Abstract
Background
Vitamin D plays an important role in bone mineralization, remodeling, and maintenance and therefore its deficiency may be implicated in the pathogenesis of osteoarthritis (OA). Vitamin D status was evaluated in patients with knee or hip OA scheduled for joint replacement. The impact of anthropometric parameters such as gender, age, and body mass index on vitamin D levels was also examined. The study was conducted in a Mediterranean country (Greece).
Materials and methods
We included 164 patients with knee or hip OA scheduled for joint replacement in this study. Serum levels of 25-hydroxyvitamin D (vitamin D) were measured in routine blood samples taken from the patients at their pre-admission visit, a week before the operation, using radioimmunoassay.
Results
The majority of patients were vitamin D deficient (81.7 %); 15.2 % of them were vitamin D insufficient (hypovitaminosis). Only 3 % of patients were vitamin D sufficient. There was a significantly positive association between vitamin D levels and male gender.
Conclusion
These findings indicate a large percentage of vitamin D deficient patients with knee or hip OA, which is unexpected considering the high annual insolation in northern Greece. Many other possible predisposing factors for OA should be taken into consideration. Whether treatment with vitamin D supplements may provide beneficial effects to these patients and the stage of disease in which this treatment should commence remains an issue for further scientific investigation.
Level of evidence
Level IV.
Keywords: Vitamin D, Osteoarthritis, Knee osteoarthritis, Hip osteoarthritis
Introduction
Vitamin D deficiency is one of the most common and under-diagnosed medical conditions in the world, since a significant proportion of the population in many countries and regions around the world have low vitamin D levels [1–4]. The 25-hydroxyvitamin D level depends on various parameters, including the amount of solar ultraviolet B (UVB) irradiation (determined by the time of day, season [5–7] latitude, skin pigmentation, and use of sunscreen), age [7], dietary habits, gender, obesity [8], and many others [9].
Vitamin D plays an important role in bone mineralization, remodeling, and maintenance and therefore its deficiency may be implicated in the pathogenesis of osteoarthritis (OA) [10, 11]. Although the pathogenesis of OA is still unclear, recent evidence suggests that changes in subchondral bone remodeling—phases of bone absorption and of bone sclerosis––may be responsible for cartilage damage. Vitamin D has been shown to modulate the activity of metalloproteinase enzymes. Low levels of 25(OH)D3 lead to an increased production of degradative enzymes [12]. The theory behind changes in the bone is that low levels of 25-hydroxyvitamin D slow the remodeling response of subarticular bone, resulting in thickening of the subchondral bone, osteophyte formation, and resultant cartilage damage [13].
Prospective epidemiological studies have found an association between dietary intake and serum levels of 25-hydroxyvitamin D and the development or progression of radiographic hip [14, 15] and knee OA [22]. Low serum levels of 25-hydroxyvitamin D have been reported in a significant proportion of patients with OA of hip and knee joints [14, 16–24]. Some authors suggest that achieving vitamin D sufficiency may prevent and/or delay cartilage loss in knee OA [15, 25]. In patients with hip OA who underwent total hip replacement, 25-hydroxyvitamin D levels were found to correlate positively with both pre- and post-operative Harris hip scores. Therefore, it seems that vitamin D deficiency in patients undergoing total hip replacement may be a risk factor for a suboptimal outcome [19].
However, results of other studies do not support an association between the low level of serum 25(OH)D and the development of OA [27–29]. An association of serum 25(OH)D levels with hip or knee OA has therefore not yet been fully established. The authors recommended serum 25(OH)D measurement in any patient with symptoms suggestive of knee OA, particularly at the initial stage of disease [23].
The main purpose of this study was to evaluate the vitamin D status in patients with knee or hip OA scheduled for joint replacement in a Mediterranean country. Associations between vitamin D serum levels and gender, age, and body mass index (BMI) were also investigated.
Materials and methods
This uncontrolled cohort study was conducted from December 2011 to October 2012 in a Mediterranean country. The study was approved by the hospital’s scientific ethics committee and all patients provided informed consent.
Patients with hip or knee OA scheduled for hip or knee replacement were included in this study. Exclusion criteria were inflammatory arthritis, malignancy, renal failure, or anaemia.
The clinical examination of patients combined with a knee or hip plain radiograph set the diagnosis of OA. The Kellgren and Lawrence scale [26] was used and patients with grade 3 or 4 OA were scheduled for joint replacement. Age, gender, BMI, and co-morbidities were also recorded.
Blood samples were taken from the patients at their pre-admission visit by a resident orthopaedic surgeon, a week before the operation. The serum levels of 25-hydroxyvitamin D were measured by the Department of Nuclear Medicine, using radioimmunoassay (RIA) (radioactive material supplied by DiaSorin Inc., USA). The patients were categorized into three groups according to their vitamin D status. Vitamin D deficiency was defined as a 25-hydroxyvitamin D level below 20 ng/ml (50 nmol/L) and vitamin D insufficiency as a 25-hydroxyvitamin D level of 21–29 ng/ml (52.5–72.5 nmol/L) [30].
Clinical measurements were recorded by the biochemical laboratory of the Biopathology Department in order to exclude other bone disorders or systemic diseases. The following normal ranges were used: hematocrit (men, normal range: 40–54 %; women, normal range: 37–47 %), haemoglobin (men, normal range: 13.0–18.8 g/dL; women, normal range: 11.6–16.4g/dL), C-reactive protein (normal range: 0.07–8.2 mg/L), urea-BUN (normal range: 9–20 mg/dL), serum creatinine (men, normal range: 0.2-0.6 mg/dL; women, normal range: 0.6–1.0 mg/dL), serum glucose (normal range: 17–43 mg/dL), serum calcium (normal range: 8.1–10.4 mg/dL), and serum phosphorus (normal range: 2.5–4.5 mg/dL).
Results
In this study, 164 patients were included, 42 (25.6 %) of whom were men and 122 (74.3 %) were women. Age range was 48–86 years [mean = 68.9, standard deviation (SD) = 7.7 years]. All patients were Caucasian; 128 (78 %) of them suffered from knee OA and 36 (22 %) from hip OA. 19.5 % of patients belonged to the Muslim minority.
The levels of vitamin D ranged from 1.61 to 52.19 ng/ml (mean = 13.4, SD = 7.8 ng/ml). It is noteworthy that most patients were vitamin D deficient (81.7 %). 15.2 % of patients were vitamin D insufficient (hypovitaminosis). Only 3 % of patients were vitamin D sufficient (Table 1). Regarding BMI, 6.1 % of patients had optimal weight (BMI range 22–24.9 kg/m2), 36.6 % were overweight (BMI range 25–29.9 kg/m2) and more than half of the patients (56.7 %) were obese (BMI over 30 kg/m2) (Table 1). Additionally, 12 patients, all postmenopausal women, were under medication for osteoporosis with calcium and vitamin D supplements; 7 of them were vitamin D deficient, 4 were vitamin D insufficient and only 1 was vitamin D sufficient.
Table 1.
Patient groups according to gender, condition, BMI, and vitamin D serum levels
| Number | Percent | |
|---|---|---|
| Gender | ||
| Female | 122 | 74.4 |
| Male | 42 | 25.6 |
| Condition | ||
| OA knee | 128 | 78.0 |
| OA hip | 36 | 22.0 |
| ΒΜΙ groups | ||
| <22 kg/m2 | 1 | 0.6 |
| 22–24.9 kg/m2 | 10 | 61 |
| 25–29.9 kg/m2 | 60 | 36.6 |
| >30 kg/m2 | 93 | 56.7 |
| Vitamin D groups | ||
| Deficiency | 134 | 81.7 |
| Insufficiency | 25 | 15.2 |
| Normal | 5 | 3.0 |
| Total | 164 | 100.0 |
A linear regression model was used to assess links between vitamin D levels and age, gender, and BMI. The regression equation was: VitD levels = 15.238 – (0.226 × BMI) + (0.005 × age) + (3.952 × gender), with r2 = 0.074 and p = 0.006. Male gender had both the highest statistical significance (p = 0.004) and impact on the model (β = 3.952) in contrast to age (p = 0.951, β = 0.005). Male gender correlated positively with vitamin D serum levels (p = 0.004) (Fig. 1). ΒΜΙ was borderline statistically insignificant (p = 0.061) and correlated negatively with vitamin D levels (β = −0.226).
Fig. 1.
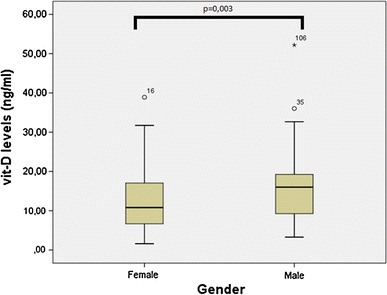
Correlation of vitamin D serum levels and gender
Further analysis of vitamin D levels between men and women showed that, on average, men had levels higher by 4.12 ng/ml than women (p = 0.003). All analyses were undertaken using the statistical package SPSS for Windows (version 19.0; SPSS Inc., Chicago, USA).
Discussion
The most important finding of this study is the high prevalence (96.9 % deficiency and insufficiency) of low serum levels of 25-hydroxyvitamin D in a population with OA, in a sunny region of a Mediterranean country. Our study showed that over 4 out of 5 patients with knee or hip OA were vitamin D deficient with serum levels below 20 ng/ml. Several studies have shown a high incidence of vitamin D deficiency in patients with OA of hip or knee [17–19, 23, 24]. Considering the commonness of sunshine in Greece and in relation with existing studies [1–3], we had expected a higher vitamin D status in Greek patients with knee or hip OA. Moreover, the prevalence of vitamin D deficiency in patients with OA scheduled for total hip or knee replacement in our study was higher than the reported values of studies carried out in northern European countries such as Finland [17], Germany [18], and the UK [19] where annual insolation is significantly lower. However, in these countries consumption of vitamin D enriched foods is very common.
We also tried to correlate serum levels of vitamin D with related anthropometric predisposing factors such as age, gender, and BMI. A significant association with gender was observed, with female patients having lower serum levels of vitamin D. A higher prevalence of severe deficiency of vitamin D has also been demonstrated among US adult women compared to men [30]. In a study that took place in Quebec, Canada, gender was not associated with 25(OH)D concentration [31]. In addition, the same study showed that age and BMI were not correlated with 25(OH)D deficiency. This result corresponds to our findings regarding age and BMI. However, considering that BMI was a borderline insignificant predictor of vitamin D levels in our sample, it may be possible that other anthropometric obesity measurements may have stronger association with vitamin D levels. Such measurements could include waist-to-hip ratio and waist-to-height ratio. The notion of association of obesity with low vitamin D levels is supported by Lagunova et al. [32], who found that the prevalence of vitamin D deficiency is dependent on BMI and age separately. The results of that study suggested that 1 in 3 women and 1 in 2 men with BMI ≥40 kg/m2 are vitamin D deficient.
The limitations of this study include a small sample size, particularly patients with hip OA. Another limitation is the absence of a control group and the scarcity of available data concerning the vitamin D status in the general population in our region. Despite the aforementioned limitations, the high prevalence of vitamin D deficiency in patients with knee or hip OA scheduled for joint arthroplasty is alarming.
Conflict of interest
All named authors hereby declare that they have no conflicts of interest to disclose.
Ethical standards
The study conforms to the Declaration of Helsinki [34]. An approval by the scientific ethical committee of our University General Hospital was obtained. All the patients provided informed consent prior to enrollment.
Contributor Information
Thomais Goula, Phone: +306974889349, Email: thetigoula@hotmail.com.
Alexandros Kouskoukis, Email: akouskoukis@gmail.com.
Georgios Drosos, Email: drosos@otenet.gr.
Alexandros-Savvas Tselepis, Email: savvalex@gmail.com.
Athanasios Ververidis, Email: averver@otenet.gr.
Christos Valkanis, Email: valkanisxr@yahoo.gr.
Athanasios Zisimopoulos, Email: azisimop@med.duth.gr.
Konstantinos Kazakos, Email: k.kazakos@med.duth.gr.
References
- 1.Endocrine Society Practice Guidelines JCEM (2011). Evaluation, treatement and prevention of vitamin D deficiency. http://www.endo-society.org/guidelines/final/upload/FINAL-Standalone-Vitamin-D-Guideline.pdf
- 2.Holick MF. The vitamin D epidemic and its health consequences. J Nutr. 2005;135(11):2739S–2748S. doi: 10.1093/jn/135.11.2739S. [DOI] [PubMed] [Google Scholar]
- 3.Kull M, Jr, Kallikorm R, Tamm A, Lember M. Seasonal variance of 25-(OH) vitamin D in the general population of Estonia, a Northern European country. BMC Public Health. 2009;19(9):22. doi: 10.1186/1471-2458-9-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van der Wielen RP, Lφwik MR, van den Berg H, de Groot LC, Haller J, Moreiras O, van Staveren WA. Serum vitamin D concentrations among elderly people in Europe. Lancet. 1995;346(8969):207–210. doi: 10.1016/S0140-6736(95)91266-5. [DOI] [PubMed] [Google Scholar]
- 5.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 6.Holick MF, Chen TC, Lu Z, et al. Vitamin D and skin physiology: a D-lightful story. J Bone Miner Res. 2007;22(Suppl 2):V28–V33. doi: 10.1359/jbmr.07s211. [DOI] [PubMed] [Google Scholar]
- 7.Renier JC, Bernat M, Rebel A, et al. Study of circulating 25-hydroxyvitamin D. Rev Rhum Mal Osteoartic. 1976;43(7–9):481–489. [PubMed] [Google Scholar]
- 8.Briot K, Audran M, Cortet B, et al. Vitamin D: skeletal and extraskeletal effects recommendations for good practice. Presse Med. 2009;38(1):43–54. doi: 10.1016/j.lpm.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 9.Ovesen L, Andersen R, Jakobsen J. Geographical differences in vitamin D status, with particular reference to European countries. Proc Nutr Soc. 2003;62(4):813–821. doi: 10.1079/PNS2003297. [DOI] [PubMed] [Google Scholar]
- 10.Samuels J, Krasnokutsky S, Abramson SB. Osteoarthritis: a tale of three tissues. Bull NYU Hosp Jt Dis. 2008;66:244e50. [PubMed] [Google Scholar]
- 11.Kwan Tat S, Lajeunesse D, Pelletier JP, Martel-Pelletier J. Targeting subchondral bone for treating osteoarthritis: what is the evidence? Best Pract Res Clin Rheumatol. 2010;24:51–70. doi: 10.1016/j.berh.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dean DD, Schwartz Z, Schmitz J, et al. Vitamin D regulation of metalloproteinase activity in matrix vesicles. Connect Tissue Res. 1996;35:331–336. doi: 10.3109/03008209609029208. [DOI] [PubMed] [Google Scholar]
- 13.Radin EL, Rose RM. Role of subchondral bone in the initiation and progression of cartilage damage. Clin Orthop. 1986;213:34–40. [PubMed] [Google Scholar]
- 14.McAlindon TE, Felson DT, Zhang Y, et al. Relation of dietary intake and serum levels of vitamin D to progression of osteoarthritis of the knee among participants in the Framingham Study. Ann Intern Med. 1996;125:353–359. doi: 10.7326/0003-4819-125-5-199609010-00001. [DOI] [PubMed] [Google Scholar]
- 15.Bergink AP, Uitterlinden AG, Van Leeuwen JP, Buurman CJ, Hofman A, Verhaar JA, Pols HA. Vitamin D status, bone mineral density, and the development of radiographic osteoarthritis of the knee: the Rotterdam Study. J Clin Rheumatol. 2009;15(5):230–237. doi: 10.1097/RHU.0b013e3181b08f20. [DOI] [PubMed] [Google Scholar]
- 16.Glowacki J, Hurwitz S, Thornhill TS, Kelly M, Leboff MS. Osteoporosis and vitamin D deficiency among postmenopausal women with osteoarthritis undergoing total hip arthroplasty. J Bone Joint Surg Am. 2003;85-A:2371–2377. doi: 10.2106/00004623-200312000-00015. [DOI] [PubMed] [Google Scholar]
- 17.Makinen TJ, Alm JJ, Laine H, Svedstrφm E, Aro HT. The incidence of osteopenia and osteoporosis in women with hip osteoarthritis scheduled for cementless total joint replacement. Bone. 2007;40(4):1041–1047. doi: 10.1016/j.bone.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 18.Breijawi N, Eckardt A, Pitton MB, Hoelzl AJ, Giesa M, von Stechow D, Haid F, Drees P. Bone mineral density and vitamin D status in female and male patients with osteoarthritis of the knee or hip. Eur Surg Res. 2009;42(1):1–10. doi: 10.1159/000166164. [DOI] [PubMed] [Google Scholar]
- 19.Nawabi DH, Chin KF, Keen RW, Haddad FS. Vitamin D deficiency in patients with osteoarthritis undergoing total hip replacement: a cause for concern? J Bone Joint Surg Br. 2010;92(4):496–499. doi: 10.1302/0301-620X.92B3.23535. [DOI] [PubMed] [Google Scholar]
- 20.Chaganti RK, Parimi N, Cawthon P, Dam TL, Nevitt MC, Lane NE. Association of 25-hydroxyvitamin D with prevalent osteoarthritis of the hip in elderly men: the osteoporotic fractures in men study. Arthritis Rheum. 2010;62(2):511–514. doi: 10.1002/art.27241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bischoff-Ferrari HA, Zhang Y, Kiel DP, Felson DT. Positive association between serum 25-hydroxyvitamin D level and bone density in osteoarthritis. Arthritis Rheum. 2005;53(6):821–826. doi: 10.1002/art.21601. [DOI] [PubMed] [Google Scholar]
- 22.Lane NE, Gore LR, Cummings SR, Hochberg MC, Scott JC, Williams EN, Nevitt MC. Serum vitamin D levels and incidence changes of radiographic hip osteoarthritis: a longitudinal study: study of Osteoporotic Fractures Research Group. Arthritis Rheum. 1999;42:854–860. doi: 10.1002/1529-0131(199905)42:5<854::AID-ANR3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 23.Heidari B, Heidari P, Hajian-Tilaki K. Association between serum vitamin D deficiency and knee osteoarthritis. Int Orthop. 2011;35(11):1627–1631. doi: 10.1007/s00264-010-1186-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Al-Jarallah KF, Shehab D, Al-Awadhi A, Nahar I, Haider MZ, Moussa MA. Are 25(OH)D levels related to the severity of knee osteoarthritis and function? Med Princ Pract. 2012;21(1):74–78. doi: 10.1159/000330025. [DOI] [PubMed] [Google Scholar]
- 25.Ding C, Cicuttini F, Parameswaran V, Burgess J, Quinn S, Jones G. Serum levels of vitamin D, sunlight exposure, and knee cartilage loss in older adults: the Tasmanian older adult cohort study. Arthritis Rheum. 2009;60(5):1381–1389. doi: 10.1002/art.24486. [DOI] [PubMed] [Google Scholar]
- 26.Kellgren JH, Lawrence JS. Radiological assessment of osteoarthrosis. Ann Rheum Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Felson DT, Niu J, Clancy M, Aliabadi P, Sack B, Guermazi A, Hunter DJ, Amin S, Rogers G, Booth SL. Low levels of vitamin D and worsening of knee osteoarthritis: results of two longitudinal studies. Arthritis Rheum. 2007;56(1):129–136. doi: 10.1002/art.22292. [DOI] [PubMed] [Google Scholar]
- 28.Muraki S, Dennison E, Jameson K, Boucher BJ, Akune T, Yoshimura N, Judge A, Arden NK, Javaid K, Cooper C. Association of vitamin D status with knee pain and radiographic knee osteoarthritis. Osteoarthr Cartil. 2011;19(11):1301–1306. doi: 10.1016/j.joca.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 29.Konstari S, Paananen M, Helifvaara M, Knekt P, Marniemi J, Impivaara O, Arokoski J, Karppinen J. Association of 25-hydroxyvitamin D with the incidence of knee and hip osteoarthritis: a 22-year follow-up study. Scand J Rheumatol. 2012;41(2):124–131. doi: 10.3109/03009742.2011.617314. [DOI] [PubMed] [Google Scholar]
- 30.Zadshir A, Tareen N, Pan D, Norris K, Martins D The prevalence of hypovitaminosis D among US adults: data from the NHANES III. Ethn Dis. 2005 Autumn;15(4 Suppl 5):S5-97-101 [PubMed]
- 31.Barakι R, Weiler H, Payette H, Gray-Donald K. Vitamin D status in healthy free-living elderly men and women living in Quebec, Canada. J Am Coll Nutr. 2010;29(1):25–30. doi: 10.1080/07315724.2010.10719813. [DOI] [PubMed] [Google Scholar]
- 32.Lagunova ZI, Porojnicu AC, Lindberg F, Hexeberg S, Moan J. The dependency of vitamin D status on body mass index, gender, age and season. Anticancer Res. 2009;29(9):3713–3720. [PubMed] [Google Scholar]
- 33.World Medical Association (2013) World medical association declaration of Helsinki: ethical principles for medical researchinvolving human. JAMA 310(20):2191–2194. doi:10.1001/jama.2013.281053 [DOI] [PubMed]


