birth to age one hundred taking into account sex, obesity, and presence of type-2 diabetes. Clin Anat. 2007;20:933–942. doi: 10.1002/ca.20543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bakker O.J., van Santvoort H., Besselink M.G., Boermeester M.A., van Eijck C., Dejong K., van Goor H., Hofker S., Ahmed Ali U., Gooszen H.G., Bollen T.L., Dutch Pancreatitis Study Group Extrapancreatic necrosis without pancreatic parenchymal necrosis: a separate entity in necrotising pancreatitis? Gut. 2013;62:1475–1480. doi: 10.1136/gutjnl-2012-302870. [DOI] [PubMed] [Google Scholar]
- 6.Banks P.A., Bollen T.L., Dervenis C., Gooszen H.G., Johnson C.D., Sarr M.G., Tsiotos G.G., Vege S.S., Acute Pancreatitis Classification Working Group Classification of acute pancreatitis–2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102–111. doi: 10.1136/gutjnl-2012-302779. [DOI] [PubMed] [Google Scholar]
- 7.Balthazar E.J., Robinson D.L., Megibow A.J., Ranson J.H. Acute pancreatitis: value of CT in establishing prognosis. Radiology. 1990;174:331–336. doi: 10.1148/radiology.174.2.2296641. [DOI] [PubMed] [Google Scholar]
- 8.Schäffler A., Hamer O., Dickopf J., Goetz A., Landfried K., Voelk M., Herfarth H., Kopp A., Büchler C., Schölmerich J., Brünnler T. Admission resistin levels predict peripancreatic necrosis and clinical severity in acute pancreatitis. Am J Gastroenterol. 2010;105:2474–2484. doi: 10.1038/ajg.2010.278. [DOI] [PubMed] [Google Scholar]
- 9.Schäffler A., Hamer O.W., Dickopf J., Goetz A., Landfried K., Voelk M., Herfarth H., Kopp A., Buechler C., Schölmerich J., Brünnler T. Admission visfatin levels predict pancreatic and peripancreatic necrosis in acute pancreatitis and correlate with clinical severity. Am J Gastroenterol. 2011;106:957–967. doi: 10.1038/ajg.2010.503. [DOI] [PubMed] [Google Scholar]
- 10.Sadr-Azodi O., Orsini N., Andrén-Sandberg Å., Wolk A. Abdominal and total adiposity and the risk of acute pancreatitis: a population-based prospective cohort study. Am J Gastroenterol. 2013;108:133–139. doi: 10.1038/ajg.2012.381. [DOI] [PubMed] [Google Scholar]
- 11.O'Leary D.P., O'Neill D., McLaughlin P., O'Neill S., Myers E., Maher M.M., Redmond H.P. Effects of abdominal fat distribution parameters on severity of acute pancreatitis. World J Surg. 2012;36:1679–1685. doi: 10.1007/s00268-011-1414-y. [DOI] [PubMed] [Google Scholar]
- 12.Yashima Y., Isayama H., Tsujino T., Nagano R., Yamamoto K., Mizuno S., Yagioka H., Kawakubo K., Sasaki T., Kogure H., Nakai Y., Hirano K., Sasahira N., Tada M., Kawabe T., Koike K., Omata M. A large volume of visceral adipose tissue leads to severe acute pancreatitis. J Gastroenterol. 2011;46:1213–1218. doi: 10.1007/s00535-011-0430-x. [DOI] [PubMed] [Google Scholar]
- 13.Funnell I.C., Bornman P.C., Weakley S.P., Terblanche J., Marks I.N. Obesity: an important prognostic factor in acute pancreatitis. Br J Surg. 1993;80:484–486. doi: 10.1002/bjs.1800800426. [DOI] [PubMed] [Google Scholar]
- 14.Abu Hilal M., Armstrong T. The impact of obesity on the course and outcome of acute pancreatitis. Obes Surg. 2008;18:326–328. doi: 10.1007/s11695-007-9298-5. [DOI] [PubMed] [Google Scholar]
- 15.Papachristou G.I., Papachristou D.J., Avula H., Slivka A., Whitcomb D.C. Obesity increases the severity of acute pancreatitis: performance of APACHE-O score and correlation with the inflammatory response. Pancreatology. 2006;6:279–285. doi: 10.1159/000092689. [DOI] [PubMed] [Google Scholar]
- 16.Porter K.A., Banks P.A. Obesity as a predictor of severity in acute pancreatitis. Int J Pancreatol. 1991;10:247–252. doi: 10.1007/BF02924162. [DOI] [PubMed] [Google Scholar]
- 17.Shin K.Y., Lee W.S., Chung D.W., Heo J., Jung M.K., Tak W.Y., Kweon Y.O., Cho C.M. Influence of obesity on the severity and clinical outcome of acute pancreatitis. Gut Liver. 2011;5:335–339. doi: 10.5009/gnl.2011.5.3.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sempere L., Martinez J., de Madaria E., Lozano B., Sanchez-Paya J., Jover R., Perez-Mateo M. Obesity and fat distribution imply a greater systemic inflammatory response and a worse prognosis in acute pancreatitis. Pancreatology. 2008;8:257–264. doi: 10.1159/000134273. [DOI] [PubMed] [Google Scholar]
- 19.Evans A.C., Papachristou G.I., Whitcomb D.C. Obesity and the risk of severe acute pancreatitis. Minerva Gastroenterol Dietol. 2010;56:169–179. [PubMed] [Google Scholar]
- 20.Chen S.M., Xiong G.S., Wu S.M. Is obesity an indicator of complications and mortality in acute pancreatitis? An updated meta-analysis. J Dig Dis. 2012;13:244–251. doi: 10.1111/j.1751-2980.2012.00587.x. [DOI] [PubMed] [Google Scholar]
- 21.Bollen T.L., Singh V.K., Maurer R., Repas K., van Es H.W., Banks P.A., Mortele K.J. A comparative evaluation of radiologic and clinical scoring systems in the early prediction of severity in acute pancreatitis. Am J Gastroenterol. 2012;107:612–619. doi: 10.1038/ajg.2011.438. [DOI] [PubMed] [Google Scholar]
- 22.Singh V.K., Bollen T.L., Wu B.U., Repas K., Maurer R., Yu S., Mortele K.J., Conwell D.L., Banks P.A. An assessment of the severity of interstitial pancreatitis. Clin Gastroenterol Hepatol. 2011;9:1098–1103. doi: 10.1016/j.cgh.2011.08.026. [DOI] [PubMed] [Google Scholar]
- 23.Cosen-Binker L.I., Binker M.G., Wang C.C., Hong W., Gaisano H.Y. VAMP8 is the v-SNARE that mediates basolateral exocytosis in a mouse model of alcoholic pancreatitis. J Clin Invest. 2008;118:2535–2551. doi: 10.1172/JCI34672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fallon M.B., Gorelick F.S., Anderson J.M., Mennone A., Saluja A., Steer M.L. Effect of cerulein hyperstimulation on the paracellular barrier of rat exocrine pancreas. Gastroenterology. 1995;108:1863–1872. doi: 10.1016/0016-5085(95)90151-5. [DOI] [PubMed] [Google Scholar]
- 25.Gaisano H.Y., Lutz M.P., Leser J., Sheu L., Lynch G., Tang L., Tamori Y., Trimble W.S., Salapatek A.M. Supramaximal cholecystokinin displaces Munc18c from the pancreatic acinar basal surface, redirecting apical exocytosis to the basal membrane. J Clin Invest. 2001;108:1597–1611. doi: 10.1172/JCI9110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lam P.P., Cosen Binker L.I., Lugea A., Pandol S.J., Gaisano H.Y. Alcohol redirects CCK-mediated apical exocytosis to the acinar basolateral membrane in alcoholic pancreatitis. Traffic. 2007;8:605–617. doi: 10.1111/j.1600-0854.2007.00557.x. [DOI] [PubMed] [Google Scholar]
- 27.Aho H.J., Sternby B., Kallajoki M., Nevalainen T.J. Carboxyl ester lipase in human tissues and in acute pancreatitis. Int J Pancreatol. 1989;5:123–134. doi: 10.1007/BF02924413. [DOI] [PubMed] [Google Scholar]
- 28.Klöppel G., Dreyer T., Willemer S., Kern H.F., Adler G. Human acute pancreatitis: its pathogenesis in the light of immunocytochemical and ultrastructural findings in acinar cells. Virchows Arch A Pathol Anat Histopathol. 1986;409:791–803. doi: 10.1007/BF00710764. [DOI] [PubMed] [Google Scholar]
- 29.Ren J., Dimitrov I., Sherry A.D., Malloy C.R. Composition of adipose tissue and marrow fat in humans by 1H NMR at 7 Tesla. J Lipid Res. 2008;49:2055–2062. doi: 10.1194/jlr.D800010-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thomas L.W. The chemical composition of adipose tissue of man and mice. Q J Exp Physiol Cogn Med Sci. 1962;47:179–188. doi: 10.1113/expphysiol.1962.sp001589. [DOI] [PubMed] [Google Scholar]
- 31.Garaulet M., Hernandez-Morante J.J., Lujan J., Tebar F.J., Zamora S. Relationship between fat cell size and number and fatty acid composition in adipose tissue from different fat depots in overweight/obese humans. Int J Obes (Lond) 2006;30:899–905. doi: 10.1038/sj.ijo.0803219. [DOI] [PubMed] [Google Scholar]
- 32.Mössner J., Bödeker H., Kimura W., Meyer F., Böhm S., Fischbach W. Isolated rat pancreatic acini as a model to study the potential role of lipase in the pathogenesis of acinar cell destruction. Int J Pancreatol. 1992;12:285–296. doi: 10.1007/BF02924368. [DOI] [PubMed] [Google Scholar]
- 33.Kimura W., Meyer F., Hess D., Kirchner T., Fischbach W., Mössner J. Comparison of different treatment modalities in experimental pancreatitis in rats. Gastroenterology. 1992;103:1916–1924. doi: 10.1016/0016-5085(92)91452-a. [DOI] [PubMed] [Google Scholar]
- 34.Durgampudi C., Noel P., Patel K., Cline R., Trivedi R.N., DeLany J.P., Yadav D., Papachristou G.I., Lee K., Acharya C., Jaligama D., Navina S., Murad F., Singh V.P. Acute lipotoxicity regulates severity of biliary acute pancreatitis without affecting its initiation. Am J Pathol. 2014;184:1773–1784. doi: 10.1016/j.ajpath.2014.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Navina S., Acharya C., DeLany J.P., Orlichenko L.S., Baty C.J., Shiva S.S., Durgampudi C., Karlsson J.M., Lee K., Bae K.T., Furlan A., Behari J., Liu S., McHale T., Nichols L., Papachristou G.I., Yadav D., Singh V.P. Lipotoxicity causes multisystem organ failure and exacerbates acute pancreatitis in obesity. Sci Transl Med. 2011;3:107ra110. doi: 10.1126/scitranslmed.3002573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Panek J., Sztefko K., Drozdz W. Composition of free fatty acid and triglyceride fractions in human necrotic pancreatic tissue. Med Sci Monit. 2001;7:894–898. [PubMed] [Google Scholar]
- 37.Sztefko K., Panek J. Serum free fatty acid concentration in patients with acute pancreatitis. Pancreatology. 2001;1:230–236. doi: 10.1159/000055816. [DOI] [PubMed] [Google Scholar]
- 38.Acharya C., Cline R.A., Jaligama D., Noel P., Delany J.P., Bae K., Furlan A., Baty C.J., Karlsson J.M., Rosario B.L., Patel K., Mishra V., Dugampudi C., Yadav D., Navina S., Singh V.P. Fibrosis reduces severity of acute-on-chronic pancreatitis in humans. Gastroenterology. 2013;145:466–475. doi: 10.1053/j.gastro.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fu C.Y., Yeh C.N., Hsu J.T., Jan Y.Y., Hwang T.L. Timing of mortality in severe acute pancreatitis: experience from 643 patients. World J Gastroenterol. 2007;13:1966–1969. doi: 10.3748/wjg.v13.i13.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carnovale A., Rabitti P.G., Manes G., Esposito P., Pacelli L., Uomo G. Mortality in acute pancreatitis: is it an early or a late event? JOP. 2005;6:438–444. [PubMed] [Google Scholar]
- 41.Mutinga M., Rosenbluth A., Tenner S.M., Odze R.R., Sica G.T., Banks P.A. Does mortality occur early or late in acute pancreatitis? Int J Pancreatol. 2000;28:91–95. doi: 10.1385/IJGC:28:2:091. [DOI] [PubMed] [Google Scholar]
- 42.Lowe M.E. The triglyceride lipases of the pancreas. J Lipid Res. 2002;43:2007–2016. doi: 10.1194/jlr.r200012-jlr200. [DOI] [PubMed] [Google Scholar]
- 43.Miller R., Lowe M.E. Carboxyl ester lipase from either mother's milk or the pancreas is required for efficient dietary triglyceride digestion in suckling mice. J Nutr. 2008;138:927–930. doi: 10.1093/jn/138.5.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Singh V.P., Bhagat L., Navina S., Sharif R., Dawra R.K., Saluja A.K. Protease-activated receptor-2 protects against pancreatitis by stimulating exocrine secretion. Gut. 2007;56:958–964. doi: 10.1136/gut.2006.094268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bøyum A., Løvhaug D., Tresland L., Nordlie E.M. Separation of leucocytes: improved cell purity by fine adjustments of gradient medium density and osmolality. Scand J Immunol. 1991;34:697–712. doi: 10.1111/j.1365-3083.1991.tb01594.x. [DOI] [PubMed] [Google Scholar]
- 46.Dettelbach M.A., Deftos L.J., Stewart A.F. Intraperitoneal free fatty acids induce severe hypocalcemia in rats: a model for the hypocalcemia of pancreatitis. J Bone Miner Res. 1990;5:1249–1255. doi: 10.1002/jbmr.5650051210. [DOI] [PubMed] [Google Scholar]
- 47.Hussain N., Wu F., Zhu L., Thrall R.S., Kresch M.J. Neutrophil apoptosis during the development and resolution of oleic acid-induced acute lung injury in the rat. Am J Respir Cell Mol Biol. 1998;19:867–874. doi: 10.1165/ajrcmb.19.6.3118. [DOI] [PubMed] [Google Scholar]
- 48.Wu R.P., Liang X.B., Guo H., Zhou X.S., Zhao L., Wang C., Li R.S. Protective effect of low potassium dextran solution on acute kidney injury following acute lung injury induced by oleic acid in piglets. Chin Med J (Engl) 2012;125:3093–3097. [PubMed] [Google Scholar]
- 49.Inoue H., Nakagawa Y., Ikemura M., Usugi E., Nata M. Molecular-biological analysis of acute lung injury (ALI) induced by heat exposure and/or intravenous administration of oleic acid. Leg Med (Tokyo) 2012;14:304–308. doi: 10.1016/j.legalmed.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 50.Lai J.P., Bao S., Davis I.C., Knoell D.L. Inhibition of the phosphatase PTEN protects mice against oleic acid-induced acute lung injury. Br J Pharmacol. 2009;156:189–200. doi: 10.1111/j.1476-5381.2008.00020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hirota M., Nozawa F., Okabe A., Shibata M., Beppu T., Shimada S., Egami H., Yamaguchi Y., Ikei S., Okajima T., Okamoto K., Ogawa M. Relationship between plasma cytokine concentration and multiple organ failure in patients with acute pancreatitis. Pancreas. 2000;21:141–146. doi: 10.1097/00006676-200008000-00006. [DOI] [PubMed] [Google Scholar]
- 52.Messmann H., Vogt W., Falk W., Vogl D., Zirngibl H., Leser H.G., Schölmerich J. Interleukins and their antagonists but not TNF and its receptors are released in post-ERP pancreatitis. Eur J Gastroenterol Hepatol. 1998;10:611–617. doi: 10.1097/00042737-199807000-00016. [DOI] [PubMed] [Google Scholar]
- 53.Brivet F.G., Emilie D., Galanaud P. Pro- and anti-inflammatory cytokines during acute severe pancreatitis: an early and sustained response, although unpredictable of death. Parisian Study Group on Acute Pancreatitis. Crit Care Med. 1999;27:749–755. doi: 10.1097/00003246-199904000-00029. [DOI] [PubMed] [Google Scholar]
- 54.Dambrauskas Z., Giese N., Gulbinas A., Giese T., Berberat P.O., Pundzius J., Barauskas G., Friess H. Different profiles of cytokine expression during mild and severe acute pancreatitis. World J Gastroenterol. 2010;16:1845–1853. doi: 10.3748/wjg.v16.i15.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aoun E., Chen J., Reighard D., Gleeson F.C., Whitcomb D.C., Papachristou G.I. Diagnostic accuracy of interleukin-6 and interleukin-8 in predicting severe acute pancreatitis: a meta-analysis. Pancreatology. 2009;9:777–785. doi: 10.1159/000214191. [DOI] [PubMed] [Google Scholar]
- 56.Daniel P., Leśniowski B., Mokrowiecka A., Jasińska A., Pietruczuk M., Małecka-Panas E. Circulating levels of visfatin, resistin and pro-inflammatory cytokine interleukin-8 in acute pancreatitis. Pancreatology. 2010;10:477–482. doi: 10.1159/000276986. [DOI] [PubMed] [Google Scholar]
- 57.Ueda T., Takeyama Y., Yasuda T., Matsumura N., Sawa H., Nakajima T., Ajiki T., Fujino Y., Suzuki Y., Kuroda Y. Significant elevation of serum interleukin-18 levels in patients with acute pancreatitis. J Gastroenterol. 2006;41:158–165. doi: 10.1007/s00535-005-1735-4. [DOI] [PubMed] [Google Scholar]
- 58.Regnér S., Appelros S., Hjalmarsson C., Manjer J., Sadic J., Borgstrom A. Monocyte chemoattractant protein 1, active carboxypeptidase B and CAPAP at hospital admission are predictive markers for severe acute pancreatitis. Pancreatology. 2008;8:42–49. doi: 10.1159/000114866. [DOI] [PubMed] [Google Scholar]
- 59.Domschke S., Malfertheiner P., Uhl W., Büchler M., Domschke W. Free fatty acids in serum of patients with acute necrotizing or edematous pancreatitis. Int J Pancreatol. 1993;13:105–110. doi: 10.1007/BF02786078. [DOI] [PubMed] [Google Scholar]
- 60.Ranson J.H., Rifkind K.M., Roses D.F., Fink S.D., Eng K., Spencer F.C. Prognostic signs and the role of operative management in acute pancreatitis. Surg Gynecol Obstet. 1974;139:69–81. [PubMed] [Google Scholar]
- 61.Ishola D.A., Jr., Post J.A., van Timmeren M.M., Bakker S.J., Goldschmeding R., Koomans H.A., Braam B., Joles J.A. Albumin-bound fatty acids induce mitochondrial oxidant stress and impair antioxidant responses in proximal tubular cells. Kidney Int. 2006;70:724–731. doi: 10.1038/sj.ki.5001629. [DOI] [PubMed] [Google Scholar]
- 62.Moran J.H., Nowak G., Grant D.F. Analysis of the toxic effects of linoleic acid, 12,13-cis-epoxyoctadecenoic acid, and 12,13-dihydroxyoctadecenoic acid in rabbit renal cortical mitochondria. Toxicol Appl Pharmacol. 2001;172:150–161. doi: 10.1006/taap.2001.9149. [DOI] [PubMed] [Google Scholar]
- 63.Forsmark C.E., Baillie J., AGA Institute Clinical Practice and Economics Committee; AGA Institute Governing Board AGA Institute technical review on acute pancreatitis. Gastroenterology. 2007;132:2022–2044. doi: 10.1053/j.gastro.2007.03.065. [DOI] [PubMed] [Google Scholar]
- 64.Lerch M.M., Gorelick F.S. Models of acute and chronic pancreatitis. Gastroenterology. 2013;144:1180–1193. doi: 10.1053/j.gastro.2012.12.043. [DOI] [PubMed] [Google Scholar]
- 65.Pini M., Rhodes D.H., Castellanos K.J., Hall A.R., Cabay R.J., Chennuri R., Grady E.F., Fantuzzi G. Role of IL-6 in the resolution of pancreatitis in obese mice. J Leukoc Biol. 2012;91:957–966. doi: 10.1189/jlb.1211627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cuzzocrea S., Mazzon E., Dugo L., Centorrino T., Ciccolo A., McDonald M.C., de Sarro A., Caputi A.P., Thiemermann C. Absence of endogenous interleukin-6 enhances the inflammatory response during acute pancreatitis induced by cerulein in mice. Cytokine. 2002;18:274–285. doi: 10.1006/cyto.2002.0883. [DOI] [PubMed] [Google Scholar]
- 67.Guice K.S., Oldham K.T., Remick D.G., Kunkel S.L., Ward P.A. Anti-tumor necrosis factor antibody augments edema formation in caerulein-induced acute pancreatitis. J Surg Res. 1991;51:495–499. doi: 10.1016/0022-4804(91)90171-h. [DOI] [PubMed] [Google Scholar]
- 68.Dahlhoff M., Algül H., Siveke J.T., Lesina M., Wanke R., Wartmann T., Halangk W., Schmid R.M., Wolf E., Schneider M.R. Betacellulin protects from pancreatitis by activating stress-activated protein kinase. Gastroenterology. 2010;138:1585–1594. doi: 10.1053/j.gastro.2009.12.045. 1594 e1581–1583. [DOI] [PubMed] [Google Scholar]
- 69.Mareninova O.A., Sung K.F., Hong P., Lugea A., Pandol S.J., Gukovsky I., Gukovskaya A.S. Cell death in pancreatitis: caspases protect from necrotizing pancreatitis. J Biol Chem. 2006;281:3370–3381. doi: 10.1074/jbc.M511276200. [DOI] [PubMed] [Google Scholar]