Abstract
Background
There remains no FDA approved medication for the treatment of cocaine dependence. Preclinical studies and early pilot clinical investigations have suggested that N-acetylcysteine (NAC) may be useful in the treatment of the disorder.
Objective
The present report assessed the efficacy of NAC in the treatment of cocaine dependence.
Methods
Cocaine-dependent volunteers (n = 111) were randomized to receive daily doses of 1200mg of NAC, 2400mg of NAC or placebo. Participants were followed for 8 weeks (up to 3 visits weekly). At each of these visits, urine samples were collected, along with self-reports of cocaine use. Urine samples were assessed for quantitative levels of benzoylecognine (i.e., cocaine metabolite).
Results
Overall, the primary results for the clinical trial were negative. However, when considering only subjects who entered the trial having already achieved abstinence, results favored the 2400mg NAC group relative to placebo, with the 2400mg group having longer times to relapse and lower craving ratings.
Conclusion
While the present trial failed to demonstrate that NAC reduces cocaine use in cocaine-dependent individuals actively using, there was some evidence it prevented return to cocaine use in individuals who had already achieved abstinence from cocaine. Scientific significance: N-Acetylcysteine may be useful as a relapse prevention agent in abstinent cocaine-dependent individuals.
INTRODUCTION
At present, there exists no FDA-approved medication for the treatment of cocaine dependence. However, there are a number of preclinical animal studies that have suggested that N-acetylcysteine (NAC) may be a candidate medication for the treatment of this disorder. Previous research has shown that following chronic cocaine administration1-3, basal extracellular glutamate levels within the nucleus accumbens are reduced, which contributes to the reinstatement of cocaine-seeking behaviors in animal models of relapse 1, 4. NAC, a cystine pro-drug, can prevent reinstated cocaine-seeking behaviors by normalizing basal glutamate levels via activation of the cystine-glutamate exchanger 1, 4. Research on the administration of NAC to animals trained to self-administer cocaine has shown that NAC also hastens extinction learning and provides lasting protection against the reinstatement of cocaine-seeking behaviors5-6.
Consistent with the preclinical findings in animals, preliminary double blind clinical studies have demonstrated that NAC reduces craving initiated by conditioned cues or a cocaine injection in cocaine-dependent volunteers7-8. In addition, an open-label trial found that participants taking NAC reported reduced use of cocaine over a 4-week period9. Importantly, clinical studies thus far have found NAC to be safe and well-tolerated among cocaine-dependent individuals9-10.
Together, these studies supported the initiation of a placebo-controlled double-blind trial of NAC for the treatment of cocaine dependence. We hypothesized that treatment-seeking, cocaine-dependent subjects receiving weekly cognitive therapy combined with a daily oral dose of 1200mg or 2400mg of NAC (600mg or 1200mg twice daily, respectively) would show dose-dependent reductions in cocaine use as compared to subjects receiving cognitive therapy coupled with placebo.
METHODS
Participants
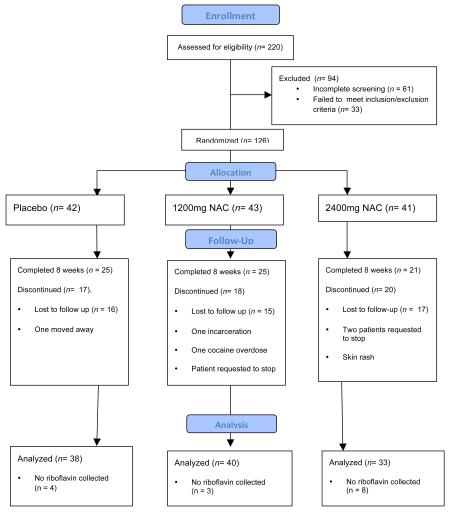
Participants were treatment-seeking adult males and females who met DSM-IV criteria for cocaine dependence11. Participants were excluded if they required medical detoxification from alcohol or if they met dependence criteria for any substance other than alcohol, nicotine, or marijuana. Other exclusionary criteria included females who were pregnant or nursing, serious medical conditions, psychiatric disorders that would impair ability to participate safely in the study, a past medical history of asthma or seizures, or recent use (<14 days) of medications felt to be hazardous if taken with N-acetylcysteine. Participants had to be willing to attend research visits three times weekly and to participate in weekly counseling for cocaine dependence. Two-hundred and twenty individuals were screened. Initially, 126 were randomized, but 15 dropped out of the trial before riboflavin (i.e. compliance) data were collected and were therefore excluded from any further analysis.
The final participant pool consisted of 111 individuals that included 48 Caucasians, 61 African Americans, and 2 Hispanics. Twenty-eight were women. The average participant age was 43.2 (SD = 9.2). Participants reported using cocaine an average of 13.9 (SD = 8.1) years, with 72% reporting primarily smoking cocaine (i.e., crack). Thirty-nine participants met criteria for alcohol abuse or dependence, 28 met criteria for mood disorders (substance-induced or otherwise), while 7 patients met criteria for anxiety-related disorders.
Measures
Time Line Follow Back (TLFB)12
This measure assesses self-reported cocaine use (in number of days) occurring in the 30 days prior to engaging in the treatment study. At each subsequent research visit, participants reported updates of their on-going cocaine use, as well as their use of alcohol, nicotine, and marijuana.
Riboflavin levels
Riboflavin levels were used to assess compliance. Study medication and placebo capsules each contained 50mg of riboflavin. Each week, one urine sample was assessed for riboflavin levels using fluorometry. Based on previous work13, a cut-off level of 1500ng/ml was used as an indication of medication compliance. For each participant, percent compliance was computed by dividing the number of weeks after Week 1 with riboflavin levels >1500ng/ml by the number of weeks completed by the participant.
Evaluation of Side Effects and Adverse Events
At each visit, participants were asked to report any and all physical symptoms, side effects, and adverse events experienced. Vital signs and changes in non-study medications were also assessed at each visit. Side effects and adverse events were evaluated weekly by a nurse practitioner or the PI (last author), a licensed physician.
Urine Screens for Quantitative Benzoylecognine Levels
Because qualitative urine drug screen results do not always reflect subtle changes in patterns of drug use, urine samples were analyzed for quantitative levels of benzoylecognine (BE) levels (i.e., cocaine metabolite) for each research visit. Data were processed at either Northwest Toxicology Inc. (Salt Lake City, UT) or Clinical Reference Laboratories (Lenexa, KS). Quantitative BE levels were log-transformed, and mean log transformed BE levels were calculated for each study week.
Days of Confirmed Abstinence
Each participant reported days of use and non-use of cocaine, and reports were verified using urine drug screens. Since urine screens were often collected within close succession, there was a frequent possibility of “carry over” of positive results (i.e. BE levels could be >300ng/ml on successive screens despite the absence of use between observations). To address this, each quantitative BE level was classified as “new use” or “non-new use” using criteria adapted from those initially suggested by Preston and colleagues14. A urine sample was classified as “new use” based on the following criteria: 1) in the absence of any preceding urine samples within 4 days, any BE level >300ng/ml, 2) if BE a level was >300ng/ml and the previous urine sample collected within 48 hours prior was negative (<300ng/ml), 3) if a BE level was >300ng/ml and more than 1/2 the level of the preceding specimen value, if the preceding specimen was collected less than 48 hours prior, and 4) if BE a level was >300ng/ml and more than 1/4 of the level of the preceding specimen, if the preceding specimen was collected between 48 and 96 hours prior. BE levels were used to confirm reported non use days using the following criteria: 1) Urines with BE levels >300ng/ml but determined to be “non new use” could confirm the previous day as a non-use day, assuming the person reported no use on that day, and 2) urines with BE levels <300ng/ml could confirm up to 3 reverse-consecutive non-use self report days, assuming non-use was reported for those days. Note if any day in 3 reverse-consecutive days was reported as a use day, then it was counted as a use day, and any days prior to that within the 3-day window could not be confirmed as non-use. All days reported as use days were counted as such, regardless of urine drug screen results. For each participant, the number of confirmed abstinence days was totaled for each study week.
Brief Substance Craving Scale (BSCS)
The BSCS15 includes three items formatted on a 5-point Likert scale that assess intensity, length, and frequency of cocaine craving. Responses to these items were summed providing a total score for craving for each study week.
Cocaine Selective Severity Assessment (CSSA)
The CSSA16 is an 18-item clinician-administered instrument with well-established reliability and validity that assesses the severity of early cocaine abstinence symptoms (e.g., cocaine cravings, sleep disturbances, appetite changes). Each of the 18 items is rated on a scale of 0 (no symptoms) to 7 (maximum severity). Items were summed to provide a total score for each study week.
Procedures
Individuals were recruited from the community and clinics at a southeastern medical university and its VAMC affiliate through media advertisements, flyers, and by word-of-mouth. Volunteers provided oral and written consent during an IRB-approved informed consent process that fully explained study procedures. Each participant received a thorough medical history and physical examination by a physician or nurse practitioner. In addition, electrocardiogram, and blood tests that included hematology and chemistry panels were collected and evaluated by the study PI, a licensed physician. Participant psychiatric histories were collected by trained research personnel using semi-structured diagnostic interviewing procedures developed to screen for DSM-IV criteria for drug abuse, dependence, and other psychiatric conditions17. All eligible participants were randomized based on a stratification procedure that accounted for gender, frequency of use prior to initiating the study (greater than 10 v. less than or equal to 10 days of use in the 30 prior to the study), and whether they were positive for cocaine on the first study day. A prior open-label study suggested that daily doses of 1200mg and 2400mg of NAC had acceptable rates of side effects and acceptable levels of tolerability9. Thus, participants were randomized to receive daily doses of either 1200mg or 2400mg of NAC (consisting of 600mg and 1200mg twice daily, respectively) or to receive an identically appearing (and smelling) placebo capsule, after which they entered an 8-week medication phase with three scheduled visits per week.
On the first day of medication administration, participants first completed self-report measures, the BSCS and CSSA, and then received their first dose of NAC or placebo. Participants remained under medical supervision and were observed for allergic reactions and other side effects for the following hour. Participants were then given a one-week supply of study capsules in blister packs. Participants were instructed to bring back any unused capsules at the beginning of the following week. During the remainder of the first week, participants were scheduled to attend two more research visits. During each subsequent week, participants were scheduled to attend three research visits per week. At each research visit, reports of adverse events/side effects, urine samples, vital signs, and self-reported updates of cocaine and other drug use were collected. During the first research visit of each week, unused study capsules were collected and an additional one-week supply of study capsules was given. During that same first visit, participants completed the BSCS and CSSA. Participants were also scheduled to attend a 1-hour weekly session of manual-based cognitive therapy for cocaine dependence after one of the thrice weekly research visits. Cognitive therapy was provided by Masters- and Ph.D.-level counselors18. Participants were compensated travel costs for each study visit (between $5 and $15).
Statistical Methods
Data from the final participant pool (n = 111) were analyzed using SPSS 18.0 (SPSS Inc., Chicago IL). Baseline subject characteristics were examined to assess the success of the randomization procedure using one-way ANOVAs for continuous variables, χ2 and Fisher Exact tests for categorical data, and Cox Regression for time to drop out. Group differences in frequencies of participants reporting side effect were analyzed using χ2 and Fisher exact tests.
Primary Analyses
To determine whether NAC reduced overall weekly cocaine use, the Generalized Estimating Equations (GEE) method was used. Independent variables included Treatment Group (between-subjects variable) and Treatment Week (repeated-measure, within-subject variable). GEE methods assumed missing data were missing completely at random. Initially, it was intended that Baseline Cocaine Use (number of days of use within the 30 days prior to study participation) was to be used as a baseline covariate. However, preliminary analyses revealed that it significantly interacted with Treatment Group and Treatment Week. Therefore, it was instead included as an additional between-subjects variable. In order the equalize cell sizes in subsequent analyses, a cutoff of 11 days (the median) was used to differentiate high versus low baseline use. Mean weekly log transformed BE levels, craving, and measures of cocaine withdrawal, were analyzed using this 3-way GEE model. Number of days of confirmed abstinence was analyzed with GEE as well, and included number of total urine screens collected within each week as a covariate. Post-hoc comparisons were conducted to clarify main and interactive effects, with t-tests used to assess between group effects and Wald χ2 used to assess time-related effects.
Exploratory Analyses
While previous preclinical animal studies have found strong and enduring effects of NAC, these studies were designed to model relapse, and NAC was administered while subjects were abstinent from cocaine19. Given that the present trial was designed as a cessation trial and not a relapse prevention trial per se, an exploratory analysis was conducted to determine if NAC reduced time to relapse among a subset of participants who were abstinent during the week prior to receiving medication. These individuals had reported at least one day of use within the 30 days prior to starting the study and demonstrated sufficient compliance (i.e. greater or equal to 50%). Relapse was defined as first observed “new use” of cocaine (as defined above) or as early dropout from the study. Time to relapse was quantified as study day (ranging from 0 to 55) during which the positive urine was collected or the last day of contact with the participant prior to dropout. For the purpose of survival analyses, patients who never relapsed received a value of 56 (days) and were treated as censored in the analysis. Time to relapse was assessed using a Cox regression analysis, controlling for baseline use (days of use in the 30 prior to starting the study), and included Treatment Group as the primary factor of interest.
A final exploratory analysis examined whether NAC would reduce craving in these initially abstinent individuals, as has been observed in previous laboratory studies7-8. Using craving data from the BSCS and the single craving item from the CSSA, a 2-way GEE analysis was conducted with Treatment Week as a within-subjects variable, and Treatment Group as a between subjects variable. Only data from Weeks 1 through 7 were included in this analysis as missing data points prohibited inclusion of Weeks 0 and 8.
RESULTS
Baseline Participant Characteristics
Groups did not differ with respect to distribution of race, gender, mood and anxiety disorders, alcohol abuse/dependence, method of cocaine delivery (i.e. smoked vs. snorted), rates of study completion, nor were there significant differences between groups for age, education, years of cocaine use, years of alcohol use, or number of weeks completed (Table 1). Baseline characteristics did not differ when groups were further subdivided based on high versus low baseline use levels.
Table 1. Baseline Subject Characteristics (n = 111).
| Placebo | 1200mg | 2400mg | |||
|---|---|---|---|---|---|
| n | n | n | χ2(df =2) | p | |
| Group Total | 38 | 40 | 33 | ||
| Male1 | 28 | 30 | 25 | .04 | .98 |
| # Positive for cocaine at 1st visit1 | 27 | 30 | 24 | .16 | .93 |
| More than 10 days use at Baseline1 | 22 | 26 | 24 | 1.71 | .43 |
| Non White | 23 | 21 | 19 | .54 | .77 |
| Mood DO | 11 | 10 | 7 | .56 | .76 |
| Anxiety DO | 5 | 3 | 0 | 4.58 | .10 |
| ETOH Abuse/Dependence | 14 | 15 | 11 | .10 | .95 |
| Smoked Cocaine | 25 | 29 | 26 | .91 | .64 |
| High Baseline Use2 | 18 | 22 | 18 | .55 | .76 |
| M(SD) | M(SD) | M(SD) | F3 | p | |
| Age | 42.8 (8.7) | 43.5 (10.1) | 43.3 (8.9) | .07 | .93 |
| Years Education | 13.0 (1.9) | 12.6 (2.6) | 13.5 (2.1) | 1.41 | .25 |
| Years Cocaine Use | 12.5 (7.8) | 15.9 (8.0) | 14.2 (8.3) | 1.74 | .18 |
| Years ETOH Use | 20.3 (11.6) | 20.0 (13.3) | 19.8 (11.5) | .01 | .99 |
Variable used in stratification for randomization procedures
Median split, >11 days use in 30 days prior to study, used for analysis
df = 2,108, except for education (2,104) and ETOH (2,107), due to missing data.
Drop Out Rates
Rate of dropout was not associated with group. Number of 8-week completers was 25, 25, and 21 in the placebo, 1200mg NAC, and 2400mg NAC groups respectively. Cox regression survival analysis revealed that time to dropout was not associated with group membership. Mean number of weeks completed was 6.9 (SD=1.8), 6.6 (SD=2.0), and 6.7 (SD= 1.9) in the placebo, 1200mg NAC, and 2400mg NAC groups respectively.
Riboflavin Results (Percent Compliance)
Groups did not differ with respect to percent compliance levels. The overall mean percent compliance was 70.8% (SD = 31.0). The median was 75% and mode was 100% (n = 41). Eight individuals had a compliance rate of 0.0%. Exclusion of these eight individuals from subsequent analyses did not significantly change the overall pattern of findings.
Adverse Events/Side Effects
Most side effects were coded as mild by the study physician or nurse practitioner. The frequency of participants reporting side effect/adverse events are summarized in Table 2. The most frequent side effects involved gastrointestinal (GI) discomfort that included heartburn, flatulus, and cramps. Results of χ2 and Fisher exact tests revealed that groups did not differ with respect to the overall number of participants who reported side effects.
Table 2. Number of Participants who Reported Side Effects by Group.
| ADVERSE EVENT | Placebo n = 38 |
1200mg n = 40 |
2400mg n = 33 |
TOTAL | χ2 | df | p |
|---|---|---|---|---|---|---|---|
| GI Symptoms | 16 | 15 | 19 | 50 | 3.15 | 2 | .20 |
| Headache | 9 | 3 | 3 | 15 | 5.15 | 2 | .07 |
| Dermatological | 2 | 3 | 5 | 10 | * | - | .41 |
| Respiratory | 6 | 1 | 3 | 10 | * | - | .13 |
| Musculosketetal | 4 | 2 | 2 | 8 | * | - | .66 |
| Sleepiness | 2 | 2 | 3 | 7 | * | - | .78 |
| Appetite | 3 | 1 | 2 | 7 | * | - | .59 |
| Hyperactivity | 2 | 3 | 1 | 6 | * | - | .87 |
| Insomnia | 2 | 2 | 2 | 6 | * | - | .99 |
| Urinary Problems | 1 | 1 | 1 | 3 | * | - | .43 |
| Infrequent SEs (<3)** | 7 | 8 | 3 | 18 | 1.79 | 2 | .41 |
|
Participants reporting
any side effects |
30 | 32 | 27 | 89 | .09 | 2 | .95 |
Fisher exact probability test performed due to small cell size
Infrequent Side Effects included two observations of the following: chest pain, decreased sex drive, dehydration, difficulty swallowing capsules, dizziness, fever. Infrequent side effects included a single observation of each of the following: auto accident, reported bad taste, cold intolerance, cold sweat, feeling dazed, dry mouth, heavy bleeding, High BP, neurological problems, numbness, rectal bleeding, replacement of dental fillings, tachycardia, tremor, vaginal infection
Three subjects were removed from the study by the investigators. One subject in the placebo group was removed for a low neutrophil count. One subject in the 1200mg NAC Group was evaluated for chest pain while using cocaine. This was not believed to be related to NAC use. One subject in the 2400mg NAC group developed a rash that was successfully treated with diphenhydramine.
Results of BE Levels
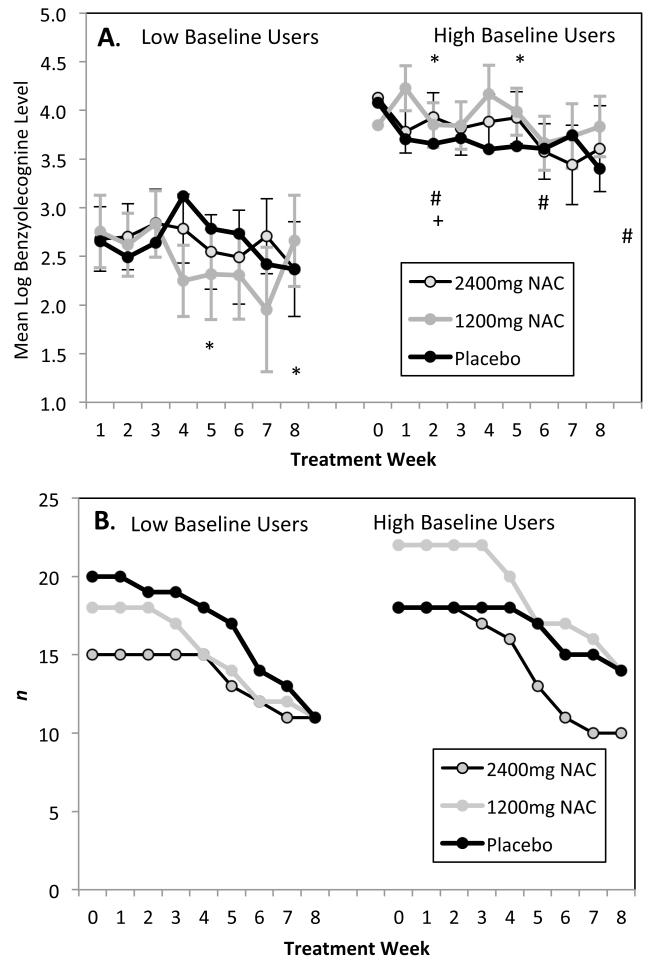
There were no between-group differences in BE levels. However, BE levels changed over time, as indicated by a significant effect for Treatment Week, Wald χ2 = 16.6, df = 8, p< .05. BE levels were higher among those with high levels of baseline use, Wald χ2= 23.2, df = 1, p< .001. While there were no between group differences, the pattern of change in BE levels differed across groups, as indicated by a significant effect for the 3-way Treatment Group × Baseline Use × Treatment Week, Wald χ2= 31.0, df = 16, p< .05. Further post-hoc inspection revealed that the 1200mg NAC group with low baseline cocaine use showed reduction in urine BE levels, Wald χ2= 24.5, df = 8, p< .01, with significant reductions relative to Week 0 at Weeks 4 and 7, Wald χ2= 6.7, 7.1, respectively, df = 1, p < .01 for both. Neither the low baseline placebo nor 2400mg NAC group showed time-related changes. Conversely, among the high baseline users, the 1200mg group had higher BE levels, Wald χ2= 16.1, df = 8, p < .05, with elevated levels relative to Week 0 at Weeks 1 and 4, Wald χ2= 5.0, 3.9, respectively, df = 1, p< .05 for both. The high baseline Placebo group showed reductions relative to Week 0, Wald χ2= 17.2, df = 8, p < .05, with reductions at Weeks 1, 5, and 8 Wald χ2= 7.9, 4.9, 6.1, respectively, df = 1, p < .05 for all three. The 2400mg group showed reductions as well, Wald χ2= 21.3, df = 8, p < .01, with levels at Week 1 lower than those at Week 0, Wald χ2= 5.4, df = 1, p < .05.
Days of Confirmed Abstinence
For number of confirmed abstinence days, there was no main effect for Treatment Group. None of the interactions with Treatment Group were significant. Treatment Week was significant, Wald χ2= 23.9, p < .01, as was Baseline Use, Wald χ2= 17.1, p < .001. The number of urines collected within each week accounted for a significant amount of variance, Wald χ2= 61.1, p < .001, suggesting that those who provided more urine screens had more days of confirmed abstinence.
BSCS and CSSA Self Report Measures
The self-report measures did not reveal any effect of NAC relative to placebo. Ratings of craving, as assessed by the BSCS, showed a time-related reduction, Wald χ2= 104.1, df = 8, p< .001, and higher levels in those who reported high baseline use, Wald χ2= 10.9, df = 1, p< .01. For the CSSA, there was evidence that those with high baseline levels of use produced higher ratings overall, Wald χ2= 6.7, df = 1, p< 0.01. There was evidence for reduction in CSSA ratings over time, Wald χ2= 102.1, df = 8, p< .001.
Results of Exploratory Relapse Analysis
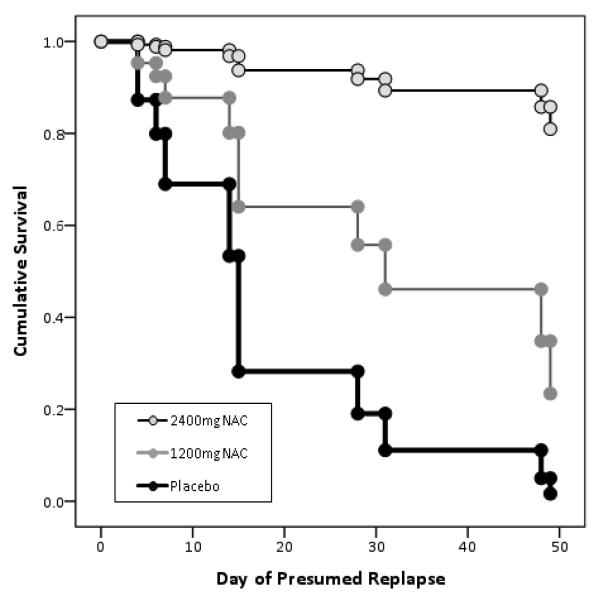
There were 17 participants who were abstinent for at least one week immediately prior to beginning the trial (n = 8 for the placebo group, n = 4 for the 1200mg NAC group, and n = 5 for the 2400mg NAC group). While these individuals reported using fewer days in the month prior to the study than those in the main sample (M = 8.2 days, SD = 4.6, versus M = 14.7 days, SD = 8.0, F[1, 109] = 10.4, p < .01), they did not differ from the main sample on any other baseline characteristic. Six of 8 individuals with the placebo group relapsed (75%), 3 of 4 within the 1200mg NAC group relapsed (75%), and 2 of 5 within the 2400mg group relapsed (40%). Although rates of relapse were not significantly different across groups, time to relapse differed across groups, Wald χ2= 7.8, p < .05. Those receiving 2400mg of NAC had longer time to relapse compared to receiving placebo, OR = .05 (CI95 = .01, .41), β = − .29 (SE = 1.06), p < .01, with time to relapse in those receiving 1200mg NAC falling between the two (though not significantly different from either). Baseline use was also significantly associated with time to relapse, β = − .36 (SE = .12), p < .01.
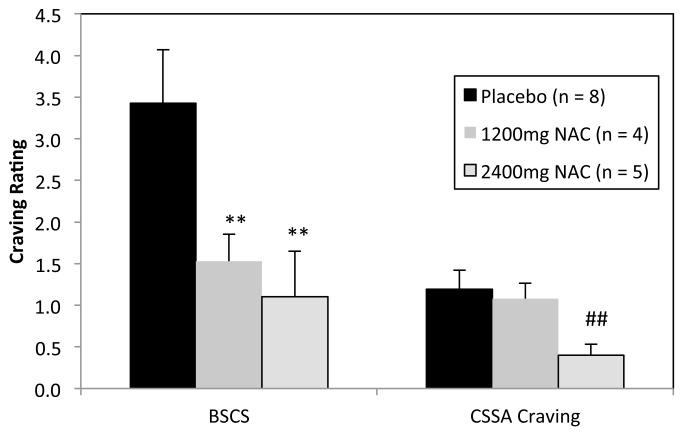
For self-report ratings, GEE analyses for this subsample indicated a significant effect on BSCS ratings for Treatment Week, Wald χ2=, 28.1, p < .001, Treatment Group, Wald χ2= 8.7, p < .05, and a significant Treatment Week × Group Effect, Wald χ2= 33.4, p < .01. The effect for Treatment Group is depicted in Figure 3. Post hoc analyses indicated significantly lower BSCS ratings in both NAC groups relative to placebo, t’s = −2.6, −2.8, p < .01 for 1200mg NAC and 2400mg NAC, respectively. NAC groups did not differ from one another. The CSSA craving item also indicated a significant effect for Treatment Week, Wald χ2=, 41.1, p < .001, Treatment Group, Wald χ2= 13.5, p < .01, and a significant Treatment Week × Group Effect, Wald χ2= 146.4, p < .001. Overall, the 2400mg NAC group reported less craving than the placebo group and the 1200mg NAC group, t’s = −3.0, −2.9, respectively, p < .05 for both (Figure 3).
Figure 3. Exploratory analysis of overall mean craving ratings among initially abstinent individuals across the trial.
Note: Due to excessive missing data points at Weeks 0 and 8, means included data from only Weeks 1 through 7. Error bars represent standard error of measurement.
** = BSCS level is significantly less than placebo, p < .001
## = CSSA craving rating significantly less than NAC 1200mg and placebo, p < .01.
DISCUSSION
Overall, the primary results of this double blind, single site trial failed to demonstrate that NAC reduces cocaine use among cocaine dependent individuals who are actively using cocaine. There were no between group differences for BE levels, days of confirmed abstinence, craving, or withdrawal severity. This lack of findings is somewhat unexpected, given numerous preclinical and clinical studies that have found positive effects for NAC. For instance, in rats trained to self-administer cocaine, chronic NAC administration leads to normalization of glutamate transmission in the accumbens and results in enduring protection from conditioned cue-, context- and cocaine-induced reinstatement of cocaine-seeking (measured by operant lever pressing) for up to 3 weeks after the last daily NAC administration20-21. These preclinical findings consistently suggest that NAC may ameliorate cellular neuropathologies induced by chronic cocaine administration. Moreover, double-blind clinical laboratory studies have demonstrated that NAC is associated with reduced craving/desire to use cocaine, 7-8 as well as reduced motivation to use other addictive substances as well, including nicotine22, marijuana23, and methamphetamine24.
When considering why the above-described results were not replicated in the primary results of the present trial, it is essential to underscore the key difference between the design the preclinical/early human studies and the present trial. The earlier preclinical and human laboratory cocaine studies were designed to examine whether NAC would prevent “relapse” (i.e. reinstated lever pressing in response to a cue or priming dose of cocaine), or, in the case of the human studies, to prevent the reinstatement of desire to use. In all of these studies, NAC was administered in conditions of abstinence from cocaine (i.e. no longer receiving cocaine through self-administration or no current use). In contrast, the present trial followed a conventional cocaine clinical trial design that assesses whether a medication reduces cocaine use in those actively using cocaine25-27. Thus, the majority of individuals in the present trial were still actively using cocaine, and under these conditions NAC did not appear to be effective. Interestingly, when only the subsample of individuals who were abstinent at the beginning of the trial were evaluated, and relapse prevention was assessed in a manner that more closely paralleled existing preclinical and early human studies, there was evidence that NAC increased time to relapse and reduced craving, particularly in those receiving the highest dose. This pattern of results suggests that NAC may be more effective as a medication for preventing relapse than as a medication for achieving abstinence.
While the primary results of the present study were negative, NAC did show some effects on patterns of cocaine use. Indeed, those receiving the moderate 1200mg dose of NAC and who had reported low baseline use of cocaine showed decreases in BE levels relative to baseline, while those receiving the same dose who reported high baseline use showed increases in BE levels relative to baseline. Preclinical animal studies may eventually account for these discrepant findings, as these studies have uncovered that NAC-induced increases in glutamate levels not only can inhibit reinstatement of cocaine-seeking by stimulating inhibitory presynaptic mGluR2/3 receptors28-29, but can, under the right conditions, have the reverse effect by stimulating excitatory postsynaptic mGluR5 receptors30-31. What remains to be investigated is the extent to which training dose and length of access to cocaine (i.e. the animal equivalent of “baseline use”) interacts with the impact of variations in NAC-induced glutamate release on the stimulation of these receptors. In sum, although the present clinical findings have limited clinical applicability, these data nevertheless complement the on-going pre-clinical efforts to uncover the basic mechanisms involved in maintaining addictive processes.
This study has a number of limitations. Although care was made to randomize individuals based on baseline use levels, the study was not originally powered to assess 3-way interactions. Additionally, while the mean level of compliance was relatively high, there still remained a number of individuals who were non-compliant, and future studies must strive to ensure medication is taken properly and consistently. Another factor that may have obscured medication effects is the inclusion of the behavioral treatment platform, as has been observed in a previous large scale trial in which individuals receiving therapy combined with placebo medications fared comparably well to those receiving medication alone32. Lastly, there were numerous exploratory analyses that may have increased the rate at which the null hypothesis was falsely rejected.
Despite these weaknesses, these results provide useful insights into when and how one might expect NAC to be a useful intervention for the treatment of cocaine dependence. These results underscore several parameters that should be addressed in future studies, such as the impact of baseline use, dosage levels, and the need to assess NAC in the context of abstinence. N-acetylcysteine remains a promising agent for treatment in that its impact on the basic neuropathology induced by cocaine in cortico-striatal synaptic plasticity is perhaps better understood than any other candidate medications under investigation. Moreover, there is a growing body of evidence indicating that NAC is not only potentially useful as a treatment for cocaine dependence7-8, but also for a broad range of compulsive/addictive disorders, including nicotine, marijuana, and methamphetamine use7-8, 22-24, 33, as well as gambling or trichotillomania34-35. Thus, further trials assessing the efficacy of NAC for compulsive behaviors in general, and for cocaine specifically, are warranted.
Figure 1. Mean benzoylecognine levels and sub-sample size by group, baseline use, and treatment week.
Panel A. BE levels # -- within Placebo group, levels from this week are lower relative to Week 0
* -- within NAC 1200 group, levels from this week are lower relative to Week 0
+ -- within NAC 2400 group, levels from this week are lower relative to Week 0
Significant Group × Baseline Use × Time interaction, Wald χ2= 31.0, df = 16, p< .05. . No group differences are noted at any Treatment Week (n = 111). Error bars represent standard error of measurement.
Panel B. Sample size (n) per group per week.
Figure 2. Results of exploratory analysis of time to relapse for subjects who were abstinent for at least 1 week prior to entering the medication trial.
Note: Placebo n=8, 1200mg NAC n=4, 2400mg NAC n= 5
Acknowledgements
Support provided by NIDA grant DA0199903 (Malcolm, PI). Thanks are extended to Kristi Huebner and Kelly Barnes for the assistance in project coordination and data management, Dr. Kelly Barth for her assistance in clinical management of patients, and Drs. Carrie Randall and Melissa Milanak for their comments and feedback.
Appendix A. Patient recruitment, randomization, and attrition
Footnotes
The authors report no competing or conflicting interests. This trial was registered at ClinicalTrials.gov, NCT00218491.
REFERENCES
- 1.Baker DA, McFarland K, Lake RW, et al. Neuroadaptation in cystine-glutamate exchange underlie cocaine relapse. Nature Neuroscience. 2003;6(7):743–749. doi: 10.1038/nn1069. [DOI] [PubMed] [Google Scholar]
- 2.McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. Journal Of Neuroscience. 2003;23(8):3531–3537. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Madayag A, Lobner D, Kau KS, et al. Repeated N-acetylcysteine administration alters plasticity-dependent effects of cocaine. The Journal of Neuroscience. 2007;27(51):13968–13976. doi: 10.1523/JNEUROSCI.2808-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moran MM, McFarland K, Melendez RI, Kalivas PW, Seamans JK. Cystine/glutamate exchange regulates metabotropic glutamate receptor presynaptic inhibition of excitatory transmission and vulnerability to cocaine seeking. Journal of Neuroscience. 2005;25(27):6389–6393. doi: 10.1523/JNEUROSCI.1007-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.LaRowe SD, Kalivas PW. The role of N-actylcysteine in inhibiting responding during extinction in rats trained to self-adminster cocaine. The Open Addiction Journal. 2010;3:88–91. doi: 10.2174/1874941001003010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moussawi K, Zhou W, Shen H, et al. Reversing cocaine-induced synaptic potentiation provides enduring protection from relapse. Processings of the National Academy of Sciences. 2011;108(1):385–390. doi: 10.1073/pnas.1011265108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.LaRowe SD, Myrick H, Hedden S, et al. Is cocaine desire reduced by N-acetylcysteine? American Journal of Psychiatry. 2007;164:1115–1117. doi: 10.1176/ajp.2007.164.7.1115. [DOI] [PubMed] [Google Scholar]
- 8.Amen SL, Piacentine LB, Ahmad ME, Li S-J, Matsch JR, Risinger RC. Repeated N-acetyl cysteine reduces cocaine seeking in rodents and craving in cocaine-dependnece humans. Neuropsychopharmacology. 2011;36(4):871–878. doi: 10.1038/npp.2010.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mardikian P, LaRowe SD, Hedden S, Kalivas P, Malcolm R. An open-label trial of N-acetylcysteine for the treatment of cocaine dependence: a pilot study. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2007;31:389–394. doi: 10.1016/j.pnpbp.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 10.LaRowe SD, Mardikian P, Malcolm R, et al. Safety and tolerability of N-acetylcysteine in cocaine-dependent individuals. The American Journal on Addictions. 2006;15:105–110. doi: 10.1080/10550490500419169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.American Psychiatric Association . The Diagnostic and Statistical Manual of Mental Disorders (4th ed) APA; Washington, DC: 1994. [Google Scholar]
- 12.Sobell LC, Sobell MB. Validity of self-reports in three populations of alcoholics. J Consult Clin Psychol. 1978;46:901–907. doi: 10.1037//0022-006x.46.5.901. [DOI] [PubMed] [Google Scholar]
- 13.Malcolm R, LaRowe S, Cochran K, et al. A controlled trial of amlodipine for cocaine dependence: a negative report. J Subst Abuse Treat. 2005 Mar;28(2):197–204. doi: 10.1016/j.jsat.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 14.Preston KL, Silverman K, Schuster CR, Cone EJ. Assessment of cocaine use with quantitative urinalysis and estimate of new uses. Addiction. 1997;92(6):717–727. [PubMed] [Google Scholar]
- 15.Mezinskis JP, Honos-Webb L, Kropp F, Somoza E. The measurement of craving. Journal of Addictive Diseases. 2001;20(3):67–85. doi: 10.1300/J069v20n03_07. [DOI] [PubMed] [Google Scholar]
- 16.Kampman KM, Volpicelli JR, NcGinnis DE, et al. Reliability and validity of the cocaine selective severity assessment. Addictive Behaviors. 1998;23(4):449–461. doi: 10.1016/s0306-4603(98)00011-2. [DOI] [PubMed] [Google Scholar]
- 17.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders -- Non-patient Edition (SCID-I/NP, 11/2002 revision) Biometrics Research Department, New York State Psychiatric Institute; New York, NY: 2002. [Google Scholar]
- 18.Brief DJ, Bollinger AR, Horton GE, LoCastro JS. Integrated treatment for addictions: education, motivational enhancement, and skills building for cocaine addiction. Medication Development Division, NIDA; Bethesda, MD: 2000. [Google Scholar]
- 19.Moussawi K, Zhou W, Shen H, et al. Reversing cocaine-induced synaptic potentiation provides enduring protection from relapse. Proc Natl Acad Sci U S A. 2011 Jan 4;108(1):385–390. doi: 10.1073/pnas.1011265108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moussawi K, Zhou W, Shen H, et al. Reversing cocaine-induced synaptic potentiation provides enduring protection from relapse. Proceedings of the National Academy of Sciences. 2011;108(1):385–390. doi: 10.1073/pnas.1011265108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reichel CM, Moussawi K, Do PH, Kalivas PW, See RE. Chronic N-acetylcysteine during abstinence or extinction after cocaine self-adminsitration proceduces enduring reductions in drug seeking. The Journal of Pharmacology and Experimental Therapeutics. 2011;337(2):487–493. doi: 10.1124/jpet.111.179317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knackstedt LA, LaRowe S, Mardikian P, et al. The role of cystine-glutamate exchange in nicotine dependence in rats and humans. Biological Psychiatry. 2009;65:841–845. doi: 10.1016/j.biopsych.2008.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gray KM, Carpenter MJ, Baker SM, DeSantis SM, McRae-Clark AL, Brady KT. A randomized controlled trial of N-acetylcysteine in cannabis-dependent adolescents: Main findings; 73rd Annual Scientific Meeting of the College on Problems of Drug Dependence; Hollywood, FL. 2011. [Google Scholar]
- 24.Haile CN, DeLaGarza R, Mahoney JJ, et al. N-acetyl-cysteine alters drug-cue enhanced subjective effects of smoked methamphetamine in METH-dependent volunteers; 73rd Annual Scientific Meeting of the College on Problems of Drug Dependence; Hollywood, FL. 2011. [Google Scholar]
- 25.Dackis CA, Kampman KM, Lynch KG, Pettinati HM, O’Brien CP. A Double-Blind, Placebo-Controlled Trial of Modafinil for Cocaine Dependence. Neuropsychopharmacology. 2005 Jan;30(1):205–211. doi: 10.1038/sj.npp.1300600. [DOI] [PubMed] [Google Scholar]
- 26.Malcolm RJ, LaRowe SD, Cochran K, et al. A controlled trial of amlodipine for cocaine dependence: a negative report. Journal of Substance Abuse Treatment. 2005;28:197–204. doi: 10.1016/j.jsat.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 27.Shoptaw S, Yang X, Rotheram-Fuller EJ, et al. Randomized placebo-controlled trial of baclofen for cocaine dependence: Preliminary effects for individuals with chronic patterns of cocaine use. Journal of Clinical Psychiatry. 2003 Dec;64(12):1440–1448. doi: 10.4088/jcp.v64n1207. [DOI] [PubMed] [Google Scholar]
- 28.Peters J, Kalivas PW. The group II metabotropic glutamate receptor agonist, LY379268, inhibits both cocaine- and food-seeking behavior in rats. Psychopharmacology. 2006;186:143–149. doi: 10.1007/s00213-006-0372-9. [DOI] [PubMed] [Google Scholar]
- 29.Baptista MAS, Martin-Fardon R, Weiss F. Preferential effects of the mGlu2/3 receptor agonist, LY379268, on conditioned reinstatement vs. primary reinforcement: Comparison between cocaine and a potent non-drug reinforcer. The Journal of Neuroscience: Brief Communications. 2004;24:4723–4727. doi: 10.1523/JNEUROSCI.0176-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kupchik YM, Moussawi K, Tang X-C, et al. The effect of N-acetylcysteine in the nucleus accumbens on neurotransmission and relapse to cocaine. Biological Psychiatry. 2012;71(11):978–986. doi: 10.1016/j.biopsych.2011.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moussawi K, Pacchioni A, Moran M, et al. N-Acetylcysteine reverses cocaine-induced metaplasticity. Nature Neuroscience. 2009;12(2):182–189. doi: 10.1038/nn.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anton RF, Ciraulo D, Cisler R, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. Journal of the American Medical Association. 2006;295(17):2003–2017. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- 33.Gray KM, Watson NL, Carpenter MJ, LaRowe SD. N-Acetylcysteine (NAC) in young marijuana users: an open-label study. The American Journal on Addictions. 2010;19:187–189. doi: 10.1111/j.1521-0391.2009.00027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grant JE, Odlaug BL, Kim SW. N-acteylcysteine, a glutamate modulator, in the treatment of tricotillomania. Archives of General Psychiatry. 2009;66(7):756–763. doi: 10.1001/archgenpsychiatry.2009.60. [DOI] [PubMed] [Google Scholar]
- 35.Grant JE, Kim SW, Odlaug BL. N-acetyl cysteine, a glutamate-modulating agent, in the treatment of pathological gambling: a pilot study. Biological Psychiatry. 62:652–657. doi: 10.1016/j.biopsych.2006.11.021. [DOI] [PubMed] [Google Scholar]