Abstract
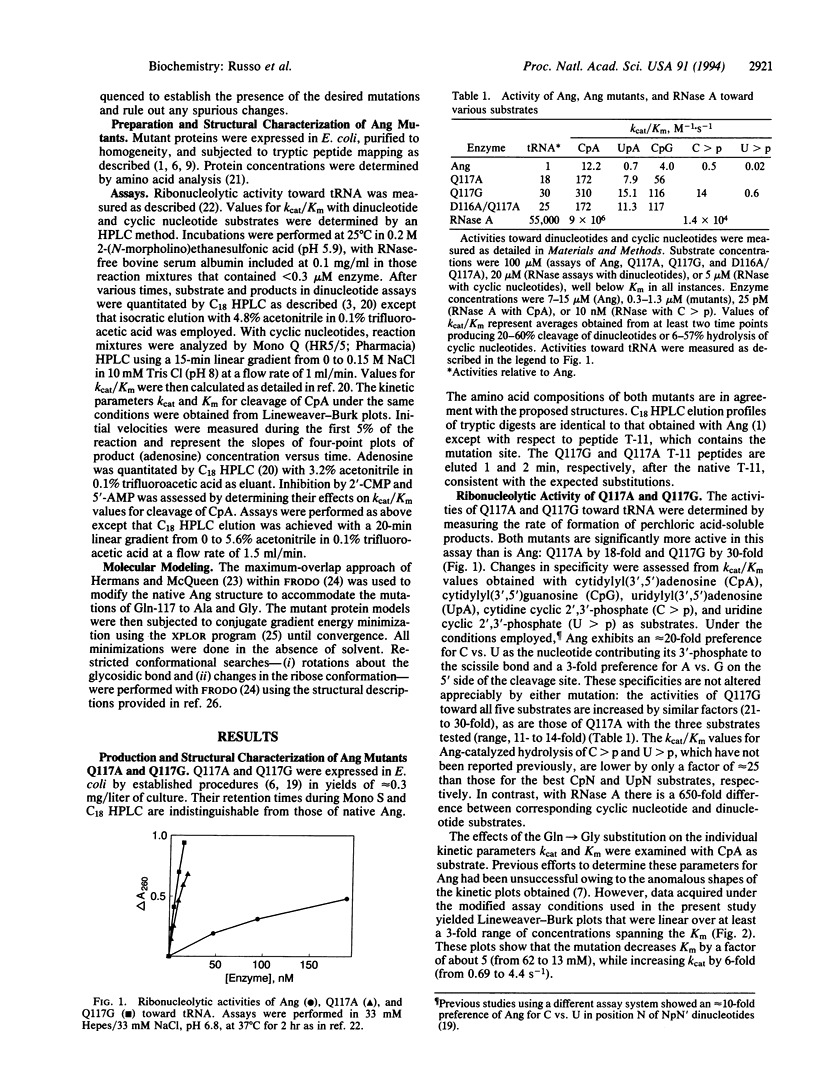
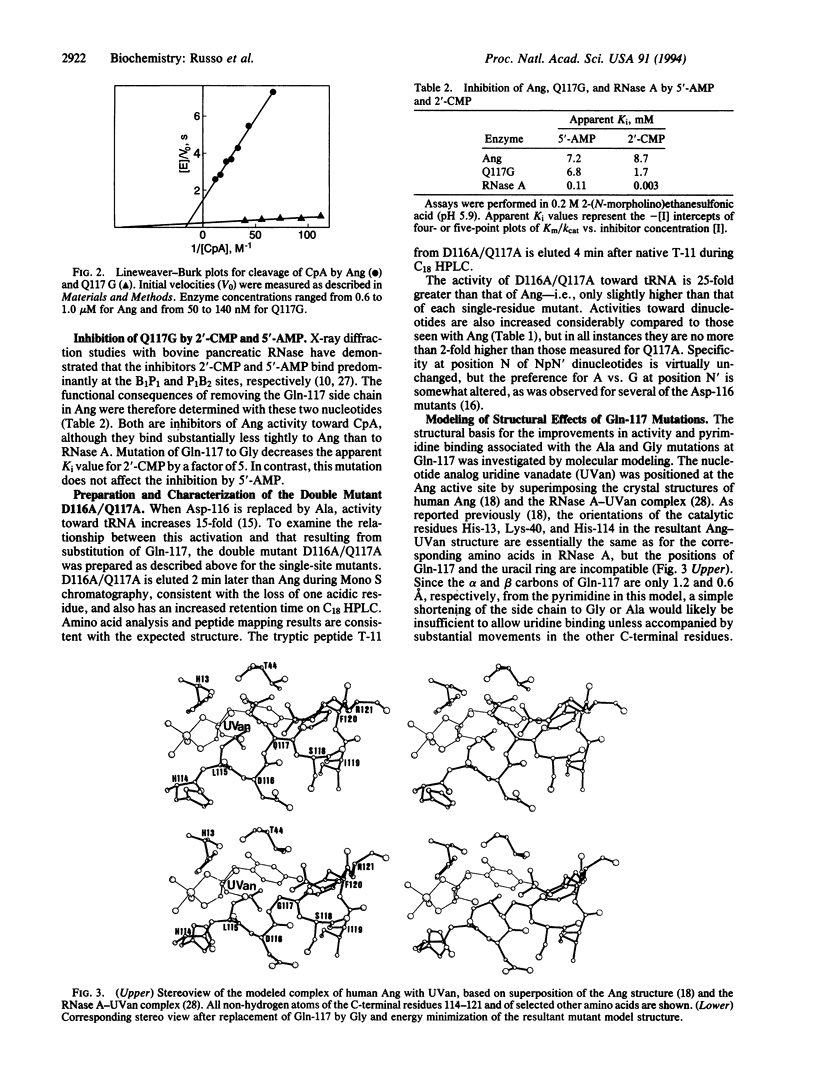
The crystal structure of human angiogenin (reported in the preceding paper in this issue) reveals that the site that corresponds to the pyrimidine binding site of RNase A is obstructed by Gln-117. Mutation of this residue to Ala and Gly is here found to increase activity 11- to 18-fold and 21- to 30-fold, respectively, toward dinucleotide, polynucleotide, and cyclic nucleotide substrates, but without changing specificity. The enhanced activity of Q117G toward CpA is due to a 5-fold decrease in Km and a 6-fold increase in kcat. Its Ki value for 2'-CMP is 5-fold lower than that of native angiogenin, whereas its Ki value for 5'-AMP is unchanged. It has been reported previously that mutating Asp-116 to Ala increases activity 15-fold. The double mutant D116A/Q117A is shown to be only slightly more active than each individual mutant. The present results demonstrate that Gln-117 impedes the ribonucleolytic activity of angiogenin, as predicted by x-ray crystallography. Moreover, they suggest that prior to or during catalysis angiogenin must undergo a conformational change to reorient the C-terminal segment that contains this residue, and that a similar reorganization is required for the mutants as well. This view is supported by molecular modeling of an angiogenin-uridine vanadate complex. These in vitro findings have implications for the angiogenic activity of angiogenin in vivo.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acharya K. R., Shapiro R., Allen S. C., Riordan J. F., Vallee B. L. Crystal structure of human angiogenin reveals the structural basis for its functional divergence from ribonuclease. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):2915–2919. doi: 10.1073/pnas.91.8.2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borah B., Chen C. W., Egan W., Miller M., Wlodawer A., Cohen J. S. Nuclear magnetic resonance and neutron diffraction studies of the complex of ribonuclease A with uridine vanadate, a transition-state analogue. Biochemistry. 1985 Apr 9;24(8):2058–2067. doi: 10.1021/bi00329a038. [DOI] [PubMed] [Google Scholar]
- Borkakoti N. The active site of ribonuclease A from the crystallographic studies of ribonuclease-A-inhibitor complexes. Eur J Biochem. 1983 Apr 15;132(1):89–94. doi: 10.1111/j.1432-1033.1983.tb07329.x. [DOI] [PubMed] [Google Scholar]
- Cohen S. A., Michaud D. P. Synthesis of a fluorescent derivatizing reagent, 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate, and its application for the analysis of hydrolysate amino acids via high-performance liquid chromatography. Anal Biochem. 1993 Jun;211(2):279–287. doi: 10.1006/abio.1993.1270. [DOI] [PubMed] [Google Scholar]
- Curran T. P., Shapiro R., Riordan J. F. Alteration of the enzymatic specificity of human angiogenin by site-directed mutagenesis. Biochemistry. 1993 Mar 9;32(9):2307–2313. doi: 10.1021/bi00060a023. [DOI] [PubMed] [Google Scholar]
- Curran T. P., Shapiro R., Riordan J. F., Vallee B. L. Modulation of the activity of angiogenin by mutagenesis at Asp-116. Biochim Biophys Acta. 1993 Oct 6;1202(2):281–286. doi: 10.1016/0167-4838(93)90017-l. [DOI] [PubMed] [Google Scholar]
- Fett J. W., Strydom D. J., Lobb R. R., Alderman E. M., Bethune J. L., Riordan J. F., Vallee B. L. Isolation and characterization of angiogenin, an angiogenic protein from human carcinoma cells. Biochemistry. 1985 Sep 24;24(20):5480–5486. doi: 10.1021/bi00341a030. [DOI] [PubMed] [Google Scholar]
- Hallahan T. W., Shapiro R., Strydom D. J., Vallee B. L. Importance of asparagine-61 and asparagine-109 to the angiogenic activity of human angiogenin. Biochemistry. 1992 Sep 1;31(34):8022–8029. doi: 10.1021/bi00149a036. [DOI] [PubMed] [Google Scholar]
- Hallahan T. W., Shapiro R., Vallee B. L. Dual site model for the organogenic activity of angiogenin. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2222–2226. doi: 10.1073/pnas.88.6.2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper J. W., Auld D. S., Riordan J. F., Vallee B. L. Enzymatically active angiogenin/ribonuclease A hybrids formed by peptide interchange. Biochemistry. 1988 Jan 12;27(1):219–226. doi: 10.1021/bi00401a033. [DOI] [PubMed] [Google Scholar]
- Harper J. W., Vallee B. L. Mutagenesis of aspartic acid-116 enhances the ribonucleolytic activity and angiogenic potency of angiogenin. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7139–7143. doi: 10.1073/pnas.85.19.7139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges R. S., Merrifield R. B. Synthetic study of the effect of tyrosine at position 120 of ribonuclease. Int J Pept Protein Res. 1974;6(6):397–405. doi: 10.1111/j.1399-3011.1974.tb02401.x. [DOI] [PubMed] [Google Scholar]
- Hodges R. S., Merrifield R. B. The role of serine-123 in the activity and specificity of ribonuclease. Reactivation of ribonuclease 1-118 by the synthetic COOH-terminal tetradecapeptide, ribonuclease 111-124, and its O-methylserine and alanine analogs. J Biol Chem. 1975 Feb 25;250(4):1231–1241. [PubMed] [Google Scholar]
- Howlin B., Harris G. W., Moss D. S., Palmer R. A. X-ray refinement study on the binding of cytidylic acid (2'-CMP) to ribonuclease A. J Mol Biol. 1987 Jul 5;196(1):159–164. doi: 10.1016/0022-2836(87)90518-3. [DOI] [PubMed] [Google Scholar]
- Jones T. A. Diffraction methods for biological macromolecules. Interactive computer graphics: FRODO. Methods Enzymol. 1985;115:157–171. doi: 10.1016/0076-6879(85)15014-7. [DOI] [PubMed] [Google Scholar]
- Moroianu J., Riordan J. F. Nuclear translocation of angiogenin in proliferating endothelial cells is essential to its angiogenic activity. Proc Natl Acad Sci U S A. 1994 Mar 1;91(5):1677–1681. doi: 10.1073/pnas.91.5.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parés X., Nogués M. V., de Llorens R., Cuchillo C. M. Structure and function of ribonuclease A binding subsites. Essays Biochem. 1991;26:89–103. [PubMed] [Google Scholar]
- Shapiro R., Fett J. W., Strydom D. J., Vallee B. L. Isolation and characterization of a human colon carcinoma-secreted enzyme with pancreatic ribonuclease-like activity. Biochemistry. 1986 Nov 18;25(23):7255–7264. doi: 10.1021/bi00371a002. [DOI] [PubMed] [Google Scholar]
- Shapiro R., Fox E. A., Riordan J. F. Role of lysines in human angiogenin: chemical modification and site-directed mutagenesis. Biochemistry. 1989 Feb 21;28(4):1726–1732. doi: 10.1021/bi00430a045. [DOI] [PubMed] [Google Scholar]
- Shapiro R., Harper J. W., Fox E. A., Jansen H. W., Hein F., Uhlmann E. Expression of Met-(-1) angiogenin in Escherichia coli: conversion to the authentic less than Glu-1 protein. Anal Biochem. 1988 Dec;175(2):450–461. doi: 10.1016/0003-2697(88)90569-6. [DOI] [PubMed] [Google Scholar]
- Shapiro R., Riordan J. F., Vallee B. L. Characteristic ribonucleolytic activity of human angiogenin. Biochemistry. 1986 Jun 17;25(12):3527–3532. doi: 10.1021/bi00360a008. [DOI] [PubMed] [Google Scholar]
- Shapiro R., Vallee B. L. Identification of functional arginines in human angiogenin by site-directed mutagenesis. Biochemistry. 1992 Dec 15;31(49):12477–12485. doi: 10.1021/bi00164a026. [DOI] [PubMed] [Google Scholar]
- Shapiro R., Vallee B. L. Site-directed mutagenesis of histidine-13 and histidine-114 of human angiogenin. Alanine derivatives inhibit angiogenin-induced angiogenesis. Biochemistry. 1989 Sep 5;28(18):7401–7408. doi: 10.1021/bi00444a038. [DOI] [PubMed] [Google Scholar]
- Shapiro R., Weremowicz S., Riordan J. F., Vallee B. L. Ribonucleolytic activity of angiogenin: essential histidine, lysine, and arginine residues. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8783–8787. doi: 10.1073/pnas.84.24.8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strydom D. J., Fett J. W., Lobb R. R., Alderman E. M., Bethune J. L., Riordan J. F., Vallee B. L. Amino acid sequence of human tumor derived angiogenin. Biochemistry. 1985 Sep 24;24(20):5486–5494. doi: 10.1021/bi00341a031. [DOI] [PubMed] [Google Scholar]
- Trautwein K., Holliger P., Stackhouse J., Benner S. A. Site-directed mutagenesis of bovine pancreatic ribonuclease: lysine-41 and aspartate-121. FEBS Lett. 1991 Apr 9;281(1-2):275–277. doi: 10.1016/0014-5793(91)80410-5. [DOI] [PubMed] [Google Scholar]
- Wodak S. Y. The structure of cytidilyl(2',5')adenosine when bound to pancreatic ribonuclease S. J Mol Biol. 1977 Nov;116(4):855–875. doi: 10.1016/0022-2836(77)90275-3. [DOI] [PubMed] [Google Scholar]
- deMel V. S., Martin P. D., Doscher M. S., Edwards B. F. Structural changes that accompany the reduced catalytic efficiency of two semisynthetic ribonuclease analogs. J Biol Chem. 1992 Jan 5;267(1):247–256. [PubMed] [Google Scholar]



