Abstract
In autoimmune inflammatory diseases, including juvenile idiopathic arthritis (JIA), which leads to joint destruction, there is an imbalance between production of reactive oxygen species (ROS) and their neutralization which, as a consequence, leads to “oxidative stress.” The aim of the study was to assess the concentration of oxidative stress markers: nitric oxide (NO), a degree of lipid membrane damage, and total antioxidant plasma capacity in children with JIA. Thirty-four children with JIA were included into the study. A degree of lipid membrane damage (lipid peroxidation products) was estimated as thiobarbituric acid-reactive substances (TBARs), NO concentration as NO end-products: nitrite/nitrate (NO2 −/NO3 −) and total antioxidant plasma capacity as ferric reducing ability of plasma (FRAP). NO2 −/NO3 − serum concentration in children with JIA was statistically significantly higher than that in healthy children (p = 0.00069). There was no significant difference in TBAR levels between children with JIA and the control group. FRAP in sera of children with JIA was lower than that in healthy children, but the difference was not statistically significant. A statistically significant positive correlation was observed between NO end products and the 27-joint juvenile arthritis disease activity score (JADAS-27) and ESR, and a negative correlation was observed between FRAP and C-reactive protein (CRP) and white blood cell count (WBC). Our results confirm the increased oxidative stress in children with JIA. Overproduction of NO and decrease in the antioxidant plasma capacity may be involved in JIA pathogenesis.
Keywords: Ferric reducing ability of plasma (FRAP), Juvenile idiopathic arthritis (JIA), Nitrates (NO3−), Nitric oxide (NO), Nitrites (NO2−), Thiobarbituric acid-reactive substances (TBARs)
Introduction
Juvenile idiopathic arthritis (JIA) is a heterogeneous group of autoimmune diseases of unknown origin, with onset before the age of 16 years. JIA is considered to be the most common connective tissue disease of childhood. It is characterized by leukocyte infiltration in the synovium leading to a chronic inflammation in the joints, which persists for more than 6 weeks, with consequent destruction of articular tissue [1, 2]. Recently, considerable interest has been generated in determining the JIA pathogenesis, looking for new therapeutic strategies and for the prognostic factors, which would permit an early identification of patients with a poor prognosis, justifying the application of an aggressive treatment in the early stages of the disease [2, 3].
Reactive oxygen species (ROS) play a pivotal role in physiological processes. ROS mediate and regulate many cell functions, including cytokine production, signal transduction, mitochondrial functions, immune processes, gene expression, and apoptosis. The effects of ROS depend on their serum concentration. Nitric oxide (NO), a unique biological messenger molecule, is a short-lived gaseous free radical. It is synthesized from l-arginine by NO synthase in various cell types such as chondrocytes, synoviocytes, and leukocytes, e.g., phagocytic neutrophils. Direct measurement of NO and other free radicals in standard laboratories is difficult because of their biochemical instability. Due to the above, concentrations of NO end-products nitrite/nitrate (NO2 −/NO3 −) or the activity of NO synthase is usually evaluated. NO is involved in immune regulation, neurotransmission, and vasodilatation. Recently, the imbalance between formation of ROS and body antioxidant defense in homeostasis disturbances is widely discussed [4]. The currently reported data demonstrated a significant role of ROS in the etiopathogenesis of autoimmune diseases. In chronic inflammatory processes, after long stimulation, the exhaustion of the body’s antioxidative reserves results in a harmful activity of ROS, as they gain an advantage over the antioxidant system—this phenomenon is called “oxidative stress” [5].
Many studies are conducted on the role of ROS in the etiopathogenesis of rheumatoid arthritis (RA), which seem to be a main direct factor destroying the joint tissues, but in the pediatric population with JIA, their role has still not been elucidated [5–9]. It could be hypothesized that patients with JIA have defective defense mechanisms against ROS and these mechanisms vary according to the JIA subtypes.
The aim of the study was to assess markers of the oxidative stress: concentrations of NO end-products nitrite/nitrate (NO2 −/NO3 −), thiobarbituric acid-reactive substances (TBARs), and total antioxidant capacity of plasma via ferric reducing ability of plasma (FRAP) in the sera of children with JIA in comparison with healthy children. Moreover, the study also aimed to correlate these markers with disease characteristics (type of JIA onset, disease activity).
Patients and methods
Patients
Thirty-four children (22 girls, 12 boys) with JIA were included into the study. The patients were entered in regard to the 2001 International League Against Rheumatism (ILAR) classification criteria [10]. Twenty-six children had polyarticular JIA type of onset, and eight had oligoarticular JIA. They were aged 7–18 years (mean 13.8 ± 1.2 years, range 7–18), had disease duration time of 0.5–7.5 years (mean 3.5 ± 0.8 years), and were treated with DMARDs (methotrexate, 28 patients or sulfasalazine, 6 patients) and low doses of steroids (prednisone <10 mg/day). All of the study group children were biologically naive (Table 1).
Table 1.
Demographic, clinical, and laboratory parameters of children with JIA
| Characteristics of children with JIA (n = 34) | |
|---|---|
| Female/male, n (%) | 22/12 (64.7/35.3) |
| Age (years)/range | 13.8 ± 1.2/7–18 |
| Type of JIA onset | |
| Polyarticular, n (%) | 26 (76.5) |
| IgM-RF-negative | 24 |
| IgM-RF-positive | 2 |
| Oligoarticular, n (%) | 8 (23.5) |
| Persistent oligoarticular JIA | 7 |
| Extended oligoarticular JIA | 1 |
| Disease duration time (years) | 3.5 ± 0.8 |
| ESR (mm/h) | 19.4 ± 20.5 (median 10) |
| ESR above cutoff value (>20 mm/h), n (%) | 8 (23.5) |
| CRP (mg/dL) | 2.67 ± 6.26 (median 0.39) |
| CRP above cutoff value (>0.5 mg/dL), n (%) | 14 (41.2) |
| IgM-RF positivity (≥24 RU/mL), n (%) | 2 (5.9) |
| Disease activity | |
| JADAS-27 | 23.94 ± 10.10 (median 25) |
Data are means ± standard deviation (SD), unless otherwise indicated
n number of children, ERS erythrocyte sedimentation ratio, CRP C-reactive protein
Twenty sex- and age-matched healthy children (without autoimmune diseases) from the control group were also examined.
Serum samples (3 mL) were obtained simultaneously with routine laboratory tests (white blood cell (WBC) count, red blood cell (RBC) count, thrombocyte count, hemoglobin, C-reactive protein (CRP)). The activity of the rheumatoid process was assessed according to the 27-joint juvenile arthritis disease activity score (JADAS-27) [11]. Each patient was evaluated for the physician’s global assessment of overall disease activity, which was measured on a 10-cm visual analog scale (VAS; 0 = no activity and 10 = maximum activity); parent’s global assessment of the child’s overall well-being, which was measured on a 10-cm VAS (0 = very good and 10 = very poor); and 27-joint count including the cervical spine, elbows, wrists, metacarpophalangeal joints (from the first to the third), proximal interphalangeal joints, hips, knees, ankles, and ESR.
The study was approved by the local ethical committee. In every case, a written informed consent was obtained from the patient and parents before study entry.
Methods
NO assay
The concentration of NO was assessed with an indirect method by measurement of NO end-products nitrite/nitrate (NO2 −/NO3 −) (Nitrate/Nitrite Colorimetric Assay Kit, Sigma-Aldrich Chemie GmbH, Switzerland). Total NO2 −/NO3 − concentrations in the serum samples were determined by microcolorimetric Griess reaction for nitrite [12] following nitrate reductase-mediated nitrate ion reduction [13]. The absorbance of the chromophore was read at a wavelength of λ = 548 nm.
Determination of TBARs
Measurement of lipid peroxidation products was based on thiobarbituric acid-reactive substance (TBAR) concentration in plasma. Briefly, 1 mL of 0.05 mol/L H2SO4 and 0.5 mL of 1.23 mol/L trichloroacetic acid (TCA) were added to 0.02 mL of plasma, mixed, and then centrifuged for 10 min (1,500 × g, 20 °C). Fluorescence was measured at an excitation wavelength of λ = 515 nm and emission wavelength of λ = 546 nm.
FRAP assay
The total antioxidant plasma capacity was assayed in terms of the ferric reducing ability of plasma (FRAP) according to the original description by Benzie and Strain [14]. This method measures the ability of antioxidants contained in a sample to reduce ferric tripyridyltriazine (Fe3+-TPTZ) to a ferrous form (Fe2+-TPTZ) that absorbs light at λ = 593 nm.
Statistical analysis
Data was expressed as mean and standard deviation (±SD). Results were analyzed using nonparametric Mann-Whitney tests, and correlation was found by the Spearman coefficient. Confidence level was 95 % and statistical significance was considered at a level of 0.05.
Results
The demographic, clinical, and laboratory features of children with JIA are shown in Table 1.
NO assay/concentration of NO2−/NO3−
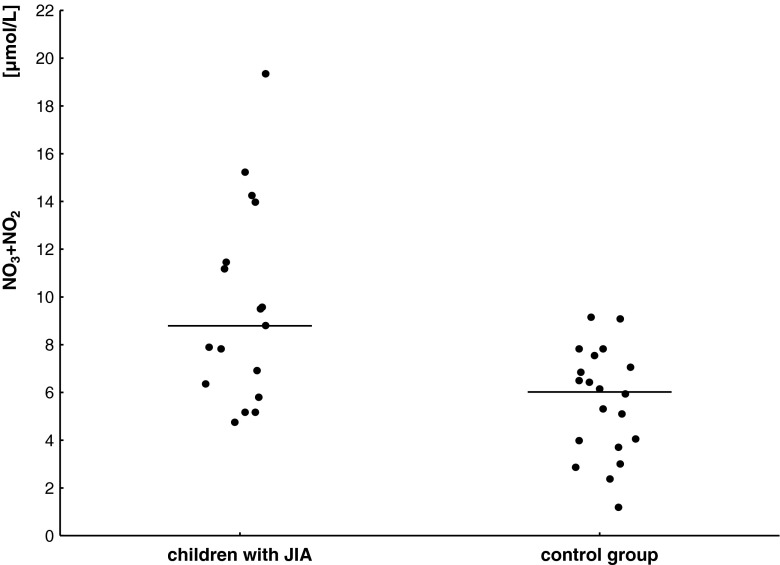
Figure 1 shows higher serum concentrations of NO end-products and mean obtained from children with JIA compared to 20 healthy children.
Fig. 1.
Nitrite/nitrate (NO2 −/NO3 −) serum concentration and mean value (horizontal line) in children with JIA and in the control group
Total NO2 −/NO3 − concentrations in the serum samples of 34 children with JIA were significantly higher (9.59 ± 4.14 μmol/L) than those measured in sera of 20 sex- and age-matched children from the control group (5.59 ± 2.25 μmol/L; p = 0.00069). There was no statistically significant difference in serum NO2 −/NO3 − concentrations between children with polyarthritis and oligoarthritis (p > 0.05) (Table 2).
Table 2.
Concentration of nitrite/nitrate (NO2 −/NO3 −), thiobarbituric acid-reactive substance (TBARs), and the ferric reducing ability of plasma (FRAP) in sera of children with JIA and the control group
| Children with JIA (n = 34) | Children from control group (n = 20) | p value | |
|---|---|---|---|
| NO2 −/NO3 − (μmol/L) | 9.59 ± 4.14 | 5.59 ± 2.25 | 0.00069 |
| TBARs (μmol/L) | 0.59 ± 0.2 | 0.61 ± 0.18 | 0.836 |
| FRAP (mmol/L) | 0.75 ± 0.1 | 0.89 ± 0.17 | 0.048 |
A positive correlation was observed between NO2 −/NO3 − concentrations and JADAS-27 and ESR (R = 0.542, p = 0.039; R = 0.621, p = 0.009).
Determination of TBARs
There were no statistically significant differences in TBAR levels between children with JIA and the control group (0.59 ± 0.2 and 0.61 ± 0.18 μmol/L, respectively) (Table 2). No statistically significant difference was observed in serum TBAR levels between children with polyarthritis and oligoarthritis (p > 0.05).
TBARs did not correlate with either clinical or laboratory variables of disease activity.
FRAP assay
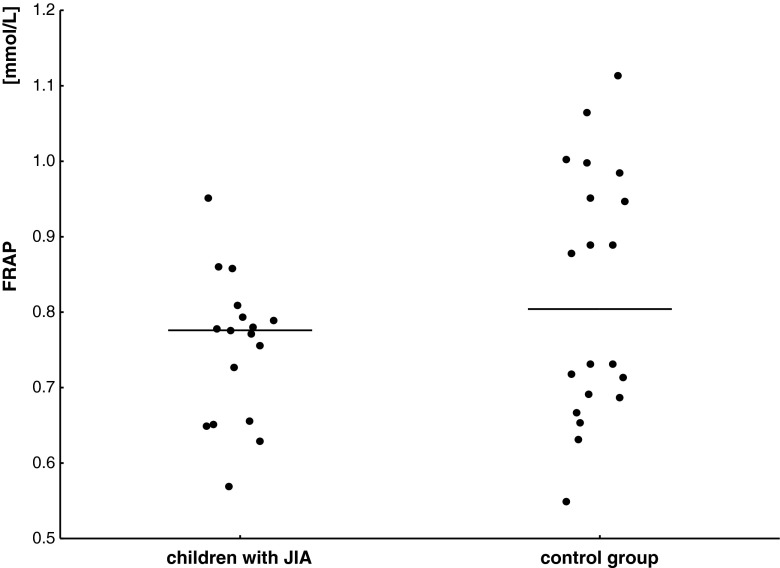
In the group of children with JIA, FRAP was lower (0.75 ± 0.1 mmol/L) than in healthy subjects (0.89 ± 0.17 mmol/L, p = 0.048) (Fig. 2). However, no statistically significant difference was observed in serum FRAP levels between children with polyarthritis and oligoarthritis (p > 0.05).
Fig. 2.
FRAP serum concentration and mean value (horizontal line) in children with JIA and in the control group
Furthermore, statistically significant negative correlations between WBC vs. FRAP levels (R = −0.583, p = 0.013) as well as CRP vs. FRAP levels (R = −0.633, p = 0.0064) in the group of children with JIA were detected.
No statistically significant correlations were detected between distribution of NO end-products, TBARs, and FRAP according to sex, age, and treatment.
Discussion
Proinflammatory factors such as cytokines and prostaglandins are released at inflammation sites together with ROS [15]. Several studies in patients with RA have documented evidence for increased endogenous NO synthesis, suggesting that overproduction of NO in an inflamed joint may be important for the pathogenesis of RA, but in JIA its role is still unclear [5–9, 16]. To the best of our knowledge, this is the first study assessing the NO end-products nitrite/nitrate, the index of lipid peroxidation (TBARs), and the total antioxidant plasma capacity (FRAP) in children with JIA.
The currently reported data with adult RA patients and confirmed in animal models indicated that NO may function as a disease marker and as a proinflammatory mediator in arthritis [6–9]. Regarding the pathogenesis of RA, it seems that excessive oxidative stress in the inflamed joints is reflected as an increased concentration of NO in peripheral blood [16]. Our study demonstrated a statistically significant increase in serum concentration of NO2 −/NO3 − in children with JIA compared to healthy subjects, which is in concordance with results obtained by Lotito et al. and Beri et al. [17, 18].
Similarly to other studies, there was no significant difference in NO levels among two JIA subtypes (poly- and oligoarthritis) in our study group [18–20].
Similarly to Bica et al. and Lotito et al., we showed that the concentration of NO end-products in sera of children with JIA and active disease was higher than that in children with inactive process, however without a statistically significant difference [3, 17]. Our study indicated a significant positive correlation between JADAS-27 and ESR and NO end-products’ levels, which is in concordance with previous authors’ observations in adult patients with RA; however, in JIA such correlations were not found [3, 9, 18]. On the other hand, it was observed by Bica et al. that patients with active JIA and erosive disease had significantly higher NO levels, whereas children with an inactive rheumatoid process had similar NO levels regardless of whether erosions were present or not [3].
There is no compliance among authors about the serum concentration of lipid peroxidation products measured as TBARs in children with JIA. However, it is well established that overproduction of ROS in pathological processes, like chronic inflammation, could result in oxygenation of cell membrane compounds (protein structure damage, lipid oxidation), thus inducing loss of cell integrity and functional alteration of cell receptors and enzymes [15]. In our group of children with JIA, no statistically significant difference was detected in the concentration of TBARs either between the two JIA subtypes and healthy subjects, which is in line with previous observations of other authors [20]. In contrary, in another study, increased TBARs were found in sera of children with JIA [21]. Ramos et al. also observed such correlations; however, the authors determined different products of lipid peroxidation than we did [19]. Guney et al. who determined malonodialdehyde (MDA) by thiobarbituric acid test indicated higher MDA levels in patients with JIA, with the highest concentration in systemic JIA [15]. The discrepancies between studies may be also explained by the fact that TBARs are not a very specific marker, and perhaps it would be better to determine specific substances like MDA and lipoperoxide (LPO). Furthermore, patients with rheumatoid process have defective defense mechanisms against ROS [6–9, 22, 23]. It was also proved that inflammatory immune response is upon aging [4, 24]. Perhaps, it could be speculated that similar levels of TBARs in healthy children and those with JIA could be explained by high efficiency of the antioxidant system in children, as well as higher cell membrane stability in young subjects and less susceptibility of the membrane’s components to damaging factors [15].
It is worth noticing that this is the first study on FRAP in children with JIA. Among various methods, assessing the antioxidant capacity of plasma FRAP, which estimates the total antioxidant plasma capacity, seems to be the best, as it gathers whole scavenging abilities of plasma. It is still undecided if a lower level of FRAP is the cause or effect of rheumatoid process. Sarban et al. have described decreased levels of the antioxidant plasma capacity assayed in terms of FRAP in RA patients and postulated that decreased FRAP could contribute to the pathogenesis of rheumatoid process [23]. Although there was no statistically significant difference in FRAP levels, comparison of our study and control groups indicated that FRAP was lower in sera of children with JIA. Perhaps, a small difference between FRAP levels in the study and control groups was due to the efficient defense against ROS in young patients with JIA or an effective anti-inflammatory therapy of JIA that results in adaptive increase of FRAP [25]. Although there was no significant correlation of FRAP with disease activity, it should be underlined that FRAP negatively correlates with CRP and WBC in children with JIA.
Conclusions
The results provide some evidence for a potential role of the increased NO and decreased antioxidant plasma capacity in the pathogenetic mechanism of JIA. Measurement of NO and FRAP may provide a useful tool to aid in the assessment of the patient’s oxidative stress status and in the determination of an appropriate treatment management plan. Further studies are needed to determine the exact role of ROS and particularly NO in the pathogenesis of JIA and to show its usefulness as a marker of disease activity.
Acknowledgments
This study is supported by Grants 502-03/8-000-01/502-64-030 and 503/0-079-05/503/1 from the Medical University of Lodz, Poland.
Conflict of interest
The authors declare that they have no competing interests.
References
- 1.Cassidy JT. Juvenile idiopathic arthritis. In: Cassidy JT, Petty RE, editors. Textbook of pediatric rheumatology. Philadelphia: Saunders; 2001. pp. 218–221. [Google Scholar]
- 2.Prakken B, Albani S, Martini A. Juvenile idiopathic arthritis. Lancet. 2011;377(9783):2138–2149. doi: 10.1016/S0140-6736(11)60244-4. [DOI] [PubMed] [Google Scholar]
- 3.Bica BE, Gomes NM, Fernandea PD, Luiz RR, Koatz VL. Nitric oxide levels and the severity of juvenile idiopathic arthritis. Rheumatol Int. 2007;27(9):819–825. doi: 10.1007/s00296-007-0321-x. [DOI] [PubMed] [Google Scholar]
- 4.Labunskyy VM, Gladyshev VN. Role of reactive oxygen species-mediated signaling in aging. Antioxid Redox Signal. 2012;19(12):1362–1372. doi: 10.1089/ars.2012.4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiavaroli V, Giannini C, De Marco S, Chiarelli F, Mohn A. Unbalanced oxidant-antioxidant status and its effects in pediatric diseases. Redox Rep. 2011;16(3):101–107. doi: 10.1179/174329211X13049558293551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wruck CJ, Fragoulis A, Gurzynski A, Brandenburg LO, Kan YW, Chan K, et al. Role of oxidative stress in rheumatoid arthritis: insights from the Nrf2-knockout mice. Ann Rheum Dis. 2011;70(5):844–850. doi: 10.1136/ard.2010.132720. [DOI] [PubMed] [Google Scholar]
- 7.Stamp LK, Khalilova I, Tarr JM, Senthilmohan R, Turner R, Haigh RC, et al. Myeloperoxidase and oxidative stress in rheumatoid arthritis. Rheumatology (Oxford) 2012;51(10):1796–1803. doi: 10.1093/rheumatology/kes193. [DOI] [PubMed] [Google Scholar]
- 8.Seven A, Güzel S, Aslan M, Hamuryudan V. Lipid, protein, DNA oxidation and antioxidant status in rheumatoid arthritis. Clin Biochem. 2008;41(7–8):538–543. doi: 10.1016/j.clinbiochem.2008.01.029. [DOI] [PubMed] [Google Scholar]
- 9.Nagy G, Koncz A, Telarico T, Fernandez D, Ersek B, Buzás E, et al. Central role of nitric oxide in the pathogenesis of rheumatoid arthritis and systemic lupus erythematosus. Arthritis Res Ther. 2010;12(3):210. doi: 10.1186/ar3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petty RE, Southwood TR, Manners P, Baum J, Glass DN, Goldenberg J, et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis; second revision, Edmonton, 2001. J Rheumatol. 2004;31:390–392. [PubMed] [Google Scholar]
- 11.Consolaro A, Ruperto N, Bazso A, Pistorio A, Magni-Manzoni S, Filocamo G, et al. Paediatric Rheumatology International Trials Organisation: Development and validation of a composite disease activity score for juvenile idiopathic arthritis. Arthritis Rheum. 2009;61(5):658–666. doi: 10.1002/art.24516. [DOI] [PubMed] [Google Scholar]
- 12.Ding AH, Nathan CF, Stuehr DJ. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol. 1988;141(7):2407–2412. [PubMed] [Google Scholar]
- 13.Gilliam MB, Sherman MP, Griscavage JM, Ignarro LJ. A spectrophotometric assay for nitrate using NADPH oxidation by Aspergillus nitrate reductase. Anal Biochem. 1993;212(2):359–365. doi: 10.1006/abio.1993.1341. [DOI] [PubMed] [Google Scholar]
- 14.Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”. The FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 15.Guney T, Yildiz B, Altikat S, Kural N, Alatas O. Decreased antioxidant capacity and increased oxidative stress in patients with juvenile idiopathic arthritis. JPS. 2009;1(e3):1–6. [Google Scholar]
- 16.Brik R, Rosen I, Savulescu D, Borovoi I, Gavish M, Nagler R. Salivary antioxidants and metalloproteinases in juvenile idiopathic arthritis. Mol Med. 2010;16(3–4):122–128. doi: 10.2119/molmed.2009.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lotito AP, Muscara MN, Kiss MH, Teixeira SA, Novaes GS, Laurindo IM, et al. Nitric oxide-derived species in synovial fluid from patients with juvenile idiopathic arthritis. J Rheumatol. 2004;31(5):992–997. [PubMed] [Google Scholar]
- 18.Beri A, Singh S, Gupta A, Khullar M. Comparison of serum nitric oxide levels in active juvenile rheumatoid arthritis with those of patients in remission. Rheumatol Int. 2004;24:264–266. doi: 10.1007/s00296-003-0368-2. [DOI] [PubMed] [Google Scholar]
- 19.Ramos VA, Ramos PA, Dominguez MC. The role of oxidative stress in inflammation in patients with juvenile rheumatoid arthritis. J Peditr (Rio J) 2000;76(2):125–132. doi: 10.2223/JPED.45. [DOI] [PubMed] [Google Scholar]
- 20.Gromadzinska J, Sklodowska M, Wolkanin P, Biernacka M, Brozik H, Wasowicz W, et al. The activity of some trace element-dependent enzymes in blood of children with juvenile chronic arthritis. TEMA. 1993;8:780–783. [Google Scholar]
- 21.Sklodowska M, Gromadzińska J, Biernacka M, Wasowicz W, Wolkanin P, Marszalek A, et al. Vitamin E, thiobarbituric acid reactive substance concentrations and superoxide dismutase activity in the blood of children with juvenile rheumatoid arthritis. Clin Exp Rheumatol. 1996;14(4):433–439. [PubMed] [Google Scholar]
- 22.Hirao M, Yamasaki N, Oze H, Ebina K, Nampei A, Kawato Y, et al. Serum level of oxidative stress marker is dramatically low in patients with rheumatoid arthritis treated with tocilizumab. Rheumatol Int. 2012;32(12):4041–4045. doi: 10.1007/s00296-011-2135-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sarban S, Kocyigit A, Yazar M, Isikan UE. Plasma total antioxidant capacity, lipid peroxidation, and erythrocyte antioxidant enzyme activities in patients with rheumatoid arthritis and osteoarthritis. Clin Biochem. 2005;38:981–986. doi: 10.1016/j.clinbiochem.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 24.Lee K, Won HY, Bae MA, Hong JH, Hwang ES. Spontaneous and aging-dependent development of arthritis in NADPH oxidase 2 deficiency through altered differentiation of CD11b + and Th/Treg cells. Proc Natl Acad Sci U S A. 2011;108(23):9548–9553. doi: 10.1073/pnas.1012645108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Renke J, Szlagatys A, Hansdorfer-Korzon R, Szumera M, Kamińska B, Knap N, et al. Persistence of protein oxidation products and plasma antioxidants in juvenile idiopathic arthritis. A one-year follow-up study. Clin Exp Rheumatol. 2007;25(1):112–114. [PubMed] [Google Scholar]