Abstract
Purpose
To investigate the impact of modern postoperative radiotherapy (PORT) on overall survival (OS) for patients with N2 non–small-cell lung cancer (NSCLC) treated nationally with surgery and adjuvant chemotherapy.
Patients and Methods
Patients with pathologic N2 NSCLC who underwent complete resection and adjuvant chemotherapy from 2006 to 2010 were identified from the National Cancer Data Base and stratified by use of PORT (≥ 45 Gy). A total of 4,483 patients were identified (PORT, n = 1,850; no PORT, n = 2,633). The impact of patient and treatment variables on OS was explored using Cox regression.
Results
Median follow-up time was 22 months. On univariable analysis, improved OS correlated with younger age, treatment at an academic facility, female sex, urban population, higher income, lower Charlson comorbidity score, smaller tumor size, multiagent chemotherapy, resection with at least a lobectomy, and PORT. On multivariable analysis, improved OS remained independently predicted by younger age, female sex, urban population, lower Charlson score, smaller tumor size, multiagent chemotherapy, resection with at least a lobectomy, and PORT (hazard ratio, 0.886; 95% CI, 0.798 to 0.988). Use of PORT was associated with an increase in median and 5-year OS compared with no PORT (median OS, 45.2 v 40.7 months, respectively; 5-year OS, 39.3% [95% CI, 35.4% to 43.5%] v 34.8% [95% CI, 31.6% to 38.3%], respectively; P = .014).
Conclusion
For patients with N2 NSCLC after complete resection and adjuvant chemotherapy, modern PORT seems to confer an additional OS advantage beyond that achieved with adjuvant chemotherapy alone.
INTRODUCTION
More than a quarter million patients in the United States and more than 1 million patients worldwide are diagnosed with lung cancer each year, of which the overwhelming majority is non–small-cell lung cancer (NSCLC).1,2 Although 5-year overall survival (OS) ranges from 58% to 73% for completely resected pathologic stage I disease, OS decreases precipitously to less than 25% for pathologic stage III disease.3 For patients with node-positive disease at the time of resection, meta-analysis data demonstrate that adjuvant platinum-based chemotherapy has been shown to decrease distant metastases and locoregional recurrence (LRR), resulting in an approximately 5% OS advantage.4 Adjuvant chemotherapy is now considered standard of care for patients with resected node-positive NSCLC.
Patients with node-positive disease have a 20% to 40% risk of LRR, and LRR correlates independently with worse OS for patients with NSCLC.4,5 Thus, postoperative radiotherapy (PORT) holds great appeal as a means to reduce LRR and improve OS. However, a meta-analysis of randomized PORT trials demonstrated a decrease in OS with the use of PORT, which was felt to be related to the cardiac and pulmonary toxicity from the radiotherapy (RT) itself.6 Notably, most of these trials, conducted principally in the 1960s to 1970s, included outmoded RT techniques and doses. Significant advances in RT delivery over the last several decades have been theorized to reduce the toxicity of PORT, although this has not been confirmed prospectively.
Two Surveillance, Epidemiology, and End Results (SEER) analyses and a secondary analysis of data from the Adjuvant Navelbine International Trialist Association (ANITA) trial suggest that PORT may be safely delivered in a modern cohort of patients with a potential OS benefit for stage IIIA (N2) disease.7–9 Although the ANITA secondary analysis did include patients treated with PORT in the setting of adjuvant chemotherapy, no formal statistical analysis was performed. Neither the SEER studies nor older randomized trials reported data on chemotherapy. Therefore, the impact of modern PORT for resected stage IIIA (N2) NSCLC in the setting of standard adjuvant chemotherapy remains unknown.
The National Cancer Data Base (NCDB) is a joint program of the Commission on Cancer of the American College of Surgeons and the American Cancer Society. The NCDB draws from more than 1,500 commission-accredited cancer programs and captures approximately 70% of all newly diagnosed patients with cancer in the United States. Data elements are collected and submitted to the NCDB from commission-accredited oncology registries using standardized coding and data item definitions, including details not available from prior SEER analyses for this population, such as use and timing of chemotherapy, RT dose, surgical margin status, and comorbidity.10 We queried the NCDB to study the impact of modern PORT in the setting of standard-of-care adjuvant chemotherapy for pathologic stage IIIA (N2) NSCLC.
PATIENTS AND METHODS
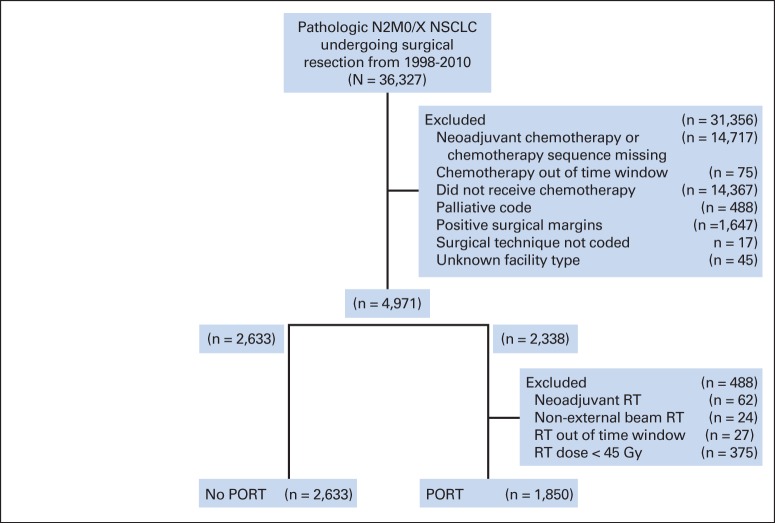
This study was exempted by the institutional review board. Deidentified data for patients with pathologic stage IIIA (N2) NSCLC were obtained from the NCDB participant user file for patients treated from 1998 to 2010. Patients with primarily resected NSCLC found to have pN2 disease treated with adjuvant chemotherapy were identified and stratified by use of PORT. Patients were excluded if they were treated with neoadjuvant chemotherapy or RT, were missing data on the timing of adjuvant therapy, had evidence of metastatic disease, were treated with palliative intent (as coded), or had incomplete resection. Patients receiving less than 45 Gy of PORT were excluded to further eliminate palliative intent therapy. To isolate patients treated with adjuvant (rather than salvage) therapy, only patients for whom at least either chemotherapy or PORT was initiated within 120 days of surgery were included. PORT was allowed to start up to 240 days after surgery to allow for reasonable delays in initiation and delivery of chemotherapy. Inclusion and exclusion criteria are summarized in the CONSORT diagram (Fig 1).
Fig 1.
CONSORT diagram. NSCLC, non–small-cell lung cancer; PORT, postoperative radiotherapy; RT, radiotherapy.
Patient, tumor, and treatment information was extracted and dichotomized when necessary. For example, patients were dichotomized as white or nonwhite, having an income greater than or less than $35,000, and living in an urban location (metro population > 250,000) or nonurban location (metro population < 250,000 or nonmetro population). The facility type to which each patient presented was dichotomized as academic or nonacademic (community cancer program or comprehensive community cancer program). Patient comorbidities were assessed using the Charlson comorbidity index, as adapted by Deyo et al,11 and were scored as 0, 1, or ≥ 2. Surgical operations were categorized as sublobar, lobectomy, or pneumonectomy. The sublobar group consisted of excisions less than one lobe, including wedge resections and segmental resections. Additional categorical variables examined included sex, readmission after surgery, chemotherapy type (single agent or multiagent), clinical stage, and use of PORT. Continuous variables examined included age, great circle distance (0 = same zip code as facility), days of inpatient stay, tumor size, and days between surgery and chemotherapy.
Categorical variables were compared using χ2 tests. Continuous variables were compared using independent sample t tests. All descriptive statistics were expressed as medians with ranges unless otherwise noted. The primary end point was OS, which was defined as the time from diagnosis to death. The Kaplan-Meier method was used to determine OS, and the Cox proportional hazards model was used to determine significant contributors to OS. Variables were included in the multivariable analysis only if significant on univariable analysis. Proportional hazards assumption for the variables was checked graphically using log-log survival plots. Inverse probability adjusted Kaplan-Meier survival curves were created using the method described by Cole and Hernan.12 All analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC). All P values were two-sided, and P < .05 was considered statistically significant.
RESULTS
A total of 4,483 patients treated from 2006 to 2010 for pathologic N2 NSCLC were identified using the aforementioned selection criteria; 1,850 of these patients (41.3%) received PORT (Fig 1). Although the original data set included patients treated from 1998 to 2010, timing of chemotherapy (as confirmed by a two-part check of both the Systemic Surgery Sequence and Chemotherapy, Days From Dx data items) was not coded before 2006; therefore, the cohort was functionally limited to 2006 to 2010. Use of PORT was stable over this time period (median, 41.3% per year; range, 39.8% to 43.3%).
For the group as a whole, 34.5% of patients had clinical stage I or II disease before surgery, 29.9% of patients had clinical stage III disease, and the remainder of patients had unknown stage or unstaged disease (35.0%) or clinical stage IV disease (0.4%). Median inpatient stay was 5 days, and the readmission rate was 6.4%. The overwhelming majority of patients (87.3%) received doublet chemotherapy a median of 44 days (range, 0 to 210 days) after surgery. For patients treated with PORT, the median dose was 54 Gy (range, 45 to 82.8 Gy), with 17.7% of patients receiving more than 60 Gy. PORT was delivered over a median of 43 days (range, 26 to 231 days) and was started a median of 73 days (range, 5 to 240 days) after surgery. There was significant variability in the relative timing of chemotherapy and PORT start dates after surgery. Of the 1,707 patients for whom the relative timing of chemotherapy and PORT could be calculated, only 47 patients (2.8%) had PORT delivered more than 45 days before start of chemotherapy. The majority of patients (n = 929, 54.4%) started PORT within 45 days before or after chemotherapy; the start date was within 7 days of chemotherapy in 691 patients (40.5%). The remainder of patients (n = 731, 42.8%) started PORT more than 45 days after chemotherapy; the start date was more than 90 days after chemotherapy in 532 patients (31.2%).
A comparison of patient and tumor characteristics in patients treated with PORT or no PORT is presented in Table 1. With the large number of patients, there were many statistically significant, although numerically small, differences between the treatment groups. Patients treated with PORT were younger and had lower Charlson comorbidity scores, although they were less likely to be treated at an academic facility. PORT patients were less likely to live in an urban area and traveled a slightly smaller median distance to the hospital. Patients treated with PORT had fewer pneumonectomies and more sublobar resections, smaller tumors, and a shorter median inpatient stay. Patients in the PORT group had a higher proportion of clinical stage III stage and unknown clinical stage. Median time between surgery and chemotherapy was shorter for the PORT group, and chemotherapy type was more likely to be documented. There was no significant difference between the groups in sex, race, income, or readmission rates after surgery.
Table 1.
Baseline Demographics and Clinical Characteristics for Patients Treated With and Without PORT
| Demographic or Clinical Characteristic | Patients (N = 4,483) |
P | |||
|---|---|---|---|---|---|
| No PORT (n = 2,633) |
PORT (n = 1,850) |
||||
| No. | % | No. | % | ||
| Age at diagnosis, years | < .001 | ||||
| Median | 66 | 64 | |||
| Range | 27-89 | 19-89 | |||
| Male | 1,226 | 46.6 | 868 | 46.9 | .831 |
| White | 2,284 | 86.7 | 1,616 | 87.4 | .558 |
| Charlson/Deyo comorbidity score | < .001 | ||||
| 0 | 1,452 | 55.1 | 1,129 | 61.0 | |
| 1 | 858 | 32.6 | 534 | 28.9 | |
| 2 | 323 | 12.3 | 187 | 10.1 | |
| Income ≥ $35,000 (n = 4,243) | 1,733 | 69.7 | 1,219 | 69.5 | .892 |
| Urban population | 1,787 | 67.9 | 1,202 | 65.0 | .046 |
| Great circle distance, miles (n = 4,279) | < .001 | ||||
| Median | 10 | 9 | |||
| Range | 0-9,454 | 0-9,382 | |||
| Facility | < .001 | ||||
| Nonacademic | 1,559 | 59.2 | 1,268 | 68.5 | |
| Academic | 1,074 | 40.8 | 582 | 31.5 | |
| Clinical stage group | < .001 | ||||
| 0 | 1 | 0.0 | 2 | 0.1 | |
| I | 701 | 26.6 | 413 | 22.3 | |
| II | 279 | 10.6 | 155 | 8.4 | |
| III | 751 | 28.5 | 589 | 31.8 | |
| IV | 13 | 0.5 | 5 | 0.3 | |
| Occult | 7 | 0.3 | 0 | 0.0 | |
| Unknown | 881 | 33.5 | 686 | 37.1 | |
| Surgery type | < .001 | ||||
| Sublobar | 262 | 10.0 | 303 | 16.4 | |
| Lobectomy | 2,109 | 80.1 | 1,439 | 77.8 | |
| Pneumonectomy | 262 | 10.0 | 108 | 5.8 | |
| Tumor size, cm (n = 4,445) | < .001 | ||||
| Median | 31 | 29 | |||
| Range | 0-170 | 2-180 | |||
| Surgical inpatient stay, days (n = 3,953) | < .001 | ||||
| Median | 6 | 5 | |||
| Range | 0-101 | 0-128 | |||
| Readmission (n = 4,298) | 166 | 6.5 | 120 | 6.9 | .663 |
| Chemotherapy type | .011 | ||||
| Not documented | 260 | 9.9 | 135 | 7.3 | |
| Single-agent chemotherapy | 95 | 3.6 | 78 | 4.2 | |
| Multiagent chemotherapy | 2,278 | 86.5 | 1,637 | 88.5 | |
| Time between surgery and chemotherapy, days (n = 4,035) | < .001 | ||||
| Median | 46 | 42 | |||
| Range | 0-120 | 0-210 | |||
| PORT dose, Gy (n = 1,850) | |||||
| Median | 54 | ||||
| Range | 45-82.8 | ||||
| Time between surgery and PORT, days (n = 1,781) | |||||
| Median | 73 | ||||
| Range | 5-240 | ||||
Abbreviation: PORT, postoperative radiotherapy.
Median follow-up for both groups was 22 months (range, 0 to 72 months). Factors associated with improved OS on univariable analysis included younger age, treatment at an academic facility, female sex, urban population, higher income, lower Charlson score, smaller tumor size, use of multiagent chemotherapy, resection with at least a lobectomy, and use of PORT (Table 2). Factors that remained independently associated with improved OS on multivariable analysis included younger age, female sex, urban population, lower Charlson score, smaller tumor size, use of multiagent chemotherapy, resection with at least a lobectomy, and use of PORT (hazard ratio, 0.888; 95% CI, 0.798 to 0.988).
Table 2.
Univariable and Multivariable Analyses of Predictors of Overall Survival
| Variable | Univariable Analysis |
Multivariable Analysis |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Age | 1.019 | 1.014 to 1.024 | < .001 | 1.017 | 1.011 to 1.022 | < .001 |
| Facility (academic v nonacademic) | 0.901 | 0.816 to 0.994 | .038 | NS | ||
| Sex (male v female) | 1.450 | 1.319 to 1.594 | < .001 | 1.379 | 1.242 to 1.531 | < .001 |
| Race (white v nonwhite) | 1.083 | 0.937 to 1.251 | .279 | |||
| Income (≥ v < $35,000) | 0.864 | 0.780 to 0.958 | .006 | NS | ||
| Population (urban v nonurban) | 0.830 | 0.752 to 0.915 | < .001 | 0.827 | 0.741 to 0.921 | .001 |
| Great circle distance | 1.000 | 1.000 to 1.000 | .075 | |||
| Charlson score | ||||||
| 1 v 0 | 1.168 | 1.052 to 1.296 | .004 | 1.137 | 1.014 to 1.274 | .028 |
| 2 v 0 | 1.335 | 1.154 to 1.544 | < .001 | 1.283 | 1.097 to 1.502 | .002 |
| Tumor size | 1.007 | 1.005 to 1.009 | < .001 | 1.008 | 1.005 to 1.010 | < .001 |
| Surgical inpatient stay | 1.005 | 0.998 to 1.013 | .161 | |||
| Chemotherapy (multiagent v single agent) | 0.686 | 0.546 to 0.861 | .001 | 0.678 | 0.536 to 0.857 | .001 |
| Days between surgery and chemotherapy | 1.0002 | 1.000 to 1.004 | .101 | |||
| Readmission | 1.149 | 0.958 to 1.378 | .135 | |||
| Lobectomy v sublobar | 0.685 | 0.599 to 0.783 | < .001 | 0.581 | 0.501 to 0.675 | < .001 |
| Pneumonectomy v sublobar | 0.799 | 0.656 to 0.973 | .026 | 0.625 | 0.497 to 0.785 | < .001 |
| PORT v no PORT | 0.873 | 0.794 to 0.961 | .005 | 0.888 | 0.798 to 0.988 | .029 |
NOTE. HRs are only reported on multivariable analysis if they remained significant.
Abbreviations: HR, hazard ratio; NS, not significant; PORT, postoperative radiotherapy.
Use of PORT, compared with no PORT, was associated with a significant increase in median OS (45.2 v 40.7 months, respectively), 3-year OS (59.3% [95% CI, 56.6% to 62.0%] v 55.2% [95% CI, 52.9% to 57.6%], respectively), and 5-year OS (39.3% [95% CI, 35.4% to 43.5%] v 34.8% [95% CI, 31.6% to 38.3%], respectively; P = .014; Fig 2A). To visualize the direct effect of PORT after the inclusion of all other variables significantly associated with OS on multivariable analysis, inverse probability adjusted Kaplan-Meier survival curves were calculated. The adjusted median, 3-year, and 5-year OS for PORT versus no PORT was 45.2 v 40.9 months, respectively; 59.9% (95% CI, 57.1% to 62.8%) v 55.7% (95% CI, 53.3% to 58.2%), respectively; and 38.4% (95% CI, 34.2% to 43.2%) v 34.6% (95% CI, 31.1% to 38.4%), respectively (P = .027; Fig 2B).
Fig 2.
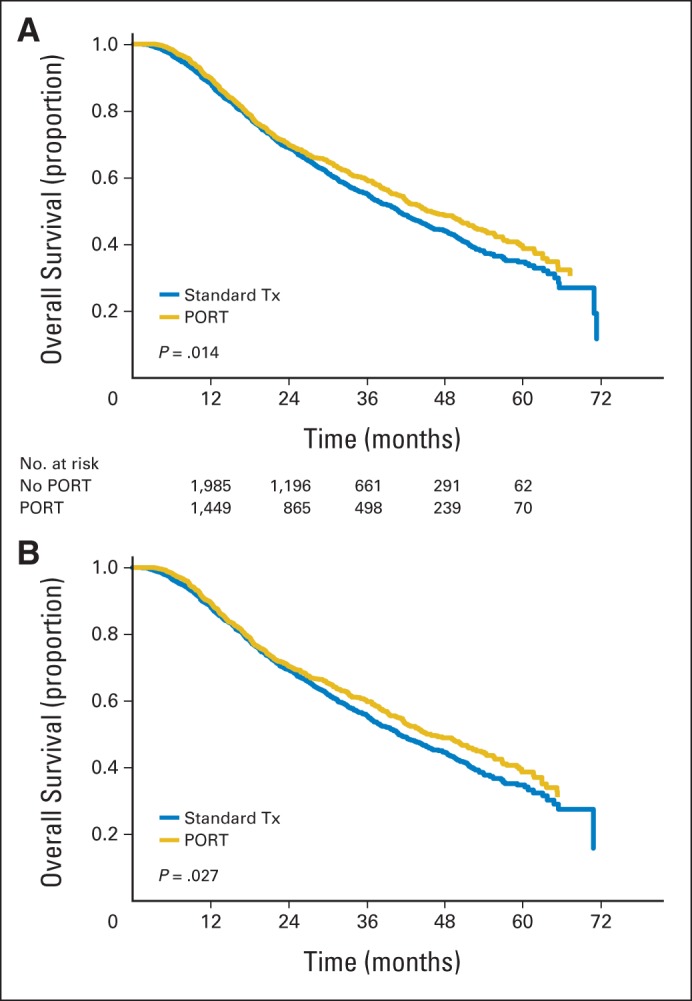
(A) Kaplan-Meier overall survival stratified by use of postoperative radiotherapy (PORT). (B) Adjusted Kaplan-Meier overall survival stratified by use of PORT. Tx, therapy.
DISCUSSION
Using a large population-based registry of patients with completely resected stage IIIA (N2) NSCLC, our results suggest an improvement in OS with PORT compared with standard-of-care adjuvant chemotherapy alone. The absolute survival improvement, although modest, is on par with that reported with adjuvant platinum-based chemotherapy in several large meta-analyses, which has now become standard of care for resected node-positive NSCLC.
Prior studies of PORT have been largely unsuccessful, with a meta-analysis of randomized trials demonstrating an overall 5% reduction in OS with PORT, driven principally by negative outcomes in patients with N0 or N1 disease and no benefit in N2 disease.6 The lack of benefit to PORT was felt to be in large part a result of competing cardiac and pulmonary toxicity from RT, with noncancer death rates of 18% and 11% with and without PORT, respectively, in the PORT meta-analysis. Whether such results are applicable in the current era remains controversial. The overwhelming majority of trials within the PORT meta-analysis were conducted in the 1960s or 1970s with outdated equipment (cobalt-60), outdated techniques, use of large treatment volumes, lack of computed tomography planning in the majority, and dose and dose per fraction considered unusual by today's standards. A recent meta-analysis of PORT trials stratified by use of cobalt-60 versus more modern linear accelerator (linac) –based treatment found an improvement in OS compared with observation only when PORT was delivered by linac.13 Multiple retrospective studies since the publication of the PORT meta-analysis have demonstrated iterative improvement in outcomes with reduced volumes,14 modern treatment techniques,13,15 standard doses less than 54 Gy,16 and standard dose per fraction ≤ 2 Gy.17
All patients in the current analysis were treated since 2006; therefore, it is a reasonable presumption that most would have been treated with modern techniques such as computed tomography simulation and at least linac-based, three-dimensional, conformal RT, although no assumption may be similarly made regarding treatment volumes. With regard to dose, the median dose in the current analysis was 54 Gy over 43 days. Assuming a standard delivery of five fractions per week, 43 days would equate to approximately 30 fractions, or 1.8 Gy per fraction. Thus, the majority of patients also received standard doses and doses per fraction. Notably, 17.7% of patients received doses in excess of 60 Gy. The most common reason for delivering definitive doses of RT in the adjuvant setting would be for positive or close margins or extracapsular extension of the nodes.18 Although patients with positive margins should have been excluded based on coding for extent of resection, it is likely that additional negative prognostic factors not captured by the NCDB influenced the decision to escalate dose in these patients.
In two serial publications by Lally et al,8,9 outcomes of patients treated with PORT for resected NSCLC were extracted from the SEER database. In an analysis of 6,148 patients with node-positive NSCLC treated with surgery from 1983 to 1998, it was demonstrated that heart disease mortality was independently associated with use of PORT and earlier year of diagnosis, with the overall contribution to a decrement in OS by PORT eliminated for patients treated after 1989 with presumably more modern techniques.8 In another analysis of 7,465 patients with stage II or III NSCLC treated with surgery from 1998 to 2002, modern PORT was still associated with decreased 5-year OS in N0 and N1 disease, but an improvement in OS was noted for N2 disease (5-year OS, 27% v 20% for PORT v no PORT, respectively; P = .0036).9 Similar findings were demonstrated in a subset analysis of the ANITA trial of adjuvant cisplatin and vinorelbine for resected NSCLC, where the greatest numeric benefit to PORT was in N2 disease, although no formal statistical analysis was performed.7 Thus, patients with stage IIIA (N2) disease seem to be the target population most likely to benefit from PORT. However, the relative benefit of modern PORT in the setting of standard-of-care platinum-based chemotherapy for stage IIIA (N2) NSCLC has not been documented to date. The Lung Adjuvant Radiotherapy Trial (LungART), a randomized trial of modern PORT versus no PORT in patients with resected NSCLC receiving platinum-based chemotherapy, is ongoing, with results not expected for several years. 19Furthermore, the SEER analysis from Lally et al9 was silent on the use of chemotherapy.
Although the previous SEER study by Lally et al9 was unable to account for the use of chemotherapy, it is heartening to see significant similarities in the Kaplan-Meier OS curves between our analyses. Remarkably, both studies consistently demonstrate no divergence in the OS curves between the no PORT and PORT groups for N2 disease until at least 2 to 2.5 years. As suggested by Lally et al,9 such results suggest the benefit to PORT is in sterilization of microscopic disease, which may not manifest its impact on OS for several years after surgery.
The current data set includes a large patient population representative of typical patients treated throughout the United States, rather than a more select trial population. As such, we were able to review the impact of multiple variables on OS. In the current analysis, we were able to account for use, type (single agent v multiagent), and timing of chemotherapy, all major potential confounders when looking at OS in this patient population. For example, we found that use of multiagent chemotherapy was independently associated with improved OS on multivariable analysis, which has been demonstrated in the metastatic setting in several other studies.20,21 However, specific chemotherapy agents were not coded in the NCDB; therefore, the actual proportion of patients receiving platinum-based chemotherapy is unknown, as is the specific combinations that may have been used. Likewise, although SEER contains specific pathologic information, data obtained from the NCDB does not specify histologic subtypes, which may have an independent impact on OS and could influence the selection of chemotherapy agents.22–24 Also representative of this large population-based cohort is the wide variability in timing of PORT relative to RT. National Comprehensive Cancer Network recommendations suggest that when PORT is to be used for completely resected N2 NSCLC that it be delivered after completion of adjuvant chemotherapy. Nonetheless, 40.5% of patients in the current cohort likely received concurrent chemotherapy and PORT by virtue of starting within 7 days of each other.
Results from our study also confirm the importance of well-established predictors of poorer OS in patients with NSCLC, including increasing age, male sex, and higher Charlson comorbidity score.3,9,25 Although the absolute effect was small relative to other factors, we also demonstrated an independent effect of tumor size, which has been recently confirmed by the large-scale international analysis of more than 80,000 patients that led to more granular breakdown of tumor size in the new seventh edition of the American Joint Committee on Cancer/International Union Against Cancer staging for NSCLC. Our data also suggest an independent improvement in OS with use of lobectomy or pneumonectomy over sublobar resection. These results fall in line with those reported from the Lung Cancer Study Group randomized study of lobectomy versus sublobar resection for clinical T1N0 NSCLC, which demonstrated improvements in LRR (6% v 17%, respectively) and OS (70% v 61%, respectively) favoring lobectomy using the predefined significance criteria (one-sided P < .10).26 In conclusion, in an analysis of the NCDB for patients with pathologic N2 NSCLC, all of whom received adjuvant chemotherapy, PORT seemed to confer an additional improvement in OS. Investigators are strongly encouraged to enroll patients on randomized trials such as LungART.
Glossary Terms
- Charlson comorbidity index:
a weighted index that takes into account the number and seriousness of 19 comorbid diseases to categorize comorbidity burden. The Charlson comorbidity index has prognostic significance in assessing disease outcomes and health resource use and has been validated in the cancer population.
- conformal radiation therapy:
an irradiation technique developed to limit the highest radiation dose to volumes at risk for tumors while sparing surrounding normal tissues. Treatment planning is based on three-dimensional reconstructions of individual patient anatomy.
- Surveillance, Epidemiology, and End Results (SEER):
a national cancer registry that collects information from all incident malignancies in multiple geographic areas of the United States.
Footnotes
Supported by Grants No. NIH K07CA178120 (V.P.) and K12CA167540-02 (Paul Calabresi award) from the National Institutes of Health, Bethesda, MD.
Presented in part at the 50th Annual Meeting of the American Society of Clinical Oncology, May 30-June 3, 2014, Chicago, IL.
Terms in blue are defined in the glossary, found at the end of this article and online at www.jco.org.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Cliff G. Robinson, Jeffrey D. Bradley, Daniel Morgensztern, Varun Puri
Collection and assembly of data: Cliff G. Robinson, Aalok P. Patel, Todd DeWees, Daniel Morgensztern, Varun Puri
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Postoperative Radiotherapy for Pathologic N2 Non–Small-Cell Lung Cancer Treated With Adjuvant Chemotherapy: A Review of the National Cancer Data Base
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Cliff G. Robinson
Speakers' Bureau: ViewRay
Research Funding: Varian Medical Systems
Travel, Accommodations, Expenses: ViewRay
Aalok P. Patel
No relationship to disclose
Jeffrey D. Bradley
Research Funding: ViewRay
Travel, Accommodations, Expenses: Varian
Todd DeWees
Consulting or Advisory Role: Traxxsson
Saiama N. Waqar
Research Funding: Agennix (Inst), Lilly (Inst), Pfizer (Inst), Daiichi Sankyo (Inst), Spectrum Pharmaceuticals, Astex Therapeutics (Inst), Novartis (Inst), Genentech (Inst), Transgene (Inst), Eisai (Inst), New Link Genetics Corp, Merck (Inst), Puma (Inst), Roche (Inst), Incyte (Inst)
Daniel Morgensztern
Honoraria: Boehringer Ingelheim, Celgene
Consulting or Advisory Role: Celgene
Speakers' Bureau: Boehringer Ingelheim
Maria Q. Baggstrom
Research Funding: Novartis (Inst), Merck (Inst), Wyeth (Inst), ImClone Systems (Inst), Boehringer Ingelheim (Inst), Merrimack (Inst), Lilly (Inst), Bristol-Myers Squibb (Inst), Oncocyte (Inst), Astex Therapeutics (Inst), Onyx (Inst)
Ramaswamy Govindan
Consulting or Advisory Role: Boehringer Ingelheim, GlaxoSmithKline, Pfizer, Merck, Bayer, Covidien, Bristol-Myers Squibb, Roche/Genentech, Mallinckrodt
Jennifer M. Bell
No relationship to disclose
Tracey J. Guthrie
No relationship to disclose
Graham A. Colditz
No relationship to disclose
Traves D. Crabtree
Honoraria: Ethicon
Consulting or Advisory Role: Ethicon
Research Funding: Ethicon
Daniel Kreisel
No relationship to disclose
Alexander S. Krupnick
No relationship to disclose
G. Alexander Patterson
No relationship to disclose
Bryan F. Meyers
Consulting or Advisory Role: Varian, Ethicon, Covidien
Research Funding: Ethicon
Varun Puri
No relationship to disclose
REFERENCES
- 1.Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 3.Detterbeck FC, Boffa DJ, Tanoue LT. The new lung cancer staging system. Chest. 2009;136:260–271. doi: 10.1378/chest.08-0978. [DOI] [PubMed] [Google Scholar]
- 4.NSCLC Meta-analyses Collaborative Group. Arriagada R, Auperin A, et al. Adjuvant chemotherapy, with or without postoperative radiotherapy, in operable non-small-cell lung cancer: Two meta-analyses of individual patient data. Lancet. 2010;375:1267–1277. doi: 10.1016/S0140-6736(10)60059-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Le Péchoux C. Role of postoperative radiotherapy in resected non-small cell lung cancer: A reassessment based on new data. Oncologist. 2011;16:672–681. doi: 10.1634/theoncologist.2010-0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.PORT Meta-analysis Trialists Group. Postoperative radiotherapy for non-small cell lung cancer. Cochrane Database Syst Rev. 2005;2:CD002142. doi: 10.1002/14651858.CD002142.pub2. [DOI] [PubMed] [Google Scholar]
- 7.Douillard JY, Rosell R, De Lena M, et al. Impact of postoperative radiation therapy on survival in patients with complete resection and stage I, II, or IIIA non-small-cell lung cancer treated with adjuvant chemotherapy: The Adjuvant Navelbine International Trialist Association (ANITA) randomized trial. Int J Radiat Oncol Biol Phys. 2008;72:695–701. doi: 10.1016/j.ijrobp.2008.01.044. [DOI] [PubMed] [Google Scholar]
- 8.Lally BE, Detterbeck FC, Geiger AM, et al. The risk of death from heart disease in patients with nonsmall cell lung cancer who receive postoperative radiotherapy: Analysis of the Surveillance, Epidemiology, and End Results database. Cancer. 2007;110:911–917. doi: 10.1002/cncr.22845. [DOI] [PubMed] [Google Scholar]
- 9.Lally BE, Zelterman D, Colasanto JM, et al. Postoperative radiotherapy for stage II or III non-small-cell lung cancer using the surveillance, epidemiology, and end results database. J Clin Oncol. 2006;24:2998–3006. doi: 10.1200/JCO.2005.04.6110. [DOI] [PubMed] [Google Scholar]
- 10.Newman LA, Lee CT, Parekh LP, et al. Use of the National Cancer Data Base to develop clinical trials accrual targets that are appropriate for minority ethnicity patients: A report from the American College of Surgeons Oncology Group (ACOSOG) Special Population Committee. Cancer. 2006;106:188–195. doi: 10.1002/cncr.21592. [DOI] [PubMed] [Google Scholar]
- 11.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 12.Cole SR, Hernán MA. Adjusted survival curves with inverse probability weights. Comput Methods Programs Biomed. 2004;75:45–49. doi: 10.1016/j.cmpb.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 13.Billiet C, Decaluwé H, Peeters S, et al. Modern post-operative radiotherapy for stage III non-small cell lung cancer may improve local control and survival: A meta-analysis. Radiother Oncol. 2014;110:3–8. doi: 10.1016/j.radonc.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 14.Miles EF, Kelsey CR, Kirkpatrick JP, et al. Estimating the magnitude and field-size dependence of radiotherapy-induced mortality and tumor control after postoperative radiotherapy for non-small-cell lung cancer: Calculations from clinical trials. Int J Radiat Oncol Biol Phys. 2007;68:1047–1052. doi: 10.1016/j.ijrobp.2007.02.028. [DOI] [PubMed] [Google Scholar]
- 15.Phlips P, Rocmans P, Vanderhoeft P, et al. Postoperative radiotherapy after pneumonectomy: Impact of modern treatment facilities. Int J Radiat Oncol Biol Phys. 1993;27:525–529. doi: 10.1016/0360-3016(93)90375-6. [DOI] [PubMed] [Google Scholar]
- 16.Machtay M, Lee JH, Shrager JB, et al. Risk of death from intercurrent disease is not excessively increased by modern postoperative radiotherapy for high-risk resected non-small-cell lung carcinoma. J Clin Oncol. 2001;19:3912–3917. doi: 10.1200/JCO.2001.19.19.3912. [DOI] [PubMed] [Google Scholar]
- 17.Dautzenberg B, Arriagada R, Chammard AB, et al. A controlled study of postoperative radiotherapy for patients with completely resected nonsmall cell lung carcinoma: Groupe d'Etude et de Traitement des Cancers Bronchiques. Cancer. 1999;86:265–273. doi: 10.1002/(sici)1097-0142(19990715)86:2<265::aid-cncr10>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 18.Bradley JD, Paulus R, Graham MV, et al. Phase II trial of postoperative adjuvant paclitaxel/carboplatin and thoracic radiotherapy in resected stage II and IIIA non-small-cell lung cancer: Promising long-term results of the Radiation Therapy Oncology Group–RTOG 9705. J Clin Oncol. 2005;23:3480–3487. doi: 10.1200/JCO.2005.12.120. [DOI] [PubMed] [Google Scholar]
- 19.Le Pechoux C. Radiation therapy in treating patients with non small cell lung cancer that has been completely removed by surgery (LUNG ART) https://clinicaltrials.gov/ct2/show/NCT00410683.
- 20.Lilenbaum RC, Herndon JE, 2nd, List MA, et al. Single-agent versus combination chemotherapy in advanced non-small-cell lung cancer: The Cancer and Leukemia Group B (study 9730) J Clin Oncol. 2005;23:190–196. doi: 10.1200/JCO.2005.07.172. [DOI] [PubMed] [Google Scholar]
- 21.Zukin M, Barrios CH, Pereira JR, et al. Randomized phase III trial of single-agent pemetrexed versus carboplatin and pemetrexed in patients with advanced non-small-cell lung cancer and Eastern Cooperative Oncology Group performance status of 2. J Clin Oncol. 2013;31:2849–2853. doi: 10.1200/JCO.2012.48.1911. [DOI] [PubMed] [Google Scholar]
- 22.Simon GR, Manegold C, Barker SS, et al. Pemetrexed use in the adjuvant setting for completely resectable non-small-cell lung cancer. Clin Lung Cancer. 2013;14:601–608. doi: 10.1016/j.cllc.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 23.Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26:3543–3551. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]
- 24.Chansky K, Sculier JP, Crowley JJ, et al. The International Association for the Study of Lung Cancer Staging Project: Prognostic factors and pathologic TNM stage in surgically managed non-small cell lung cancer. J Thorac Oncol. 2009;4:792–801. doi: 10.1097/JTO.0b013e3181a7716e. [DOI] [PubMed] [Google Scholar]
- 25.Firat S, Byhardt RW, Gore E. Comorbidity and Karnofsky performance score are independent prognostic factors in stage III non-small-cell lung cancer: An institutional analysis of patients treated on four RTOG studies. Int J Radiat Oncol Biol Phys. 2002;54:357–364. doi: 10.1016/s0360-3016(02)02939-5. [DOI] [PubMed] [Google Scholar]
- 26.Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer: Lung Cancer Study Group. Ann Thorac Surg. 1995;60:615–622. doi: 10.1016/0003-4975(95)00537-u. [DOI] [PubMed] [Google Scholar]