Abstract
Guidelines recommend lifestyle modification for individuals with coronary heart disease (CHD). Few data demonstrate which lifestyle modifications, if sustained, reduce recurrent CHD and mortality risk among cardiac patients after the post-acute rehabilitation phase. We determined the association between ideal lifestyle factors and recurrent CHD and all-cause mortality among REasons for Geographic and Racial Differences in Stroke study participants with CHD (n=4,174). Ideal lifestyle factors (physical activity ≥4 times/week, non-smoking, highest quartile of Mediterranean diet score, waist circumference <88/<102 cm for women/men) were assessed through questionnaires and an in-home study visit. There were 447 recurrent CHD events and 745 deaths over a median 4.3 and 4.5 years, respectively. After multivariable adjustment, physical activity ≥4 versus no times/week and non-smoking versus current smoking were associated with reduced hazard ratios (HR) [95% confidence interval] for recurrent CHD (0.69 [0.54–0.89] and 0.50 [0.39–0.64], respectively) and death (0.71 [0.59–0.86] and 0.53 [0.44–0.65], respectively). The multivariable adjusted HR (95% CI) for recurrent CHD and death comparing the highest versus lowest quartile of Mediterranean diet adherence were 0.77 (0.55–1.06) and 0.84 (0.67–1.07), respectively. Neither outcome was associated with waist circumference. Comparing participants with 1, 2, and 3 versus 0 ideal lifestyle factors (non-smoking, physical activity ≥4 times/week, highest quartile of Mediterranean diet score), the HR (95% CI) were 0.60 (0.44–0.81), 0.49 (0.36–0.67) and 0.38 (0.21–0.67), respectively, for recurrent CHD and 0.65 (0.51–0.83), 0.57 (0.43–0.74) and 0.41 (0.26–0.64), respectively, for death. In conclusion, maintaining smoking cessation, physical activity and Mediterranean diet adherence is important for secondary CHD prevention.
Keywords: recurrent coronary heart disease, lifestyle
Introduction
Although lifestyle modification has clear benefits and is recommended for secondary prevention of coronary heart disease (CHD)1–3, < 20% of cardiac patients complete cardiac rehabilitation programs.4,5 Few data demonstrate which secondary prevention lifestyle modifications, if sustained following the post-acute CHD event period, reduce the risk for recurrent CHD or all-cause mortality.1–3 Additionally, the long-term CHD risk reduction benefits of multiple lifestyle factors have not been extensively studied. Therefore, we determined the association of (1) ideal levels of individual lifestyle factors that are the focus of cardiac rehabilitation programs, including waist circumference, physical activity, adherence to a Mediterranean diet, and smoking status, and (2) multiple ideal lifestyle factors with recurrent CHD and all-cause mortality. Determining these associations with recurrent CHD events and all-cause mortality can justify maintaining lifestyle modifications for secondary prevention and reinforce current guidelines. To do so, we analyzed a large population-based cohort of US adults with existent CHD enrolled in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study.
Methods
The REGARDS study has been described previously.6 In brief, 30,239 adults ≥ 45 years of age from all 48 continental US states and the District of Columbia were enrolled between January 2003 and October 2007. By design, the REGARDS study oversampled blacks and residents of the Southeastern US (North Carolina, South Carolina, Georgia, Alabama, Mississippi, Tennessee, Arkansas and Louisiana). The current analysis was restricted to REGARDS participants reporting a history of CHD (defined below) at baseline and had recurrent CHD follow-up data (n = 4,174). Each participating center’s Institutional Review Board governing human subject research approved the REGARDS study protocol. All participants provided informed consent.
Baseline data were collected through a telephone interview, self-administered questionnaires, and an in-home examination. During computer-assisted telephone interviews administered by trained staff, participants’ age, race, gender, smoking status, education, annual household income, physical activity, self-rated health, regular aspirin use, and self-report of prior diagnosed co-morbid conditions (e.g. diabetes, myocardial infarction (MI), coronary revascularization procedures). Since awareness of a CHD event could prompt behavior changes, history of CHD was defined as self-reported MI, angioplasty or stenting of a coronary artery, or coronary bypass surgery. During the in-home examination, technicians measured waist circumference and blood pressure and collected blood and spot urine samples (described previously).6 Prescription and over the counter pill bottles were reviewed for medications taken during the 2 weeks prior to the in-home study visit. The use of clopidogrel, beta-blockers, statins, angiotensin-converting-enzyme inhibitors (ACEi), and angiotensin-receptor-blockers (ARBs) are considered in this analysis. Following the in-home examination, participants completed and mailed a self-administered Block 98 Food Frequency Questionnaire (FFQ) to the coordinating center.
Low-density-lipoprotein cholesterol (LDL-C) was calculated using the Friedewald equation. Diabetes was defined by self-report with concurrent use of insulin or oral hypoglycemic medications or fasting serum glucose ≥126 mg/dL or non-fasting serum glucose ≥200 mg/dL. High-sensitivity C-reactive protein (CRP) was measured by particle enhanced immunonephelometry. Estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation.7 Reduced eGFR was defined as levels <60 ml/min/1.73 m2. Albuminuria was defined as urinary albumin to urinary creatinine ratio ≥30 mg/g.
Four lifestyle factors were evaluated: abdominal obesity, physical activity, adhering to a Mediterranean-style diet, and cigarette smoking. Compared to body mass index, waist circumference has been shown to have a stronger association with cardiovascular (CV) events8 and, thus, was chosen as a measure of adiposity in the current analysis. Abdominal obesity was defined as a waist circumference > 102 cm and > 88 cm for men and women, respectively. Physical activity was assessed with the question “How many times per week do you engage in intense physical activity, enough to work up a sweat?” Response options were “none”, “1 to 3”, or “≥4” times per week. The FFQ was processed with NutritionQuest software to estimate the average dietary nutrient intake for 1 year prior to participants’ in-home visit. A Mediterranean diet score was created with 14 all-inclusive food groups and nutrients (e.g., potatoes, vegetables, legumes, fruits and nuts, dairy products, cereals, meats, fish, eggs, monounsaturated lipids, polyunsaturated lipids, saturated lipids and margarines, sugar and sweets, non-alcoholic beverages), using a monounsaturated to saturated fats ratio similar to methods described by Trichopoulou et al.9 Participants were grouped into quartiles based on the study populations distribution of Mediterranean diet scores (cut-points: ≤3, 4 to <5, 5, and ≥5 [higher quartiles: better adherence]). Current smoking was defined as responding “yes” to both questions: “Have you smoked at least 100 cigarettes in your lifetime?” and “Do you smoke cigarettes now, even occasionally?” Ideal lifestyle factors were (1) not having abdominal obesity, (2) physical activity ≥4 times per week, (3) Mediterranean diet score in the highest quartile, and (4) being a non-smoker.
Two outcomes were studied: recurrent CHD or all-cause mortality. Following the baseline visit, living participants or their proxies were contacted bi-annually via telephone to assess potential recurrent CHD events and vital status. When a CHD-related hospitalization or a death was reported, medical records were retrieved and trained clinicians adjudicated events following published guidelines.10–12 Online sources (e.g., Social Security Death Index), the National Death Index, and reports from next-of-kin were used to detect participant deaths. Circumstances of the death were obtained by interviewing proxies or next-of-kin, from death certificates, autopsy reports and medical records. Definite or probable CHD events (nonfatal MI or acute CHD death) and all-cause mortality through December 31, 2009 were analyzed.
Participant characteristics were calculated by number of ideal lifestyle factors (0, 1, 2, or ≥ 3). Crude rates for recurrent CHD were calculated for levels of each lifestyle factor. Cox proportional hazard models with progressive adjustment were used to calculate the hazard ratios (HR) for recurrent CHD associated with each lifestyle factor. An initial model (Model 1) included adjustment for age, sex, race, and geographic region of residence. A second model (Model 2) included additional adjustment for LDL-C, BP, education, annual household income, self-rated health, diabetes, albuminuria, eGFR, CRP, and use of aspirin, clopidogrel, beta-blockers, ACEI’s, ARBs, and statins. Next, the cumulative incidence of recurrent CHD was calculated by number of ideal lifestyle factors (physical activity ≥ 4 times per week, highest quartile of Mediterranean diet adherence, and non-smoking). As waist circumference was not associated with recurrent CHD or all-cause mortality, it was excluded from the analysis of number of ideal lifestyle factors. Cumulative incidence curves were plotted by number of ideal lifestyle factors. HRs for recurrent CHD associated with the number of ideal lifestyle factors were calculated with similar adjustment as described above. Identical analyses were repeated for all-cause mortality. Missing data were imputed with 10 data sets using chained equations.13 The number (%) of imputed lifestyle factors were: waist circumference: 28 (0.7%); physical activity: 62 (1.5%); smoking: 12 (0.3%); Mediterranean diet score: 1,355 (32.5%). Analyses were conducted using Stata/I.C. 12.1 (Stata Corporation, College Station, TX).
RESULTS
The mean age and the proportion of participants taking aspirin and statins were higher among those with more ideal lifestyle factors (Table 1). Women and blacks were less likely to have more ideal lifestyle factors. The percentage of participants with less than a high school education, an annual household income < $20,000, albuminuria, and diabetes was lower with more ideal lifestyle factors. The mean systolic and diastolic BP, waist circumference, LDL-C, and CRP were lower as the number of ideal lifestyle factors increased. Other participant characteristics were similar across the number of ideal lifestyle factors.
Table 1.
Baseline characteristics of the REasons for Geographic and Racial Differences in Stroke (REGARDS) study population with coronary heart disease by number of ideal lifestyle factors.
| Number of ideal lifestyle factors‡ | ||||
|---|---|---|---|---|
| Participant characteristic | 0 (n = 240) |
1 (n = 1,383) |
2 (n = 1,452) |
3 – 4 (n = 1,100) |
| Percentage of sample | 5.7% | 33.1% | 34.8% | 26.4% |
| Age (years) | 64.8 (0.5) | 67.2 (0.3) | 69.7 (0.3) | 70.4 (0.3) |
| Female | 57.1% | 47.2% | 32.5% | 21.6% |
| Black | 40.9% | 39.8% | 33.9% | 25.9% |
| Less than a high school education | 25.1% | 20.0% | 17.0% | 12.5% |
| Household Income < $20,000 | 37.3% | 28.8% | 20.2% | 12.7% |
| Geographic Region | ||||
| Stroke belt | 36.4% | 35.3% | 34.1% | 33.5% |
| Stroke buckle | 18.9% | 21.5% | 22.2% | 20.4% |
| Other region | 44.7% | 43.2% | 43.7% | 46.1% |
| Diabetes mellitus | 46.9% | 45.4% | 33.2% | 23.9% |
| Systolic blood pressure (mm Hg) | 130.9 (1.2) | 131.6 (0.5) | 129.5 (0.5) | 128.0 (0.6) |
| Diastolic blood pressure (mmHg) | 75.8 (0.6) | 76.1 (0.3) | 75.0 (0.3) | 74.0 (0.3) |
| Low estimated GFR (<60 ml/min/1.73 m2) | 22.1% | 24.4% | 25.1% | 17.1% |
| High albumin to creatinine ratio (≥ 30 mg/g) | 32.2% | 28.7% | 23.9% | 17.4% |
| Aspirin Use | 74.6% | 74.4% | 75.0% | 82.6% |
| Clopidogrel Use | 20.0% | 19.0% | 19.5% | 17.2% |
| Statin Use | 58.2% | 62.3% | 65.7% | 68.6% |
| Beta blocker use | 55.3% | 53.9% | 53.1% | 51.9% |
| ACE inhibitor use | 44.4% | 39.9% | 40.1% | 36.8% |
| Angiotensin receptor blocker use | 15.2% | 22.2% | 18.3% | 15.8% |
| Abdominal obesity† | 100.0% | 86.8% | 44.1% | 12.2% |
| Serum LDL-cholesterol (mg/dL) | 103.5 (2.6) | 100.6 (1.0) | 98.7 (1.0) | 95.1 (1.1) |
| C-reactive protein mg/L | 4.8 (4.7 – 4.9) | 3.3 (3.2 – 3.3) | 2.4 (2.4 – 2.4) | 1.6 (1.6 – 1.6) |
Number in the table numbers is percentage or mean±standard error except C-reactive protein which is geometric mean (95% confidence interval).
GFR = estimated glomerular filtration.
Abdominal obesity = waist circumference for men: > 102 cm and women: > 88 cm.
Ideal lifestyle factors were defined as not having abdominal obesity, physical activity ≥ 4 times per week, Mediterranean diet score in the highest quartile, and being a non-smoker.
The prevalence of ideal waist circumference, physical activity ≥ 4 times per week, high Mediterranean diet adherence, and non-smoking was 46.9%, 30.1%, 25.4%, and 84.6%, respectively. There were 447 recurrent CHD events over a median follow-up of 4.3 (maximum: 6.9) years. Incidence rates of recurrent CHD were lower among individuals with higher levels of physical activity, higher Mediterranean diet adherence, and non-smokers (Table 2). Recurrent CHD rates were not substantially different for individuals with and without abdominal obesity. The HRs for recurrent CHD were lower for individuals participating in physical activity 1 to 3 or ≥ 4 times per week versus no physical activity, individuals more adherent to a Mediterranean diet, and non-smokers versus current smokers after adjustment for age, race, sex, and region of residence. Although participating in physical activity 1 to 3 or ≥ 4 times per week and not smoking remained associated with a lower HR for recurrent CHD after multivariable adjustment, the HR comparing the highest to lowest quartile of Mediterranean diet score was no longer statistically significant.
Table 2.
Crude incidence rates and hazard ratios for recurrent coronary heart disease associated with individual lifestyle factors.
| Incidence Rate (95% CI) |
Hazard Ratio (95% CI) |
||||
|---|---|---|---|---|---|
| Individual lifestyle factors | Events / persons at risk |
Crude (per 1,000 person-years) |
Model 1 | Model 2 | |
| Abdominal obesity† | |||||
| Yes | 241 / 2,215 | 27.2 (23.7 – 30.6) | 1 (reference) | 1 (reference) | |
| No | 206 / 1,959 | 25.7 (22.2 – 29.2) | 0.88 (0.73 – 1.07) | 1.07 (0.87 – 1.31) | |
| p-value | - | - | 0.207 | 0.503 | |
| Physical Activity | |||||
| None | 225 / 1,611 | 36.7 (31.8 – 41.5) | 1 (reference) | 1 (reference) | |
| 1 – 3 times/week | 119 / 1,308 | 21.6 (17.7 – 25.6) | 0.59 (0.47 – 0.75) | 0.72 (0.57 – 0.90) | |
| 4+ times/week | 104 / 1,255 | 19.7 (15.9 – 23.5) | 0.53 (0.42 – 0.68) | 0.69 (0.54 – 0.89) | |
| p-trend | - | - | < 0.001 | 0.002 | |
| Mediterranean diet score‡ | |||||
| Quartile 1 (lowest -worse score) | 175 / 1,385 | 32.2 (26.8 – 37.6) | 1 (reference) | 1 (reference) | |
| Quartile 2 | 91 / 884 | 25.5 (19.2 – 37.8) | 0.79 (0.60 – 1.03) | 0.85 (0.65 – 1.12) | |
| Quartile 3 | 85 / 845 | 24.1 (18.1 – 30.2) | 0.73(0.53 – 1.03) | 0.76 (0.54 – 1.07) | |
| Quartile 4 (highest -better score) | 96 / 1,060 | 21.9 (16.9 – 26.9) | 0.66 (0.48 – 0.90) | 0.77 (0.55 – 1.06) | |
| p-trend | - | - | 0.011 | 0.084 | |
| Current Smoker | |||||
| Yes | 102 / 642 | 43.9 (35.4 – 52.4) | 1 (reference) | 1 (reference) | |
| No | 345 / 3,532 | 23.7 (21.2 – 26.2) | 0.47 (0.37 – 0.56) | 0.50 (0.39 – 0.64) | |
| p-value | - | - | < 0.001 | < 0.001 | |
Abdominal obesity = waist circumference > 102 cm for men, > 88 cm for women.
The cut-points for the lowest to highest quartile of the Mediterranean diet scores were ≤ 3, > 3 to 4, > 4 to 5, and > 5.
Model 1 includes age, race, sex, and region of residence, education and income.
Model 2 includes variables in Model 1 plus LDL cholesterol, systolic and diastolic blood pressure, self-rated health, diabetes, albuminuria, estimated glomerular filtration rate, C-reactive protein, aspirin use, clopidogrel use, beta blocker use, ace inhibitor use, angiotensin receptor blocker use, and statin use.
Over a median follow-up of 4.5 (maximum: 6.9) years, 745 deaths occurred. More physical activity, higher Mediterranean diet adherence, and not smoking were associated with lower crude rates and age, race, sex, and region of residence adjusted HRs for mortality (Table 3). More physical activity and non-smoking remained associated with lower HRs for death after full multivariable adjustment. The highest Mediterranean diet quartile was no longer significantly associated with mortality after multivariable adjustment. Abdominal obesity was not associated with mortality before or after multivariable adjustment.
Table 3.
Crude incidence rates and hazard ratios for all-cause mortality associated with individual lifestyle factors.
| Incidence Rate (95% CI) |
Hazard Ratio (95% CI) |
||||
|---|---|---|---|---|---|
| Individual lifestyle factors | Events / persons at risk |
Crude (per 1,000 person-years) |
Model 1 | Model 2 | |
| Abdominal obesity† | |||||
| Yes | 382 / 2,215 | 41.8 (37.6 – 46.0) | 1 (reference) | 1 (reference) | |
| No | 363 / 1,959 | 43.7 (39.2 – 48.2) | 0.93 (0.80 – 1.08) | 1.15 (0.98 – 1.35) | |
| p-value | - | - | 0.320 | 0.090 | |
| Physical Activity | |||||
| None | 409 / 1,613 | 64.0 (57.8 – 70.2) | 1 (reference) | 1 (reference) | |
| 1 – 3 times/week | 166 / 1,308 | 29.2 (24.7 – 33.7) | 0.48 (0.40 – 0.58) | 0.61 (0.50 – 0.73) | |
| 4+ times/week | 171 / 1,254 | 31.7 (26.9 – 36.5) | 0.50 (0.42 – 0.61) | 0.71 (0.59 – 0.86) | |
| p-trend | - | - | < 0.001 | < 0.001 | |
| Mediterranean diet score‡ | |||||
| Quartile 1 (lowest -worse score) | 274 / 1,385 | 48.3 (42.3 – 54.3) | 1 (reference) | 1 (reference) | |
| Quartile 2 | 165 / 884 | 45.6 (37.8 – 53.4) | 0.92 (0.75 – 1.14) | 1.02 (0.82 – 1.27) | |
| Quartile 3 | 145 / 845 | 40.0 (32.0 – 48.0) | 0.78 (0.61 – 1.00) | 0.83 (0.63 – 1.08) | |
| Quartile 4 (highest -better score) | 161 / 1,060 | 35.6 (29.4 – 41.7) | 0.68 (0.55 – 0.86) | 0.84 (0.66 – 1.07) | |
| p-trend | - | - | < 0.001 | 0.061 | |
| Current Smoker | |||||
| Yes | 156 / 642 | 63.4 (53.4 – 73.3) | 1 (reference) | 1 (reference) | |
| No | 589 / 3,532 | 39.3 (36.1 – 42.5) | 0.46 (0.38 – 0.55) | 0.53 (0.44 – 0.65) | |
| p-value | - | - | < 0.001 | < 0.001 | |
Abdominal obesity = waist circumference > 102 cm for men, > 88 cm for women.
The cut-points for the lowest to highest quartile of the Mediterranean diet scores were ≤ 3, > 3 to 4, > 4 to 5, and > 5.
Model 1 includes age, race, sex, and region of residence, education and income.
Model 2 includes variables in Model 1 plus LDL cholesterol, systolic and diastolic blood pressure, self-rated health, diabetes, albuminuria, estimated glomerular filtration rate, C-reactive protein, aspirin use, clopidogrel use, beta blocker use, ace inhibitor use, angiotensin receptor blocker use, and statin use.
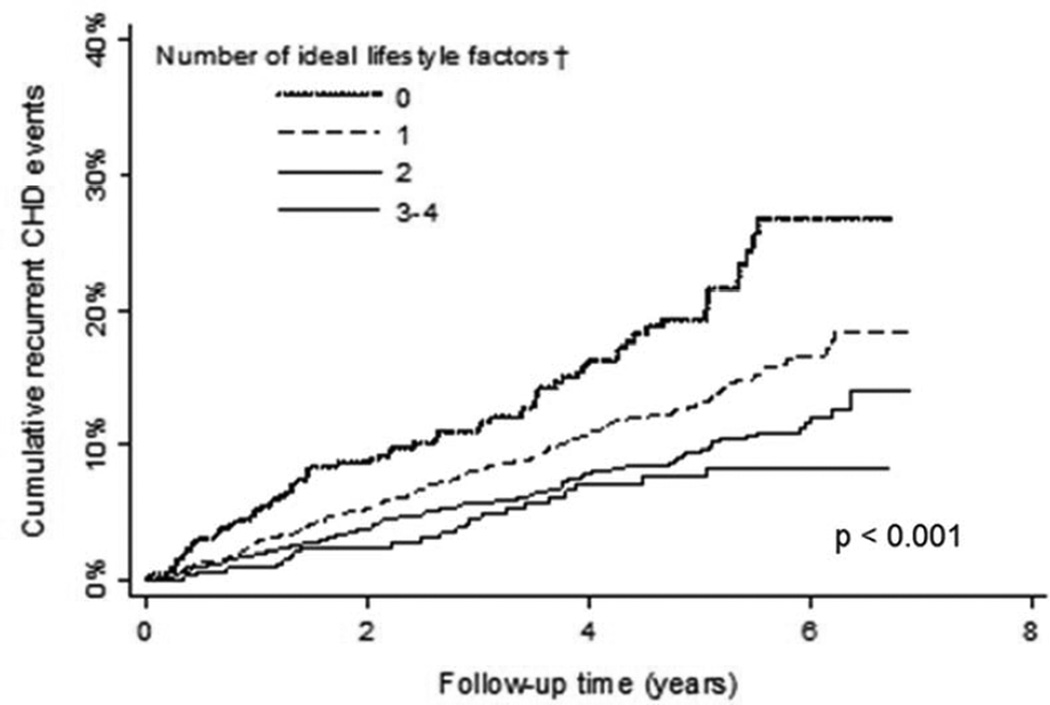
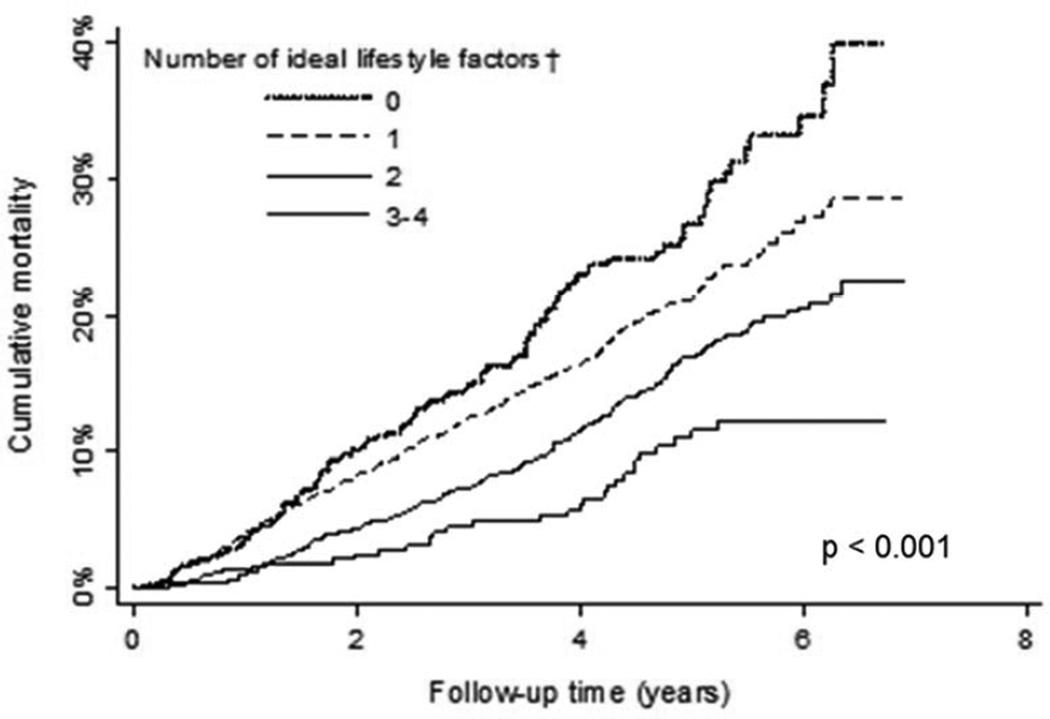
The prevalence of 0, 1, 2, or 3 ideal lifestyle factors (i.e., physical activity ≥4 times per week, Mediterranean diet in the highest quartile, non-smoking) was 10.1%, 48.2%, 33.2%, and 8.5%, respectively. The cumulative incidence for recurrent CHD and mortality were each lower with progressively more ideal lifestyle factors (Figures 1 and 2). These associations remained present after multivariable adjustment (Tables 4 and 5).
Figure 1.
Crude cumulative incidence for recurrent coronary heart disease associated with number of ideal lifestyle factors.
†Ideal lifestyle factors were defined as not having abdominal obesity, physical activity ≥ 4 times per week, Mediterranean diet score in the highest quartile, and being a non-smoker.
Figure 2.
Crude cumulative incidence for all-cause mortality associated with number of ideal lifestyle factors.
†Ideal lifestyle factors were defined as not having abdominal obesity, physical activity ≥ 4 times per week, Mediterranean diet score in the highest quartile, and being a non-smoker.
Table 4.
Crude incidence rates and hazard ratios for recurrent coronary heart disease associated with number of ideal lifestyle factors.
| Incidence rate (95% CI) |
Hazard Ratio (95% CI) |
||||
|---|---|---|---|---|---|
| Number of ideal lifestyle factors† | Events/ persons at risk |
Crude (per 1,000 person-years) |
Model 1 | Model 2 | |
| 0 | 71 / 422 | 46.2 (35.1 – 57.2) | 1 (reference) | 1 (reference) | |
| 1 | 230 / 2,011 | 28.8 (24.8 – 32.7) | 0.56 (0.42 – 0.75) | 0.60 (0.44 – 0.81) | |
| 2 | 124 / 1,386 | 21.2 (17.3 – 25.0) | 0.40 (0.30 – 0.55) | 0.49 (0.36 – 0.67) | |
| 3 | 23 / 355 | 15.0 (7.7 – 22.2) | 0.28 (0.16 – 0.49) | 0.38 (0.21 – 0.67) | |
| p-trend | - | - | < 0.001 | < 0.001 | |
Ideal lifestyle factors were defined as not having abdominal obesity, physical activity ≥ 4 times per week, Mediterranean diet score in the highest quartile, and being a non-smoker.
Model 1 includes age, race, sex, and region of residence, education and income.
Model 2 includes variables in Model 1 plus LDL cholesterol, systolic and diastolic blood pressure, waist circumference, self-rated health, diabetes, albuminuria, estimated glomerular filtration rate, C-reactive protein, aspirin use, clopidogrel use, beta blocker use, ace inhibitor use, angiotensin receptor blocker use, and statin use.
Table 5.
Crude incidence rates and hazard ratios for all-cause mortality associated with number of ideal lifestyle factors.
| Incidence rate (95% CI) |
Hazard Ratio (95% CI) |
||||
|---|---|---|---|---|---|
| Number of ideal lifestyle factors† | Events / persons at risk |
Crude (per 1,000 person-years) |
Model 1 | Model 2 | |
| 0 | 106 / 422 | 64.8 (52.0 – 77.6) | 1 (reference) | 1 (reference) | |
| 1 | 392 / 2,011 | 47.4 (42.2 – 52.6) | 0.58 (0.46 – 0.73) | 0.65 (0.51 – 0.83) | |
| 2 | 213 / 1,386 | 35.5 (29.9 – 41.1) | 0.42 (0.32 – 0.54) | 0.57 (0.43 – 0.74) | |
| 3 | 35 / 355 | 22.3 (14.1 – 30.6) | 0.26 (0.17 – 0.41) | 0.41 (0.26 – 0.64) | |
| p-trend | - | - | < 0.001 | < 0.001 | |
Ideal lifestyle factors were defined as not having abdominal obesity, physical activity ≥ 4 times per week, Mediterranean diet score in the highest quartile, and being a non-smoker.
Model 1 includes age, race, sex, and region of residence, education and income.
Model 2 includes variables in Model 1 plus LDL cholesterol, systolic and diastolic blood pressure, waist circumference, self-rated health, diabetes, albuminuria, estimated glomerular filtration rate, C-reactive protein, aspirin use, clopidogrel use, beta blocker use, ace inhibitor use, angiotensin receptor blocker use, and statin use.
DISCUSSION
In the current analysis of a nationwide sample of people with prevalent CHD, not smoking, more physical activity, and adherence to a Mediterranean diet were each associated with a lower recurrent CHD and all-cause mortality risk. However, only 40% of participants had ideal levels for 2 or 3 of these lifestyle factors. While the associations between adherence to a Mediterranean diet and recurrent CHD and all-cause mortality were attenuated after multivariable adjustment, not smoking and participating in physical activity remained protective. Additionally, there was a strong and graded association for lower recurrent CHD and mortality with more ideal lifestyle factors. Individuals with 3 ideal lifestyle factors had a 62% and 59% lower risk for recurrent CHD and mortality, respectively.
Smoking is a leading preventable cause of premature death and disability.14 The 45 – 55% lower risk for recurrent CHD events and all-cause mortality in the current study is similar to two other cohort studies of patients with a history of CHD.15,16 Considering that 15% of this sample of US adults was smoking despite having a history of CHD, smoking cessation should continue to be a high priority to lower recurrent CHD and mortality risk in individuals with CHD.
A 2005 meta-analysis demonstrated a 24% and 28% reduction in recurrent CHD and allcause mortality, respectively, comparing individuals randomized to a physical activity rehabilitation program versus usual care.1,17 The current study was unable to determine whether the participants completed a cardiac rehabilitation program. However, our study does extend prior research from the post-acute to the chronic outpatient setting and suggests that maintaining physical activity after completing cardiac rehabilitation, regardless of the frequency, may be important for reducing recurrent CHD and all-cause mortality risk.5,18,19
Despite a limited number of intervention and observational studies, the American Heart Association (AHA) and European dietary guidelines for secondary prevention recommend a healthy dietary pattern that can be largely achieved by adhering to a Mediterranean diet consisting primarily of fruits, vegetables, fish, fiber, and low amounts of saturated fats.2,20–23 Recently, the PREDIMED trial reported that adherence to a Mediterranean diet among individuals without history of CHD to be associated with a 29% and 11% lower risk for CV disease and all-cause mortality, respectively, over 4.8 years of follow-up.24 In the current study, higher Mediterranean diet scores had a strong protective benefit against recurrent CHD and mortality after adjustment for age, race, sex, and region of residence. Although this association was not statistically significant after full multivariable adjustment, many of the variables included in the final model may be on the causal pathway between diet and outcomes. With that consideration, the current study suggests that more closely following a Mediterranean diet may be beneficial for individuals with CHD.
Abdominal obesity is related to several CV risk factors (e.g., hypertension, diabetes).25 The 2011 AHA/American College of Cardiology Foundation secondary prevention guidelines recommend an initial 5–10% body weight reduction to reduce obesity-associated CV risk factors (e.g., BP) and, in the long-term, achieving a BMI of 18.5 to 24.9 kg/m2 and waist circumference ≤88 and ≤102 cm for women and men, respectively.2 In the current study, there was no association between waist circumference and recurrent CHD or mortality. Studying obesity related outcomes in the population with CHD using an observational study design is challenging. An obesity paradox, wherein obesity is protective against recurrent CHD and death among individuals with CHD has been reported in several studies.26,27 Randomized controlled trials are needed to determine whether intentional weight reduction and maintenance are important for secondary prevention of CHD and all-cause mortality. Importantly, a long-term trial of an intensive lifestyle intervention designed to achieve long-term weight loss among individuals with type 2 diabetes was recently discontinued due to futility despite improvements in individual CV risk factors.28
In the context of primary prevention, maintaining more ideal lifestyle factors has been associated with reduced CHD and all-cause mortality risk.20,29 Although the AHA and the American Association of Cardiovascular and Pulmonary Rehabilitation recommend a multifaceted, comprehensive cardiac rehabilitation program for recovering cardiac patients, there are few data demonstrating a benefit from maintaining ideal levels of individual or multiple lifestyle factors among individuals living with CHD after the post-acute rehabilitation phase.17 The current study fills this evidence gap by demonstrating a strong association with reduced risk for recurrent CHD and all-cause mortality associated with having ideal levels of lifestyle factors. The effect size for recurrent CHD and all-cause mortality risk reduction with multiple ideal lifestyle factor levels is similar to that of statins and antihypertensive medication.30 Even though the majority of participants with CHD in the current study were taking statins and antihypertensive medication, ideal lifestyle factors maintained a strong protective association with reduced recurrent CHD and mortality. These data support the use of lifestyle modification as part of optimal medical management for patients with CHD.
The results from the current study should be interpreted in the context of potential limitations. CHD at baseline was self-reported and we were unable to confirm these reports. Additionally, given the design of REGARDS, we were unable to follow participants for outcomes starting on the date of their incident CHD event. Physical activity and Mediterranean diet adherence were self-reported and assessed at only 1 time point. There may be residual confounding as more complete ascertainment of socioeconomic status was not available. Despite these limitations, the current study has a number of strengths. These include the collection of information on multiple lifestyle factors using a standardized method, adjudication of recurrent CHD events, and the inclusion of a large nationwide population-based sample of black and white adults.
Acknowledgments
Funding sources: This research project is supported by a cooperative agreement U01 NS041588 from the National Institute of Neurological Disorders and Stroke and grants R01 HL080477 and K24 HL111154 from the National Heart, Lung and Blood Institute; National Institutes of Health, Department of Health and Human Service. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke; the National Heart, Lung and Blood Institute; or the National Institutes of Health. Representatives of the funding agency have been involved in the review of the manuscript but not directly involved in the collection, management, analysis or interpretation of the data. A full list of participating REGARDS investigators and institutions can be found at http://www.regardsstudy.org.
EBL, TMB, and PM: receive research support from NIH grant RO1 HL080477 and Amgen Inc.; MEF: receives research support from Amgen Inc.; MS: receives research support from NIH grants RO1 HL080477 and K24 HL111154 and Amgen Inc. John N. Booth, III and Paul Muntner had full access to all the data and take responsibility for the integrity of the data and accuracy of the data analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Study conception: JNB, PM, MS, MEF; Data collection: MS, TMB; Data analysis: JNB, PM, EBL; Manuscript writing: JNB; Critical manuscript review: JNB, PM, MS, EBL, TMB, MEF
Disclosures: JNB: None.;
References
- 1.Graham I, Atar D, Borch-Johnsen K, Boysen G, Burell G, Cifkova R, Dallongeville J, De Backer G, Ebrahim S, Gjelsvik B, Herrmann-Lingen C, Hoes A, Humphries S, Knapton M, Perk J, Priori SG, Pyorala K, Reiner Z, Ruilope L, Sans-Menendez S, Op Reimer WS, Weissberg P, Wood D, Yarnell J, Zamorano JL, Walma E, Fitzgerald T, Cooney MT, Dudina A, Vahanian A, Camm J, De Caterina R, Dean V, Dickstein K, Funck-Brentano C, Filippatos G, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL, Altiner A, Bonora E, Durrington PN, Fagard R, Giampaoli S, Hemingway H, Hakansson J, Kjeldsen SE, Larsen ML, Mancia G, Manolis AJ, Orth-Gomer K, Pedersen T, Rayner M, Ryden L, Sammut M, Schneiderman N, Stalenhoef AF, Tokgozoglu L, Wiklund O, Zampelas A European Society of C, European Association for Cardiovascular P, Rehabilitation, Council on Cardiovascular N, European Association for Study of D, International Diabetes Federation E, European Stroke I, International Society of Behavioural M, European Society of H, European Society of General Practice/Family M, European Heart N. European guidelines on cardiovascular disease prevention in clinical practice: executive summary. Fourth Joint Task Force of the European Society of Cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of nine societies and by invited experts) Eur J Cardiovasc Prev Rehabil. 2007;14(Suppl 2):E1–E40. doi: 10.1097/01.hjr.0000277984.31558.c4. [DOI] [PubMed] [Google Scholar]
- 2.Smith SC, Jr, Benjamin EJ, Bonow RO, Braun LT, Creager MA, Franklin BA, Gibbons RJ, Grundy SM, Hiratzka LF, Jones DW, Lloyd-Jones DM, Minissian M, Mosca L, Peterson ED, Sacco RL, Spertus J, Stein JH, Taubert KA World Heart F, the Preventive Cardiovascular Nurses A. AHA/ACCF Secondary Prevention and Risk Reduction Therapy for Patients with Coronary and other Atherosclerotic Vascular Disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation. Circulation. 2011;124:2458–2473. doi: 10.1161/CIR.0b013e318235eb4d. [DOI] [PubMed] [Google Scholar]
- 3.Leon AS, Franklin BA, Costa F, Balady GJ, Berra KA, Stewart KJ, Thompson PD, Williams MA, Lauer MS American Heart A, Council on Clinical C, Council on Nutrition PA, Metabolism, American association of C, Pulmonary R. Cardiac rehabilitation and secondary prevention of coronary heart disease: an American Heart Association scientific statement from the Council on Clinical Cardiology (Subcommittee on Exercise, Cardiac Rehabilitation, and Prevention) and the Council on Nutrition, Physical Activity, and Metabolism (Subcommittee on Physical Activity), in collaboration with the American association of Cardiovascular and Pulmonary Rehabilitation. Circulation. 2005;111:369–376. doi: 10.1161/01.CIR.0000151788.08740.5C. [DOI] [PubMed] [Google Scholar]
- 4.Arena R, Williams M, Forman DE, Cahalin LP, Coke L, Myers J, Hamm L, Kris-Etherton P, Humphrey R, Bittner V, Lavie CJ American Heart Association Exercise CR, Prevention Committee of the Council on Clinical Cardiology CoE, Prevention, Council on Nutrition PA, Metabolism. Increasing referral and participation rates to outpatient cardiac rehabilitation: the valuable role of healthcare professionals in the inpatient and home health settings: a science advisory from the American Heart Association. Circulation. 2012;125:1321–1329. doi: 10.1161/CIR.0b013e318246b1e5. [DOI] [PubMed] [Google Scholar]
- 5.Suaya JA, Shepard DS, Normand SL, Ades PA, Prottas J, Stason WB. Use of cardiac rehabilitation by Medicare beneficiaries after myocardial infarction or coronary bypass surgery. Circulation. 2007;116:1653–1662. doi: 10.1161/CIRCULATIONAHA.107.701466. [DOI] [PubMed] [Google Scholar]
- 6.Howard VJ, Cushman M, Pulley L, Gomez CR, Go RC, Prineas RJ, Graham A, Moy CS, Howard G. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology. 2005;25:135–143. doi: 10.1159/000086678. [DOI] [PubMed] [Google Scholar]
- 7.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J Ckd EPI. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Czernichow S, Kengne AP, Stamatakis E, Hamer M, Batty GD. Body mass index, waist circumference and waist-hip ratio: which is the better discriminator of cardiovascular disease mortality risk?: evidence from an individual-participant meta-analysis of 82 864 participants from nine cohort studies. Obes Rev. 2011;12:680–687. doi: 10.1111/j.1467-789X.2011.00879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. New Engl J Med. 2003;348:2599–2608. doi: 10.1056/NEJMoa025039. [DOI] [PubMed] [Google Scholar]
- 10.Luepker RV, Apple FS, Christenson RH, Crow RS, Fortmann SP, Goff D, Goldberg RJ, Hand MM, Jaffe AS, Julian DG, Levy D, Manolio T, Mendis S, Mensah G, Pajak A, Prineas RJ, Reddy KS, Roger VL, Rosamond WD, Shahar E, Sharrett AR, Sorlie P, Tunstall-Pedoe H Epidemiology AHACo, Prevention, Committee AHAS, World Heart Federation Council on E, Prevention, European Society of Cardiology Working Group on E, Prevention, Centers for Disease C, Prevention, National Heart L, Blood I. Case definitions for acute coronary heart disease in epidemiology and clinical research studies: a statement from the AHA Council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; the European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; the National Heart, Lung, and Blood Institute. Circulation. 2003;108:2543–2549. doi: 10.1161/01.CIR.0000100560.46946.EA. [DOI] [PubMed] [Google Scholar]
- 11.Safford MM, Brown TM, Muntner PM, Durant RW, Glasser S, Halanych JH, Shikany JM, Prineas RJ, Samdarshi T, Bittner VA, Lewis CE, Gamboa C, Cushman M, Howard V, Howard G Investigators R. Association of race and sex with risk of incident acute coronary heart disease events. JAMA. 2012;308:1768–1774. doi: 10.1001/jama.2012.14306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prineas RJ, Crow RS, Zhang Z-M. The Minnesota code manual of electrocardiographic findings including measurement and comparison with the Novacode standards and procedures for ECG measurement in epidemiologic and clinical trials. London; New York: Springer; 2010. p. xiii.p. 328. 1 online resource. [Google Scholar]
- 13.White IR, Royston P, Wood AM. Multiple imputation using chained equations: Issues and guidance for practice. Stat Med. 2011;30:377–399. doi: 10.1002/sim.4067. [DOI] [PubMed] [Google Scholar]
- 14.Palmer RC, McKinney S. Health care provider tobacco cessation counseling among current African American tobacco users. J Natl Med Assoc. 2011;103:660–667. doi: 10.1016/s0027-9684(15)30405-3. [DOI] [PubMed] [Google Scholar]
- 15.Rea TD, Heckbert SR, Kaplan RC, Smith NL, Lemaitre RN, Psaty BM. Smoking status and risk for recurrent coronary events after myocardial infarction. Ann Intern Med. 2002;137:494–500. doi: 10.7326/0003-4819-137-6-200209170-00009. [DOI] [PubMed] [Google Scholar]
- 16.Critchley JA, Capewell S. Mortality risk reduction associated with smoking cessation in patients with coronary heart disease: a systematic review. JAMA. 2003;290:86–97. doi: 10.1001/jama.290.1.86. [DOI] [PubMed] [Google Scholar]
- 17.Balady GJ, Ades PA, Comoss P, Limacher M, Pina IL, Southard D, Williams MA, Bazzarre T. Core components of cardiac rehabilitation/secondary prevention programs: A statement for healthcare professionals from the American Heart Association and the American Association of Cardiovascular and Pulmonary Rehabilitation Writing Group. Circulation. 2000;102:1069–1073. doi: 10.1161/01.cir.102.9.1069. [DOI] [PubMed] [Google Scholar]
- 18.Cottin Y, Cambou JP, Casillas JM, Ferrieres J, Cantet C, Danchin N. Specific profile and referral bias of rehabilitated patients after an acute coronary syndrome. J Cardiopulm Rehabil. 2004;24:38–44. doi: 10.1097/00008483-200401000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Lavie CJ, Thomas RJ, Squires RW, Allison TG, Milani RV. Exercise training and cardiac rehabilitation in primary and secondary prevention of coronary heart disease. Mayo Clin Proc. 2009;84:373–383. doi: 10.1016/S0025-6196(11)60548-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB on behalf of the American Heart Association Statistics C, Stroke Statistics S. Heart Disease and Stroke Statistics--2013 Update: A Report From the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perk J, De Backer G, Gohlke H, Graham I, Reiner Z, Verschuren M, Albus C, Benlian P, Boysen G, Cifkova R, Deaton C, Ebrahim S, Fisher M, Germano G, Hobbs R, Hoes A, Karadeniz S, Mezzani A, Prescott E, Ryden L, Scherer M, Syvanne M, Scholte op Reimer WJ, Vrints C, Wood D, Zamorano JL, Zannad F European Association for Cardiovascular P, Rehabilitation, Guidelines ESCCfP. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts) Eur Heart J. 2012;33:1635–1701. doi: 10.1093/eurheartj/ehs092. [DOI] [PubMed] [Google Scholar]
- 22.Sofi F, Abbate R, Gensini GF, Casini A. Accruing evidence on benefits of adherence to the Mediterranean diet on health: an updated systematic review and meta-analysis. Am J Clin Nutr. 2010;92:1189–1196. doi: 10.3945/ajcn.2010.29673. [DOI] [PubMed] [Google Scholar]
- 23.Serra-Majem L, Roman B, Estruch R. Scientific evidence of interventions using the Mediterranean diet: a systematic review. Nutr Rev. 2006;64:S27–S47. doi: 10.1111/j.1753-4887.2006.tb00232.x. [DOI] [PubMed] [Google Scholar]
- 24.Estruch R, Ros E, Salas-Salvado J, Covas MI, Corella D, Aros F, Gomez-Gracia E, Ruiz-Gutierrez V, Fiol M, Lapetra J, Lamuela-Raventos RM, Serra-Majem L, Pinto X, Basora J, Munoz MA, Sorli JV, Martinez JA, Martinez-Gonzalez MA Investigators PS. Primary prevention of cardiovascular disease with a Mediterranean diet. New Engl J Med. 2013;368:1279–1290. doi: 10.1056/NEJMc1806491. [DOI] [PubMed] [Google Scholar]
- 25.Klein S, Burke LE, Bray GA, Blair S, Allison DB, Pi-Sunyer X, Hong Y, Eckel RH American Heart Association Council on Nutrition PA, Metabolism. Clinical implications of obesity with specific focus on cardiovascular disease: a statement for professionals from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism: endorsed by the American College of Cardiology Foundation. Circulation. 2004;110:2952–2967. doi: 10.1161/01.CIR.0000145546.97738.1E. [DOI] [PubMed] [Google Scholar]
- 26.Bashey S, Muntner P, Kini AS, Esquitin R, Razzouk L, Mathewkutty S, Wildman RP, Carson AP, Kim MC, Moreno PR, Sharma SK, Farkouh ME. Clustering of metabolic abnormalities among obese patients and mortality after percutaneous coronary intervention. Am J Cardiol. 2011;107:1415–1420. doi: 10.1016/j.amjcard.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 27.Villareal DT, Apovian CM, Kushner RF, Klein S American Society for N, Naaso TOS. Obesity in older adults: technical review and position statement of the American Society for Nutrition and NAASO, The Obesity Society. Am J Clin Nutr. 2005;82:923–934. doi: 10.1093/ajcn/82.5.923. [DOI] [PubMed] [Google Scholar]
- 28.Weight loss does not lower heart disease risk from type 2 diabetes. U.S. Department of Health and Human Services National Institutes of Health (NIH) News: National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) 2012 [Google Scholar]
- 29.Yang Q, Cogswell ME, Flanders WD, Hong Y, Zhang Z, Loustalot F, Gillespie C, Merritt R, Hu FB. Trends in cardiovascular health metrics and associations with all-cause and CVD mortality among US adults. JAMA. doi: 10.1001/jama.2012.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cholesterol Treatment Trialists C. Mihaylova B, Emberson J, Blackwell L, Keech A, Simes J, Barnes EH, Voysey M, Gray A, Collins R, Baigent C. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet. 2012;380:581–590. doi: 10.1016/S0140-6736(12)60367-5. [DOI] [PMC free article] [PubMed] [Google Scholar]