Abstract
Pharmaceuticals and cosmetics for dermal application are usually tested on healthy skin, although the primary permeation barrier, the stratum corneum, is often impaired by skin diseases or small skin lesions, especially on the hands. These skin conditions can considerably influence the permeation of chemicals and drugs. Furthermore, risk assessment for example of nanoparticles should be performed under various skin conditions to reflect the true circumstances. Therefore, an alternative and reproducible method for a high throughput of skin samples with impaired skin barrier was developed and verified by skin permeation studies (25 h) of caffeine, sorbic acid and testosterone compared to healthy (untreated) and tape-stripped skin. Skin barrier disruption was controlled by TEWL measurement.
Skin permeation of the three substances was increased in tape-stripped and abraded skin compared to untreated skin due to the reduced barrier integrity. Enhancement of drug uptake was highest for the most hydrophilic substance, caffeine, followed by sorbic acid and lipophilic testosterone. No significant difference in drug uptake studies was observed between the new abrasion method with an aluminum-coated sponge and the tape-stripping method. The obtained results demonstrate that this abrasion method is an alternative way to achieve a disturbed skin barrier for drug and chemical uptake studies.
Keywords: Disturbed skin barrier model, Abraded skin, Tape stripping, Caffeine, Sorbic acid, Testosterone
Graphical abstract

1. Introduction
Dermatological products are the first choice in the local treatment of skin diseases due to good patient compliance and low systemic exposure. The outermost layer of the skin, the stratum corneum, is formed by corneocytes imbedded in a lipid matrix primarily composed of ceramides, cholesterol and free fatty acids, representing the primary barrier [1]. This effective barrier restricts the penetration and permeation of chemicals as well as active ingredients into the skin. In contrast, skin diseases are often attended by the disorder or even a disruption of the skin barrier, such as a loss of stratum corneum integrity, and thus an increase in transepidermal water loss (TEWL) [2]. TEWL values of healthy human skin range between 3.2 and 10.9 g m−2 h−1 [3–5], whereas an impaired skin barrier increases TEWL values by up to 10 times [6–10]. Eczematous skin diseases such as atopic dermatitis, seborrheic eczema, allergic contact eczema and asteatotic eczema, as well as infectious skin diseases, lead to a loss of skin barrier function complemented by an increased TEWL [5,6,9,11,12]. Furthermore, ichthyosis and psoriasis, both complex skin disorders, tend to result in approximately 5 times [8] or even 10 times [7] higher TEWL values than healthy skin, respectively.
This dysfunction of the skin barrier can be associated with an enhanced percutaneous absorption of the applied agents [13,14] and possible undesirable side effects [15,16]. Thus, it is essential to consider the condition of the skin while developing and testing a new dermatological product. The diversity of dermatological diseases attended by skin barrier impairment and the risk of toxicity during topical treatment emphasizes the importance of an inexpensive and reproducible in vitro skin model that simulates and simplifies skin barrier impairment. Substances for the treatment of skin diseases characterized by impaired skin barrier should be tested with healthy skin, the best-case scenario, and with impaired skin, the worst-case scenario, to ensure the safety and effectiveness of dermal products. Therefore, a reliable, low-cost and easy method is needed for in vivo and ex vivo testing of formulations and substances.
An overview of different in vivo and in vitro methods to study the penetration and permeation through damaged skin is given by Gattu and Maibach [17,44]. These methods can be divided into mechanical, chemical and biological methods, as well as investigations on clinical diseased skin.
The most common method for simulating a disturbed skin barrier is the tape-stripping method [10,18-20]. The horny cell layers are gradually removed with adhesive tape. The tape-stripping method is minimally invasive and can be applied in vivo and ex vivo for humans as well as for animals (e.g., pigs and rats). The efficacy of the tape-stripping method can be influenced by the anatomical site, the application pressure, the duration of pressure, the removal rate [3] and the type of tape [21]. Furthermore, another difficulty is the inhomogeneous removal of the cell layers due to the elastic network of furrows [22,23], and the required repetitions to achieve an adequate degree of damage are quite time- and cost-intensive using the validated tape-stripping method by the Simonsen and Fullerton [10].
The objective of the present study was to develop an alternative in vitro skin model to simulate skin barrier impairment. The following requirements should be fulfilled by this model: simple and quick application, low cost, and good repeatability. Therefore, the stratum corneum was mechanically removed by a sponge with a rough surface, and the permeation of three model drugs, caffeine, sorbic acid and testosterone, which differ in physicochemical properties, such as octanol-water-coefficient (log P) and molecular weight (MW), was studied and compared with intact and tape-stripped skin. Caffeine and testosterone are marker compounds recommended by the OECD, and sorbic acid is a preservative frequently used in cosmetics.
2. Materials and methods
2.1. Skin preparation
Untreated porcine ears (domestic pig) were obtained from a local slaughterhouse and immediately transferred to the lab under cool conditions. Porcine ears were washed by rinsing with moderately warm water and wiped with paper towels, and the bristles were carefully shortened by trimming. Full-thickness skin was obtained from the outer side of the porcine auricle [24] and stored at −20 °C for up to 3 months. Porcine skin was chosen due to its similarity to human skin in terms of its morphology and permeability [25] and due to its availability. Prior to skin permeation studies, the thawed porcine full-thickness skin was prepared. Untreated skin (intact skin) and skin with an impaired barrier were used to conduct skin uptake studies. To simulate the impaired barrier, two different methods were compared. Tape-stripped porcine skin was obtained by a successive tape-stripping procedure (d-Squame® tape disks, 22 mm diameter, Cuderm Corp., USA) following Simonsen and Fullerton [10]. The impairment of the skin barrier by abrasion was induced by partial rub-off of the stratum corneum using a sponge with an aluminum coating (Spontex® Brillant scourer pad, MAPA GmbH, Germany). Therefore, the sponge was drawn in a smooth motion over the skin surface to reduce the stratum corneum. The degree of skin impairment was controlled by measuring continuous transepidermal water loss (TEWL; DermaLAB Cortex Technology, Denmark) during skin preparation. To ensure good reproducibility, the following quality criteria have been defined: initial TEWL values for skin samples (skin thickness: 1.40±0.2 mm) have to be within 10±3 g m−2 h−1. The final TEWL values for tape-stripped and abraded skin were set to 30±2 g m−2 h−1, representing serious damage of stratum corneum without complete removal (see Section 3.1). Skin samples that did not meet these requirements were discarded. Furthermore, skin biopsies were taken for histological examination (hematoxylin–eosin staining) of the skin impairment.
2.2. Quantification of caffeine, testosterone and sorbic acid
Caffeine, sorbic acid (Caesar & Loretz GmbH, Germany, both) and testosterone (Sigma Aldrich, Germany) were quantified by HPLC (LaChrom Elite® HPLC system, VWR International GmbH, Darmstadt, Germany) and UV detection at 230 nm (caffeine), 255 nm (sorbic acid) or 245 nm (testosterone), respectively. A LiChrospher® 100 RP-18e (5 µm) LiChroCART® 125-4 column (Merck KGaA, Darmstadt, Germany) was used for all test substances. The isocratic mobile phase was 10% acetonitrile (ACN) and 90% phosphate buffer (10 mM, pH 2.6: 0.34 mL/L orthophosphoric acid (85%) and 0.68 g/L NaH2PO4·H2O), delivered with a flow of 1.0 mL/min for caffeine (retention time=3.2 min, LOD: 5 ng/mL and LOQ: 14 ng/mL), and 30% ACN and 70% phosphate buffer with a flow of 1.2 mL/min for sorbic acid (retention time=2.3 min, LOD: 9 ng/mL and LOQ: 26 ng/mL); the column temperature 40 °C for each. For testosterone, the mobile phase was a gradient of ACN/phosphate buffer (45:55–85:15 v/v within 10 min followed by a washing procedure) with a flow of 1.0 mL/min. The retention time was 5.1 min, LOD: 23 ng/mL and LOQ: 69 ng/mL; the column temperature was 40 °C.
2.3. Skin permeation and drug uptake studies
Permeation studies of caffeine, sorbic acid and testosterone were performed in vitro using the Franz diffusion cell set-up [26], 15 mm in diameter (surface area 1.76 cm2) and 12 mL acceptor volume (Gauer Glas, Germany). On the day of the experiment, the prepared skin samples (see section Skin Preparation) were mounted into the Franz diffusion cell and allowed to equilibrate for 30 min at 32.5 °C. The acceptor medium was magnetically stirred at 500 rpm. To provide sink conditions, phosphate buffered saline (PBS, pH 7.4) with caffeine and sorbic acid (water solubility at 20 °C: 20 and 1.6 mg/mL, respectively) as well as PBS plus 0.5% Igepal® (Sigma Aldrich, Germany) with testosterone (determined solubility: 93.9 µg/mL) was used as acceptor medium. Then, 1 mL of the donor solution (caffeine and sorbic acid: 1 mg/mL, testosterone: 200 µg/mL with 0.4% ethanol and 2% Igepal®) was applied to the skin surface for 25 h. The donor compartments were sealed with Parafilm® to prevent evaporation of the solution. Aliquots of the acceptor medium (500 µL) were withdrawn repeatedly (every hour from 0 to 10 h and from 21 to 25 h) and replaced with fresh acceptor medium. The samples were analyzed by high-performance liquid chromatography (HPLC). At the end of the experiment, the remaining donor solution was removed by a wash-off procedure and then collected and analyzed by HPLC. Skin samples were wiped with paper towels, snap-frozen, chopped, transferred into a reaction tube and submerged with 1 mL of the extraction solution (caffeine: 10% ACN/90% phosphate buffer; sorbic acid: 30% ACN/70% phosphate buffer; testosterone: 45% ACN/55% phosphate buffer). Skin samples were incubated for 1 h at 50 °C with shaking at 1400 rpm and then centrifuged (5 min and 14,680 rpm, Centrifuge 5424, Eppendorf AG, Germany); the supernatant was subjected to HPLC analysis. The extraction was performed twice. Preliminary studies showed that recovery of the drug from the skin after two extraction steps was 91.7%±0.9% with caffeine, 94.7±2.5 % with sorbic acid and 82.7±16.0% with testosterone.
2.4. Data analysis
The cumulative amount of permeated drug, expressed in micrograms per square centimeter (µg/cm2), is plotted against time (h). The flux, the mass of test substance passing through a unit area of the membrane (1.76 cm2) per unit of time under steady-state conditions (in µg/cm2/h) and the relative lag time (abscissa intercept point, h) were calculated from the slope of the graph using an automated approach [27]. Furthermore, the total drug uptake (drug in the skin plus drug in the acceptor medium after 25 h) and the total recovery were determined. Apparent permeability (Papp) is presented in Box-and-Whiskers plot (Min to Max): The whiskers go down to the smallest value and up to the largest. The top and bottom of the box are the 25th and 75th percentiles. All experiments were performed 6 times. Images were generated using GraphPad Prism® software, version 5.03. The enhancement factor (EF) was calculated by dividing the Papp of treated skin (either tape-stripped or abraded) by the Papp of the untreated skin.
Data are given as arithmetic means and standard deviation (SD). To verify the differences in skin permeation (n=6), data were subjected to the non-parametric Kruskal–Wallis test, followed by the Dunn post-test procedure, in cases of significance (GraphPad Prism® version 5.03). Significant differences were defined as p≤0.05.
3. Results and discussion
Skin penetration and permeation are influenced by the physiochemical properties of the drug substance, the characteristics of the formulation and the skin conditions. Skin conditions can be influenced by skin diseases and often are attended by disruption of the stratum corneum. Therefore, it is essential to consider skin condition in the early stage of dermal drug formulation development because of possible modified drug transport into and across the skin [28]. To determine the drug levels within the stratum corneum, tape stripping is a well-established method [29,30]. Furthermore, this method is used to obtain an impaired skin barrier [31,32]. Producing the required degree of skin impairment (TEWL: 30±2 g m−2 h−1) needs up to 60 pieces of tape, making the validated tape-stripping method by Simonsen and Fullerton [10] expensive and less attractive for a high sample throughput. An appropriate and validated alternative method for in vivo and ex vivo testing of drug transport across the impaired skin barrier is necessary.
3.1. Method validation of skin impairment by abrasion
Skin integrity can be influenced in vivo and in vitro by various methods including chemical (by solvents or surfactants) or mechanical damage, UV radiation or clinical diseases; for detailed information, see Gattu and Maibach [17,44]. TEWL measurement is a well-established method for the determination of skin integrity, and the fraction of stratum corneum removed is related to an increase in TEWL [10,20,33]. Here, various abrasive materials, for example sandpaper and different sponge surfaces, were tested for reproducibility and effectiveness of the gradual removal of the stratum corneum as well as the increase of the TEWL value (data not shown). The Spontex® Brillant best met these demands (Fig. 1). The decrease of skin integrity was followed by TEWL measurement. After about ten repetitions, a TEWL value of 30±2 g m−2 h−1 was attained, and a plateau was reached after approximately 30 repetitions, suggesting that the complete stratum corneum was removed. The number of repetitions depends on the user and the skin sample, therefore the degree of skin damage always has to be controlled, for example by TEWL measurement. In literature, the degree of skin damage often is not reported or even measured, thus the comparison of penetration data without information of the degree of skin damage is difficult.
Fig. 1.

Transepidermal water loss value with respect to the repetition of abrasions using the sponge (Spontex® Brillant/Twist sponge, upper left inset) (mean±SD, n = 3).
Histological inspection of the porcine skin (Fig. 2) confirms the results of the TEWL measurements. After repeated treatment of the skin surface with the aluminum-coated sponge, the stratum corneum was clearly reduced after 10 repetitions (Fig. 2, center) and finally completely removed after more than 40 repetitions (Fig. 2, right).
Fig. 2.

Histological section of the pig ear: untreated skin (left), abraded skin with a TEWL value within the range of 30±2 g m−2 h−1 (center), and abraded skin with a TEWL value above 40 g m−2 h−1 (right); Magnification ×400.
Thus, the damaged skin model for skin permeation studies are characterized by a visible reduction of the stratum corneum with a TEWL value of 30±2 g m−2 h−1.
3.2. Skin permeation studies
The skin consists of two permeation compartments, the lipophilic stratum corneum, with a water content of approximately 15%, and the more hydrophilic viable epidermis and dermis, with a water content of approximately 70% [34]. Thus, permeation through the skin strongly depends on the physicochemical properties of the applied substances, including their lipophilicity and molecular weight, as well as the condition of the skin. To evaluate the treatment of the skin by abrasion with the Spontex® Brillant sponge as an alternative model of a damaged skin barrier, the percutaneous absorption and skin permeation of three model substances (caffeine, sorbic acid and testosterone) were studied using tape-stripped and abraded skin in comparison to untreated skin. Sorbic acid was used instead of the OECD proposed benzoic acid [26]. Preliminary permeation studies (n=3, data not shown) with benzoic acid were not pursued due to high variation in drug recovery (41–84%), which did not meet the requirements of corresponding guidelines [26,35]. The results of the penetration and permeation study are summarized in Table 1.
Table 1.
Summary of the results of the permeation study with untreated, tape stripped and abraded skin using caffeine, sorbic acid and testosterone (mean±SD, n=6).
| Sample |
Caffeine |
Sorbic acid |
Testosterone |
|---|---|---|---|
| TEWL (g m−2 h−1) | |||
| Untreated skin | 10.6±1.7 | 10.8±0.9 | 10.8±1.2 |
| Tape stripped skin | 28.2±0.7 | 29.8±0.7 | 30.5±0.7 |
| Abraded skin | 29.9±0.7 | 29.9±0.7 | 30.4±0.8 |
| Flux (µg/cm2/h) | |||
| Untreated skin | 1.7±1.0 | 2.1±1.1 | 0.2±0.1 |
| Tape stripped skin | 7.9±1.7 | 6.6±0.8 | 0.4±0.2 |
| Abraded skin | 13.1±4.8 | 7.7±1.6 | 0.5±0.1 |
| lag time (h) | |||
| Untreated skin | 4.3±0.7 | 4.5±0.9 | 5.5±0.5 |
| Tape stripped skin | 2.4±0.6 | 2.7±1.2 | 4.4±1.1 |
| Abraded skin | 1.8±0.9 | 3.5±0.8 | 4.8±1.1 |
| Total recovery(%) | |||
| Untreated skin | 102.5±5.7 | 94.5±5.0 | 100.9±1.7 |
| Tape stripped skin | 99.9±2.4 | 91.0±1.9 | 96.2±3.4 |
| Abraded skin | 98.1±11.2 | 87.4±1.9 | 98.0±2.7 |
| Papp (cm/s×10−7) | |||
| Untreated skin | 4.6±2.8 | 5.8±3.2 | 3.1±1.0 |
| Tape stripped skin | 21.9±4.6 | 18.2±2.2 | 5.9±2.4 |
| Abraded skin | 36.3±13.4 | 21.5±4.4 | 6.5±2.0 |
| Total drug uptakea(%) | |||
| Untreated skin | 7.1±4.3 | 9.1±4.6 | 7.8±2.6 |
| Tape stripped skin | 35.3±7.5 | 30.7±4.5 | 14.8±2.1 |
| Abraded skin | 52.0±14.4 | 36.3±8.3 | 14.9±2.8 |
Total drug uptake: drug in the skin plus drug in the acceptor medium after 25 h.
3.2.1. Caffeine skin permeation
Caffeine is a relatively small (MW = 194.19 g/mol), highly hydrophilic molecule (log P=−0.13), and its penetration into skin should be restricted mainly by the stratum corneum. The mean cumulative amount of caffeine that permeated the porcine ear skin over 25 h as well as the apparent permeability (Papp) after 25 h for the different treated skin samples are shown in Fig. 3. With intact stratum corneum (untreated skin), caffeine permeation through porcine skin was lowest, followed by the tape-stripped skin and then abraded skin. The higher permeability of caffeine with abraded skin compared to the tape-stripped skin can be associated to the skin preparation. Using the tape-stripping method, the layers of the stratum corneum are gradually removed from the skin surface resulting in reduction of stratum corneum thickness. The mechanical abrasion with an aluminum-coated sponge also leads to a reduction in the thickness of the stratum corneum thickness and a partially roughened surface of the skin, possibly resulting in a less compact stratum corneum like in certain skin diseases.
Fig. 3.

Cumulative permeation of caffeine across porcine skin over 25 h (mean±SD, n=6); ● untreated skin, ▲ abraded skin, and ■ tape-stripped skin. The upper left inset shows the apparent permeability after 25 h (Box plot with median, minimum, maximum and the 0.25 and 0.75 quartiles (n=6)); applied concentration: 1 mg/mL.
The flux and Papp values of caffeine penetration across untreated skin are consistent with the published values of 0.31×10−6 cm/s [36] and 0.46–6.82 µg/cm2/h [37]. In accordance with the permeation results, the lag time for caffeine was the shortest with the abraded skin and the tape-stripped skin and was almost twice as long for the untreated skin. The lag time depends on the lipophilicity and the molecular weight of the substance and the thickness of the skin. Tape stripping as well as the abrasion of the skin using the Spontex® Brillant decreased the thickness of the skin, more precisely the stratum corneum, and thus the main penetration barrier for caffeine, resulting in an increased flux and a decreased lag time.
3.2.2. Sorbic acid skin permeation
Sorbic acid is frequently used as a preservative in cosmetics, pharmaceuticals and food. The common concentration in cosmetics (0.1%) was applied in this experiment. Compared to caffeine, sorbic acid is more lipophilic (log P=1.268; MW =112.13 g/mol).
Sorbic acid permeation was lowest with intact stratum corneum (untreated skin) followed by the tape-stripped skin and the abraded skin (Fig. 4). The permeation of sorbic acid through the untreated skin was slightly higher than for the same concentration of caffeine. However, this was not true for the impaired skin barrier; thus, the enhancement of sorbic acid uptake after partial removal of the stratum corneum by tape-stripping (EF: 3.1) as well as abrasion (EF: 3.7) was lower than for caffeine (EF: 4.8 and 7.9, respectively). Morgan and co-workers [38] showed a remarkable increase in skin absorption of highly water-soluble antiviral drugs after complete removal of the stratum corneum by tape stripping compared to intact skin. The skin absorption of the more hydrophilic penciclovir (log P=2.1) was considerably enhanced compared to the aciclovir (log P= 1.8).
Fig. 4.
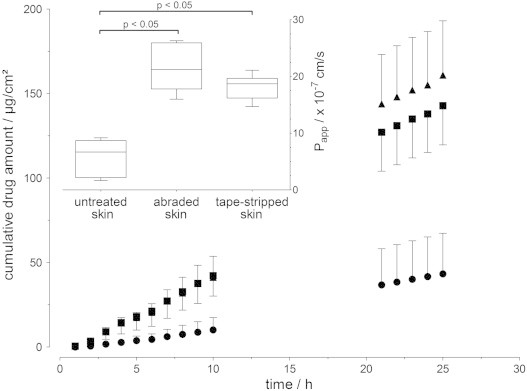
Cumulative sorbic acid permeation across porcine skin over 25 h (mean±SD, n = 6); ● untreated skin, ▲ abraded skin, and ■ tape-stripped skin. The upper left inset shows the apparent permeability after 25 h (Box plot with median, minimum, maximum and the 0.25 and 0.75 quartiles (n=6)); applied concentration: 1 mg/mL.
3.2.3. Testosterone skin permeation
Testosterone, an androgen hormone, is a lipophilic molecule (log P 3.47; MW=288.42 g/mol) that is available as a gel and as a transdermal patch for skin application. Due to its low aqueous solubility, the applied testosterone concentration was one-fifth (200 µg/mL) that of caffeine and sorbic acid. Donor concentrations of testosterone were below 2% of the maximum solubility, making sink conditions present during the entire experiment. The lag times were the highest with testosterone compared to caffeine and sorbic acid. After application onto the skin, the distribution of a model substance by passive diffusion depends on the concentration gradient and the thermodynamic activity of the substances in the different compartments (vehicle, stratum corneum, viable epidermis and dermis). Thus, the lower testosterone concentration applied and the high affinity of testosterone to the stratum corneum and viable epidermis compared to dermis [39] led to a lower skin permeation. Testosterone permeation was lowest with intact stratum corneum, followed by nearly equal permeation through tape-stripped skin and abraded skin (Fig. 5). Testosterone permeation increased by 1.9-fold and 2.1-fold through tape-stripped and abraded skin, respectively, and shows the stratum corneum is less restrictive for lipophilic compounds than for hydrophilic substances.
Fig. 5.
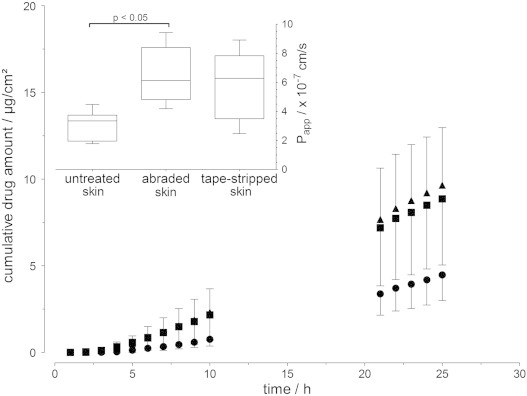
Cumulative permeability of testosterone across porcine skin over 25 h (mean±SD, n=6); ● untreated skin, ▲ abraded skin, and ■ tape-stripped skin. The upper left inset shows the apparent permeability after 25 h (Box plot with median, minimum, maximum and the 0.25 and 0.75 quartiles (n=6)); applied concentration: 200 µg/mL.
3.3. Skin uptake of caffeine, sorbic acid and testosterone
Skin uptake depends on the physicochemical properties of the applied substance, among other factors. Caffeine, sorbic acid and testosterone differ substantially in lipophilicity and slightly in molecular weight. The affinity of the studied substance to the different skin compartments as well as the applied vehicle and the acceptor medium strongly influence the permeation behavior of a molecule. Magnusson and co-workers [39] showed that the permeability coefficient across the dermis decreased with increasing lipophilicity of the applied steroids, whereas the permeability coefficient slightly increased with full-thickness skin and significantly increased using an epidermal sheet with increasing lipophilicity of the steroids. Furthermore, the stratum corneum can act as a reservoir for lipophilic substances [40].
The distribution of the caffeine, sorbic acid and testosterone between donor solution, acceptor medium and skin using intact and impaired skin is shown in Fig. 6 (left). The total recovery after 25 h meet the requirement of the skin absorption guidelines with 85–100% [35], respectively 100±10% recovery [26]. The total drug uptake was increased using both impaired skin barrier models compared to the untreated skin (Fig. 6, right). The skin permeation of a highly hydrophilic substance, such as caffeine, is primarily restricted by the stratum corneum; thus, increased caffeine uptake by the impaired skin barrier was considerably higher than for sorbic acid and testosterone. This result is consistent with published data [18,41]. The sorbic acid uptake was increased by up to 4-fold while testosterone showed only a 2-fold higher skin uptake with impaired skin barrier. Unlike with caffeine and sorbic acid, testosterone shows nearly equal amount of the drug in the acceptor medium and the skin after 25 h. Magnusson and co-workers [39] studied the skin tissue to buffer distribution of steroids of varied lipophilicity. Compared to hydrocortisone (log P=1.43), testosterone showed a clear higher affinity to the different skin layers (viable epidermis>stratum corneum>dermis). Although skin conditions were provided throughout the whole experiment, it cannot be excluded that the acceptor medium restricted the partitioning of testosterone from the skin to the acceptor medium. Furthermore, the lower percentage of testosterone uptake can be attributed to the lower concentration gradient and the dermis, which may act as a permeation barrier for the lipophilic testosterone. The more lipophilic retinol (log P=6.84) was also found at a low percentage in the receptor medium [42].
Fig. 6.
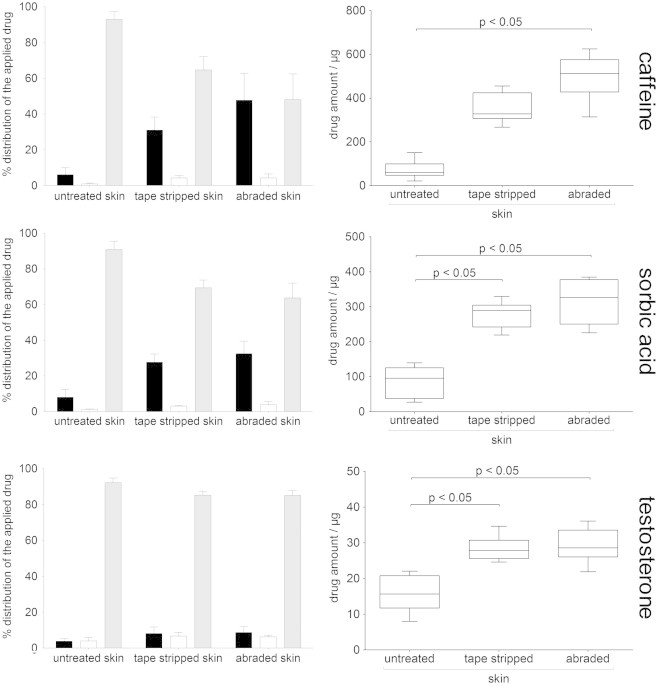
Left: percentage distribution of the applied substances between donor solution (gray bar), acceptor medium (black bar) and skin membrane (white bar); each skin sample is normalized to 100% (MW±SD, n=6). Right: Total drug uptake (amount of drug in the skin and the acceptor medium) after 25 h (Box plot with median, minimum, maximum and the 0.25 and 0.75 quartiles (n=6)).
Different skin abrasion methods described in the literature resulted in different degrees of skin barrier damage. It has been shown that the in vivo penetration of salicylic acid depends on the degree of skin barrier damage, defined by TEWL measurement, resulting in a 2.2-fold enhancement after acetone treatment (TEWL 9.1 g m−2 h−1) and up to a 157-fold enhancement after tape-stripping (TEWL 30.6 g m−2 h−1) [43]. Scratching the skin surface with the tip of a needle (1–4 abrasion lines) caused a lower enhancement than tape-stripping [18], though the use of a rotation brush leads to the opposite effect [41]. It should be noted that the different tape-stripping protocols used can lead to different skin impairments, and thus, comparison of different methods is difficult without standardization. Therefore, the control of the skin damage process by TEWL measurement is essential to track the degree of skin impairment and produce reliable results. Drug uptake from abraded skin was significantly higher compared to intact skin for caffeine, sorbic acid and testosterone; this was also true for sorbic acid and testosterone when comparing tape-stripped skin with intact skin.
4. Conclusions
The skin barrier, specifically the stratum corneum, is usually impaired at the beginning of skin therapy, thereby resulting in a modification of the permeation behavior of applied substances compared to an intact skin barrier. Furthermore, the skin is constantly changing during the healing process, and this must be considered to continuously provide a sufficient concentration of the drug at the site of action. Therefore, an accurate, easy to handle and reviewed method suitable for high sample throughput drug penetration was developed. Disruption of the skin by either abrasion with the aluminum-coated sponge or the tape-stripping methods leads to comparable results in skin penetration and permeation studies. With abraded skin, as well as tape-stripped skin, uptake of caffeine, sorbic acid and testosterone, was higher than in untreated skin. The enhancement of drug uptake was the highest with the most hydrophilic substance, caffeine, followed by sorbic acid, and it was only slightly increased with testosterone, the most lipophilic substance. Thus, the lipophilic stratum corneum is an extremely restrictive barrier, especially for hydrophilic substances. Due to the considerable influence of skin conditions on drug uptake, a valid, inexpensive and fast method is required. In particular, risk assessment of xenobiotics for example nanoparticles should be performed with impaired skin barrier models to simulate the worst case. The skin impairment have to be controlled by an adequate method such as the TEWL measurement.
These results emphasize that skin barrier disruption by abrasion using a sponge with an aluminum coating is reproducible, appropriate for high sample throughput and inexpensive, making it suitable as an alternative method for generating a disrupted skin barrier in drug uptake studies.
Acknowledgment
We would like to thank the Hessen State Ministry of Higher Education, Research and the Arts for the financial support within the Hessen initiative for scientific and economic excellence (LOEWE-Program) with a focus on Biomedical Engineering - Bioengineering & Imaging.
References
- 1.Bouwstra J.A., Honeywell-Nguyen P.L., Gooris G.S., Ponec M. Structure of the skin barrier and its modulation by vesicular formulations. Progress in Lipid Research. 2003;42:1–36. doi: 10.1016/s0163-7827(02)00028-0. 12467638 [DOI] [PubMed] [Google Scholar]
- 2.Lee W.J., Kim J.Y., Song C.H., Jung H.D., Lee S.H., Lee S.-J., Kim do W. Disruption of barrier function in dermatophytosis and pityriasis versicolor. Journal of Dermatology. 2011;38:1049–1053. doi: 10.1111/j.1346-8138.2011.01320.x. 21950511 [DOI] [PubMed] [Google Scholar]
- 3.Löffler H., Dreher F., Maibach H.I. Stratum corneum adhesive tape stripping: influence of anatomical site, application pressure, duration and removal. British Journal of Dermatology. 2004;151:746–752. doi: 10.1111/j.1365-2133.2004.06084.x. 15491413 [DOI] [PubMed] [Google Scholar]
- 4.Schäfer H., Redelmeier T.E. Skin barrier: principles of percutaneous absorption. Karger; Basel: 1996. [Google Scholar]
- 5.Seidenari S., Giusti G. Objective assessment of the skin of children affected by atopic dermatitis: a study of pH, capacitance and TEWL in eczematous and clinically uninvolved skin. Acta Dermato-Venereologica. 1995;75:429–433. doi: 10.2340/0001555575429433. 8651017 [DOI] [PubMed] [Google Scholar]
- 6.Effendy I., Loeffler H., Maibach H.I. Baseline transepidermal water loss in patients with acute and healed irritant contact dermatitis. Contact Dermatitis. 1995;33:371–374. doi: 10.1111/j.1600-0536.1995.tb02069.x. 8706392 [DOI] [PubMed] [Google Scholar]
- 7.Grice K., Sattar H., Baker H., Sharratt M. The relationship of transepidermal water loss to skin temperature in psoriasis and eczema. Journal of Investigative Dermatology. 1975;64:313–315. doi: 10.1111/1523-1747.ep12512258. 1141706 [DOI] [PubMed] [Google Scholar]
- 8.Moskowitz D.G., Fowler A.J., Heyman M.B., Cohen S.P., Crumrine D., Elias P.M., Williams M.L. Pathophysiologic basis for growth failure in children with ichthyosis: an evaluation of cutaneous ultrastructure, epidermal permeability barrier function, and energy expenditure. Journal of Pediatrics. 2004;145:82–92. doi: 10.1016/j.jpeds.2004.03.052. 15238912 [DOI] [PubMed] [Google Scholar]
- 9.Rassner G. Dermatologie: Lehrbuch und Atlas. Urban & Fischer/Elsevier; München: 2009. [Google Scholar]
- 10.Simonsen L., Fullerton A. Development of an in vitro skin permeation model simulating atopic dermatitis skin for the evaluation of dermatological products. Skin Pharmacology and Physiology. 2007;20:230–236. doi: 10.1159/000104421. 17587887 [DOI] [PubMed] [Google Scholar]
- 11.Moll I. Duale Reihe Dermatologie. Thieme; Stuttgart: 2010. [Google Scholar]
- 12.Sterry W. Kurzlehrbuch Dermatologie. Thieme; Stuttgart: 2011. [Google Scholar]
- 13.Aalto-Korte K. Improvement of skin barrier function during treatment of atopic dermatitis. Journal of the American Academy of Dermatology. 1995;33:969–972. doi: 10.1016/0190-9622(95)90288-0. 7490367 [DOI] [PubMed] [Google Scholar]
- 14.Aalto-Korte K., Turpeinen M. Transepidermal water loss and absorption of hydrocortisone in widespread dermatitis. British Journal of Dermatology. 1993;128:633–635. doi: 10.1111/j.1365-2133.1993.tb00258.x. 8338747 [DOI] [PubMed] [Google Scholar]
- 15.Fölster-Holst R., Hamm H. Ektoparasitosen im Kindesalter. Monatsschrift Kinderheilkunde Zeitschrift für Kinder-und Jugendmedizin. 2008;156:139–146. [Google Scholar]
- 16.Gladstone H.B., Darmstadt G.L. Crusted scabies in an immunocompetent child: treatment with ivermectin. Pediatric Dermatology. 2000;17:144–148. doi: 10.1046/j.1525-1470.2000.01736.x. 10792808 [DOI] [PubMed] [Google Scholar]
- 17.Gattu S., Maibach H.I. Enhanced absorption through damaged skin: an overview of the in vitro human model. Skin Pharmacology and Physiology. 2010;23:171–176. doi: 10.1159/000288163. 20185974 [DOI] [PubMed] [Google Scholar]
- 18.Bronaugh R.L., Stewart R.F. Methods for in vitro percutaneous absorption studies V: permeation through damaged skin. Journal of Pharmaceutical Sciences. 1985;74:1062–1066. doi: 10.1002/jps.2600741008. 4078703 [DOI] [PubMed] [Google Scholar]
- 19.Feldmann R.J., Maibach H.I. Penetration of 14C hydrocortisone through normal skin: the effect of stripping and occlusion. Archives of Dermatology. 1965;91:661–666. doi: 10.1001/archderm.1965.01600120093023. 14295535 [DOI] [PubMed] [Google Scholar]
- 20.Rubio L., Alonso C., López O., Rodríguez G., Coderch L., Notario J., de la Maza A., Parra J.L. Barrier function of intact and impaired skin: percutaneous penetration of caffeine and salicylic acid. International Journal of Dermatology. 2011;50:881–889. doi: 10.1111/j.1365-4632.2010.04819.x. 21699529 [DOI] [PubMed] [Google Scholar]
- 21.Jui-Chen T., Weiner N.D., Flynn G.L., Ferry J. Properties of adhesive tapes used for stratum corneum stripping. International Journal of Pharmaceutics. 1991;72:227–231. [Google Scholar]
- 22.Lademann J., Jacobi U., Surber C., Weigmann H.-J., Fluhr J.W. The tape stripping procedure—evaluation of some critical parameters. European Journal of Pharmaceutics and Biopharmaceutics: Official Journal of Arbeitsgemeinschaft für Pharmazeutische Verfahrenstechnik e.V. 2009;72:317–323. doi: 10.1016/j.ejpb.2008.08.008. 18775778 [DOI] [PubMed] [Google Scholar]
- 23.van der Molen R.G., Spies F., van ’t Noordende J.M., Boelsma E., Mommaas A.M., Koerten H.K. Tape stripping of human stratum corneum yields cell layers that originate from various depths because of furrows in the skin. Archives of Dermatological Research. 1997;289:514–518. doi: 10.1007/s004030050232. 9341971 [DOI] [PubMed] [Google Scholar]
- 24.Meyer W., Kacza J., Zschemisch N.H., Godynicki S., Seeger J. Observations on the actual structural conditions in the stratum superficiale dermidis of porcine ear skin, with special reference to its use as model for human skin. Annals of Anatomy = Anatomischer Anzeiger: Official Organ of the Anatomische Gesellschaft. 2007;189:143–156. doi: 10.1016/j.aanat.2006.09.004. 17419547 [DOI] [PubMed] [Google Scholar]
- 25.Diembeck W., Beck H., Benech-Kieffer F., Courtellemont P., Dupuis J., Lovell W., Paye M., Spengler J., Steiling W. Test guidelines for in vitro assessment of dermal absorption and percutaneous penetration of cosmetic ingredients. European cosmetic, toiletry and perfumery association. Food and Chemical Toxicology: an International Journal Published for the British Industrial Biological Research Association. 1999;37:191–205. doi: 10.1016/s0278-6915(98)00114-8. 10227743 [DOI] [PubMed] [Google Scholar]
- 26.OECD. OECD . Guideline for the Testing of Chemicals (Section 4) Organisation for Economic Co-operation and Development; Paris: 2004. Test No. 428: skin absorption: in vitro method. [Google Scholar]
- 27.Niedorf F., Schmidt E., Kietzmann M. The automated, accurate and reproducible determination of steady-state permeation parameters from percutaneous permeation data. Alternatives to Laboratory Animals. 2008;36:201–213. doi: 10.1177/026119290803600209. 18522486 [DOI] [PubMed] [Google Scholar]
- 28.Schmidts T., Marquardt K., Schlupp P., Dobler D., Heinz F., Mäder U., Garn H., Renz H., Zeitvogel J., Werfel T., Runkel F. Development of drug delivery systems for the dermal application of therapeutic DNAzymes. International Journal of Pharmaceutics. 2012;431:61–69. doi: 10.1016/j.ijpharm.2012.04.034. 22531847 [DOI] [PubMed] [Google Scholar]
- 29.Teichmann A., Jacobi U., Ossadnik M., Richter H., Koch S., Sterry W., Lademann J. Differential stripping: determination of the amount of topically applied substances penetrated into the hair follicles. Journal of Investigative Dermatology. 2005;125:264–269. doi: 10.1111/j.0022-202X.2005.23779.x. 16098036 [DOI] [PubMed] [Google Scholar]
- 30.Weigmann H.-J., Lindemann U., Antoniou C., Tsikrikas G.N., Stratigos A.I., Katsambas A., Sterry W., Lademann J. UV/vis absorbance allows rapid, accurate, and reproducible mass determination of corneocytes removed by tape stripping. Skin Pharmacology and Applied Skin Physiology. 2003;16:217–227. doi: 10.1159/000070844. 12784061 [DOI] [PubMed] [Google Scholar]
- 31.Visscher M.O., Hoath S.B., Conroy E., Wickett R.R. Effect of semipermeable membranes on skin barrier repair following tape stripping. Archives of Dermatological Research. 2001;293:491–499. doi: 10.1007/pl00007463. 11820725 [DOI] [PubMed] [Google Scholar]
- 32.Yang L., Mao-Qiang M., Taljebini M., Elias P.M., Feingold K.R. Topical stratum corneum lipids accelerate barrier repair after tape stripping, solvent treatment and some but not all types of detergent treatment. British Journal of Dermatology. 1995;133:679–685. doi: 10.1111/j.1365-2133.1995.tb02738.x. 8555016 [DOI] [PubMed] [Google Scholar]
- 33.Sekkat N., Kalia Y.N., Guy R.H. Biophysical study of porcine ear skin in vitro and its comparison to human skin in vivo. Journal of Pharmaceutical Sciences. 2002;91:2376–2381. doi: 10.1002/jps.10220. 12379922 [DOI] [PubMed] [Google Scholar]
- 34.Warner R.R., Myers M.C., Taylor D.A. Electron Probe analysis of human skin: determination of the water concentration profile. Journal of Investigative Dermatology. 1988;90:218–224. doi: 10.1111/1523-1747.ep12462252. 3339263 [DOI] [PubMed] [Google Scholar]
- 35.SCCNFP . Basic criteria for the in vitro assessment of dermal absorption of cosmetic ingredients. Scientific Committee on Cosmetic Products and Non-Food Products; 2003. [Google Scholar]
- 36.Schäfer-Korting M., Bock U., Diembeck W., Düsing H.J., Gamer A., Haltner-Ukomadu E., Hoffmann C., Kaca M., Kamp H., Kersen S., Kietzmann M., Korting H.C., Krächter H.U., Lehr C.M., Liebsch M., Mehling A., Müller-Goymann C., Netzlaff F., Niedorf F., Rübbelke M.K., Schäfer U., Schmidt E., Schreiber S., Spielmann H., Vuia A., Weimer M. The use of reconstructed human epidermis for skin absorption testing: results of the validation study. Alternatives to Laboratory Animimal. 2008;36:161187. doi: 10.1177/026119290803600207. 18522484 [DOI] [PubMed] [Google Scholar]
- 37.van de Sandt J.J., van Burgsteden J.A., Cage S., Carmichael P.L., Dick I., Kenyon S., Korinth G., Larese F., Limasset J.C., Maas W.J., Montomoli L., Nielsen J.B., Payan J.P., Robinson E., Sartorelli P., Schaller K.H., Wilkinson S.C., Williams F.M. In vitro predictions of skin absorption of caffeine, testosterone, and benzoic acid: a multi-centre comparison study. Regulatory Toxicology and Pharmacology: RTP. 2004;39:271–281. doi: 10.1016/j.yrtph.2004.02.004. 15135208 [DOI] [PubMed] [Google Scholar]
- 38.Morgan C.J., Renwick A.G., Friedmann P.S. The role of stratum corneum and dermal microvascular perfusion in penetration and tissue levels of water-soluble drugs investigated by microdialysis. British Journal of Dermatology. 2003;148:434–443. doi: 10.1046/j.1365-2133.2003.05163.x. 12653734 [DOI] [PubMed] [Google Scholar]
- 39.Magnusson B.M., Cross S.E., Winckle G., Roberts M.S. Percutaneous absorption of steroids: determination of in vitro permeability and tissue reservoir characteristics in human skin layers. Skin Pharmacology and Physiology. 2006;19:336–342. doi: 10.1159/000095254. 16931901 [DOI] [PubMed] [Google Scholar]
- 40.Wiechers J.W. The barrier function of the skin in relation to percutaneous absorption of drugs. Pharmaceutisch Weekblad. Scientific Edition. 1989;11:185–198. doi: 10.1007/BF01959410. 2694089 [DOI] [PubMed] [Google Scholar]
- 41.Akomeah F.K., Martin G.P., Muddle A.G., Brown M.B. Effect of abrasion induced by a rotating brush on the skin permeation of solutes with varying physicochemical properties. European Journal of Pharmaceutics and Biopharmaceutics: Official Journal of Arbeitsgemeinschaft für Pharmazeutische Verfahrenstechnik e.V. 2008;68:724–734. doi: 10.1016/j.ejpb.2007.06.005. 17618097 [DOI] [PubMed] [Google Scholar]
- 42.Yourick J.J., Jung C.T., Bronaugh R.L. In vitro and in vivo percutaneous absorption of retinol from cosmetic formulations: significance of the skin reservoir and prediction of systemic absorption. Toxicology and Applied Pharmacology. 2008;231:117–121. doi: 10.1016/j.taap.2008.04.006. 18511092 [DOI] [PubMed] [Google Scholar]
- 43.Benfeldt E., Serup J., Menné T. Effect of barrier perturbation on cutaneous salicylic acid penetration in human skin: in vivo pharmacokinetics using microdialysis and non-invasive quantification of barrier function. British Journal of Dermatology. 1999;140:739–748. doi: 10.1046/j.1365-2133.1999.02859.x. 10233334 [DOI] [PubMed] [Google Scholar]
- 44.Gattu S., Maibach H.I. Modest but increased penetration through damaged skin: an overview of the in vivo human model. Skin Pharmacology and Physiology. 2011;24:2–9. doi: 10.1159/000314995. 20588085 [DOI] [PubMed] [Google Scholar]


