Abstract
Background and objectives
Evaluation of glomerular hyperfiltration (GH) is difficult; the variable reported definitions impede comparisons between studies. A clear and universal definition of GH would help in comparing results of trials aimed at reducing GH. This study assessed how GH is measured and defined in the literature.
Design, setting, participants, & measurements
Three databases (Embase, MEDLINE, CINAHL) were systematically searched using the terms “hyperfiltration” or “glomerular hyperfiltration”. All studies reporting a GH threshold or studying the effect of a high GFR in a continuous manner against another outcome of interest were included.
Results
The literature search was performed from November 2012 to February 2013 and updated in August 2014. From 2013 retrieved studies, 405 studies were included. Threshold use to define GH was reported in 55.6% of studies. Of these, 88.4% used a single threshold and 11.6% used numerous thresholds adapted to participant sex or age. In 29.8% of the studies, the choice of a GH threshold was not based on a control group or literature references. After 2004, the use of GH threshold use increased (P<0.001), but the use of a control group to precisely define that GH threshold decreased significantly (P<0.001); the threshold did not differ among pediatric, adult, or mixed-age studies. The GH threshold ranged from 90.7 to 175 ml/min per 1.73 m2 (median, 135 ml/min per 1.73 m2).
Conclusion
Thirty percent of studies did not justify the choice of threshold values. The decrease of GFR in the elderly was rarely considered in defining GH. From a methodologic point of view, an age- and sex-matched control group should be used to define a GH threshold.
Keywords: hemodynamics and vascular regulation, hyperfiltration, creatinine clearance, glomerular hyperfiltration, diabetic nephropathy
Introduction
The number of patients developing CKD is increasing, reaching epidemic proportions (1). The increase is mostly secondary to diabetes mellitus and hypertension (2). Both disorders are characterized by a progressive loss of renal function. Although not a unique cause of chronic kidney function loss, glomerular hyperfiltration (GH) is thought to play an important role in the initiation of glomerular damage, especially in the diabetic patient. It is thought that GH is caused first by alteration in tubuloglomerular feedback and the activation of vasoactive mediators, such as the nitric oxide, cyclooxygenase-2–derived prostanoids, the renin-angiotensin system, protein kinase C, and endothelins, which increase glomerular capillary pressure and lead to secondary increases in GFR (detected as GH) (3).
GH has been described mainly in patients with diabetes mellitus (4) but has also been reported in patients with sickle disease (5), hypertension (6), hyperaldosteronism (7), pregnancy (8), and obesity/metabolic syndrome (3). An estimated 70% and 50% of patients with type 1 and 2 diabetes, respectively, develop GH early in their disease (9). GH has been reported as a predictor of overt diabetic nephropathy (10), although this remains debated (11). The presence of GH has also been associated with an increased risk of stroke in a large epidemiologic study (12). Finally, GH can be intentionally provoked and assessed when patients are subjected to a high-protein meal to evaluate their renal reserve (13).
Although GH plays an important role in the initiation of CKD, it is only one of several mechanisms leading to renal insufficiency. Many patients reach ESRD without going through a hyperfiltering stage.
Despite this renewed interest in GH, a clear definition in the literature is strikingly absent (9). This makes comparisons between studies difficult. Several factors contribute to the difficulty in establishing a clear definition of GH: (1) the wide variety of GFR methods used, each of them comparing differently with the gold standard (i.e., inulin clearance); (2) the naturally decline in GFR that accompanies advancing age; (3) the difference between men and women; and (4) the differences between distinct ethnic populations.
Beyond the purely methodologic aspect of this research study, a clinical impact of defining GH cutoff can be found in randomized controlled trials (RCTs) targeting hyperfiltration pharmacologically (using angiotensin-converting enzyme inhibitors) or surgically (bariatric surgery) (Supplemental Material 1). Furthermore, it is important to define a group of patients in whom hyperfiltration can be followed by a progressive decline of renal function.
We therefore systematically reviewed the literature to assess how GH is evaluated and reported in the literature. We also explored potential determinants for reporting and defining GH threshold, such as sex and age of the participants and methods used to assess GH.
Materials and Methods
The methods are reported in details in Supplemental Material 2.
Protocol and Registration
The protocol has been registered with the PROSPERO database of prospectively registered systematic reviews in health and social care (14).
Data Sources
We performed a systematic review of the literature from November 2012 to May 2014 using the following databases: MEDLINE (1951–May 2014), Embase (1980–May 2014), CINAHL (1981– May 2014). The search was conducted using the keyword “hyperfiltration” (MEDLINE and CINAHL) and “glomerular hyperfiltration” (Embase). Research strategy can be found in Supplemental Material 3. No limits on time, language, or type of study were placed on any primary database search. This systematic review was reported according to the 2009 Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement, which is included in Supplemental Material 4.
Study Selection
We included studies assessing GFR evaluation in humans. All study types except experimental studies were included (i.e., clinical and epidemiologic studies). Details of the study designs can be found in Supplemental Material 2. Two of the authors (F.C., H.C.) reviewed the abstract and then the full text of each article. All articles with a GH threshold were automatically recorded. Retrieved articles that did not report a GH threshold but explored the effect of GFR against another outcome of interest, such as BP, proteinuria, microalbuminuria, stroke, or death, were also recorded.
Data Collection
For each included study, items were extracted and recorded in duplicate by two independent reviewers, according to the Cochrane Methods Working Group on Systematic Reviews of Screening and Diagnostic Tests. Details of the recorded data can be found in Supplemental Material 5 (results: main findings).
Statistical Analyses
Study characteristics were described by percentages and were compared across publication time period using chi-squared tests or Fisher exact tests. Association between the characteristics of studies and the use of thresholds were assessed using a mixed-effect logistic regression model. A random effect on the intercept was introduced in the model to account for the first author because one author may publish several papers and an author may probably follow a similar approach in various studies. Factors associated with the threshold values were also explored by using a linear model. This analysis was conducted on the subgroup of studies reporting a single threshold value expressed in ml/min per 1.73 m2. Because few authors had two more publications in this subgroup, random effects were not introduced in the linear model. A meta-regression analysis was conducted to test the hypothesis that the proportion of participants classified as hyperfiltering depends on the chosen threshold to define GH in each particular study. Details of the meta-regression can be found in Supplemental Material 2 (15,16). All analyses were conducted with S-plus for Windows 8.0 (Insightful Corp., Seattle, WA), Stata/IC 10.1 for Windows (Stata Corp., College Station, TX), and Comprehensive Meta-Analysis 2 (Biostat, Englewood, NJ). Inter-rater concordance was evaluated using the κ concordance test. Significance was established at P value <0.05.
Results
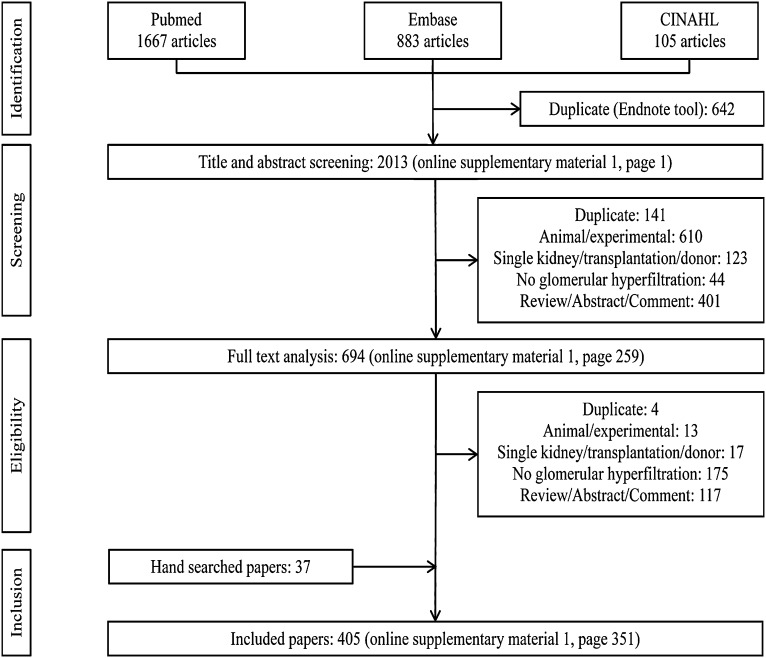
The study selection flow chart is depicted in Figure 1. All included studies appear in Supplemental Material 6 (bibliographic findings). A complete list of all retrieved but not included studies is available upon request from the corresponding author. Inter-rater agreement (Cohen κ coefficient beyond chance) for study selection was 0.615 (95% confidence interval, 0.574 to 0.655). Two studies reporting only filtration fraction and no GFR were also excluded after initial inclusion, before final analysis. Four hundred five studies were included in the final analysis. A table with references and a description of all included studies can be found in Supplemental Material 5.
Figure 1.
Flow chart of study selection process.
Studies characteristics are depicted in Table 1. Most studies were observational, with a cohort, case-control, or cross-sectional design. The sample sizes were heterogeneous, but more than half of the studies had <50 patients. Although only 27.7% of studies were published in diabetes journals, approximately 50% of studies reported on patients with diabetes or obesity/metabolic syndrome, and this percentage remained stable over time. Most patients were adults. Thirteen different methods to assess GFR were reported. The most frequent methods to assess GFR belonged to the category “isotopes, iohexol,” but no method appeared as a preferred one. Most of these characteristics varied significantly over time (Table 2). Recent studies (after 2004) more frequently had an observational design and a larger sample size. Renal reserve was the research topic in 25% of the papers before 1995, and this dropped significantly to <4% after 2005, when the literature saw a surge in studies of GH in other conditions, such as hypertension and hyperaldosteronism, sickle cell disease, and glycogen storage diseases. Over time, the use of inulin clearance decreased from 32% before 1995% to 22% after 2005, whereas the use of formulas increased during the same period from 0% to >35%.
Table 1.
Characteristics of studies (n=405) and glomerular hyperfiltration thresholds
| Characteristic | Data |
|---|---|
| Study | |
| Study type | |
| Observationala | 271 (66.9) |
| Interventionalb | 134 (33.1) |
| Context | |
| Diabetes, obesity/metabolic syndrome | 223 (55.1) |
| Renal reserve | 50 (12.3) |
| Others | 132 (32.6) |
| Patient age | |
| Pediatric | 43 (10.5) |
| Adult | 276 (68.3) |
| Pediatric and adult | 85 (21.0) |
| GFR evaluation methodc | |
| Inulin clearance | 116 (28.6) |
| Isotopes, iohexol | 149 (36.8) |
| Creatinine clearance | 77 (19.0) |
| Formulas | 65 (16.0) |
| Is binary definition of hyperfiltration used? | |
| No | 180 (44.4) |
| Yes | 225 (55.6) |
| GH threshold | |
| Single threshold | 199/225 (88.4) |
| Numerous or continuous (including equations) | 26/225 (11.6) |
| Age-dependent only | 3 |
| Sex-dependent only | 5 |
| Age- and sex-dependent | 13 |
| Unrelated to age/sexd | 5 |
| Reference for GH threshold | |
| No justification | 67/225 (29.8) |
| References/previous work | 94/225 (41.8) |
| Assessed in a control group of the same study | 52/225 (23.1) |
| Assessed on the sample itself | 12/225 (5.3) |
Values are expressed as the number (percentage) of studies. GH, glomerular hyperfiltration.
Includes prospective/retrospective cohort studies, uncontrolled longitudinal studies, case series, case-control studies, nested case-control studies, and cross-sectional studies.
Includes randomized controlled trials, quasi-randomized controlled trial, before-after trials, and crossover trials.
Three studies used several GFR evaluation methods.
One study used two methods for GFR evaluation and used specific cutoffs for each method, one study used two different cutoffs from the literature (same method for GFR evaluation), one study used two cutoffs derived from two control groups (same method for GFR evaluation), one study used a formula independent of age and sex).
Table 2.
Trends for characteristics of studies (n=405) and glomerular hyperfiltration (n=225) according to publication year
| Characteristic | 1994 or Earlier (n=132) | 1995–2004 (n=117) | 2005 or Later (n=156) | P Value |
|---|---|---|---|---|
| Study type | <0.001 | |||
| Observationala | 72 (54.5) | 80 (68.4) | 119 (76.3) | |
| Interventionalb | 60 (45.5) | 37 (31.6) | 37 (23.7) | |
| Context | <0.00 | |||
| Diabetes, obesity/metabolic syndrome | 72 (55.7) | 67 (56.0) | 84 (50.3) | |
| Renal reserve | 35 (25.2) | 11 (10.3) | 4 (3.4) | |
| Other | 25 (19.1) | 39 (33.6) | 68 (46.3) | |
| Patient age | 0.04 | |||
| Pediatric | 9 (6.9) | 8 (6.8) | 26 (16.7) | |
| Adult | 93 (71.0) | 86 (73.5) | 97 (62.2) | |
| Pediatric and adult | 29 (22.1) | 23 (19.7) | 33 (21.2) | |
| Median sample size (IQR) | 20 (12–52) | 33 (20–85) | 89 (41–291) | <0.001 |
| GFR evaluation method | ||||
| Inulin clearance | 42 (31.8) | 40 (34.2) | 34 (21.8) | 0.05 |
| Isotopes, iohexol | 63 (47.7) | 47 (40.2) | 39 (25.0) | <0.001 |
| Creatinine clearance | 27 (20.5) | 20 (17.1) | 30 (19.2) | 0.79 |
| Formulas | 0 (0.0) | 10 (8.5) | 55 (35.3) | <0.001 |
| Use of GH threshold | <0.001 | |||
| No | 75 (56.8) | 61 (52.1) | 44 (28.2) | |
| Yes | 57 (43.2) | 56 (47.9) | 112 (71.8) | |
| If yes, use of several or continuous thresholds? | 1/57 (1.8) | 6/56 (10.7) | 19/112 (17.0) | 0.01 |
| If yes, definition reported? | 51/57 (89.5) | 48/56 (85.7) | 105/112 (93.8) | 0.23 |
| If yes, what justification? | <0.001 | |||
| None | 9/57 (15.8) | 21/56 (37.5) | 37/112 (33.0) | |
| Reference/previous work | 21/57 (36.8) | 18/56 (32.1) | 55/112 (49.1) | |
| Control group same study | 26/57 (45.6) | 15/56 (26.8) | 11/112 (9.8) | |
| Sample itself | 1/57 (1.8) | 2/56 (3.6) | 9/112 (8.0) |
IQR, interquartile range.
Includes prospective/retrospective cohort studies, uncontrolled longitudinal studies, case series, case-control studies, nested case-control studies, and cross-sectional studies.
Includes randomized controlled trials, quasi-randomized controlled trials, before-after trials, and crossover trials.
More than half of studies (n=225 [56%]) defined GH; 199 of these (88.4%) used a single threshold, whereas 26 studies used several thresholds or a continuous threshold adjusted for age and/or sex (Table 1). Three studies also used several thresholds that were specific to methods for GFR assessment or derived in various control groups, without age or sex adjustment. Most studies (n=204 [91%]) using a threshold explicitly reported the value of the threshold or the age- or sex-adjusted formula, whereas 21 studies mentioned the use of a GH threshold, without reporting it. Only 23.1% of studies used a control group, and 29.8% of studies mentioned no sources or references for the reported GH threshold; GH threshold value was based on previous published work (personal or otherwise) in 41.8% of the papers. Among 57 studies reporting literature data to define GH cutoffs, 30 (53%) used the same GFR evaluation method.
Use of a binary definition of GH increased significantly over time (Table 2). However, at the same time, we observed a significant decrease in studies using a control group to define the threshold value, from 45.6% to <10%. In contrast, the use of references or previous work to define the GH threshold increased from 36.8% to 49.1%. More studies published after 2004 than before did not justify choice of GH threshold (33.0% versus 15.8%). According to a multivariate analysis (mixed-effect logistic regression model) (Table 3), interventional studies were less likely to use a GH threshold, whereas participant age did not affect GH threshold use. Studies in diabetic patients and studies using isotopes or formulas to assess GH were more likely to use a GH threshold.
Table 3.
Characteristics of studies and the use of glomerular hyperfiltration threshold (multivariate analysis, mixed-effect logistic regression model)
| Characteristic | Odds Ratio ((95% Confidence Interval) | P Value |
|---|---|---|
| Study type | ||
| Observationala | 7.21 (1.95 to 26.67) | 0.003 |
| Interventionalb | Reference | |
| Context | ||
| Diabetes, obesity/metabolic syndrome | Reference | 0.001 |
| Renal reserve | 0.02 (0.00 to 0.19) | 0.001 |
| Others | 0.31 (0.12 to 0.83) | 0.02 |
| Patient age | ||
| Pediatric | Reference | 0.18 |
| Adult | 0.46 (0.13 to 1.65) | 0.24 |
| Pediatric and adult | 1.06 (0.26 to 4.39) | 0.94 |
| GFR evaluation method | ||
| Inulin clearance | Reference | 0.03 |
| Isotopes, iohexol | 7.15 (1.81 to 28.21) | 0.005 |
| Creatinine clearance | 3.97 (0.97 to 16.26) | 0.06 |
| Formulas | 8.67 (1.76 to 42.72) | 0.008 |
Includes prospective/retrospective cohort studies, uncontrolled longitudinal studies, case series, case-control studies, nested case-control studies, and cross-sectional studies.
Includes randomized controlled trials, quasi-randomized controlled trials, before-after trials, and crossover trials.
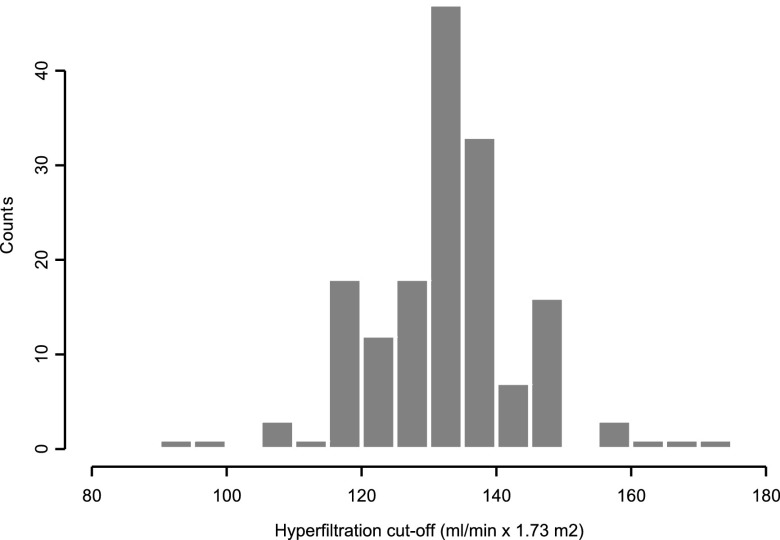
Among the 199 studies using a GH definition with a single threshold, 163 studies (81.9%) reported the threshold value with expression of GFR in ml/min per 1.73 m2. In this set of studies, the median GH threshold was 135 ml/min per 1.73 m2 (range, 90.7–175 ml/min per 1.73 m2) (Figure 2). The value of 135 was also the most frequently used (20.1% of studies). Half of studies reported a threshold value between 130 and 140 ml/min per 1.73 m2 (first and third quartiles). We also explored the associations between study characteristics and threshold values. Results are shown in Table 4. The age of patients had no effect on GH threshold. Studies using formulas to estimate GFR reported a significantly lower GH threshold than studies using other GFR evaluation methods. The percentage of patients with GH, reported in 130 studies (86.1%), ranged from 6.1% to 100.0%, indicating a high heterogeneity (I2=92%). The threshold value was not associated with the percentage of patients with GH (P=0.74): The observed heterogeneity was not explained by the threshold value (the odds ratio per 10 ml/min per 1.73 m2 was 0.98 (95% confidence interval, 0.84 to 1.13; P=0.74).
Figure 2.
Hyperfiltration cutoff. Distribution of threshold values with expression of GFR in ml/min per 1.73 m2 reported in 151 studies with a single threshold. Median (minimum, maximum), 135 (90.7, 175); first and third quartiles, 130 and 140; mean±SD, 134.6±11.7.
Table 4.
Characteristics of studies and value for glomerular hyperfiltration (multivariate analysis, linear regression analysis)
| Characteristic | No. of Studies | Mean Cutoff±SD | Adjusted Mean Difference±SEM | P Value |
|---|---|---|---|---|
| Context | ||||
| Diabetes, obesity/metabolic syndrome | 113 | 134.8±8.8 | Reference | |
| Other | 49 | 134.0±16.8 | 0.3±2.5 | 0.90 |
| Patient age | 0.24 | |||
| Pediatric | 27 | 137.7±16.1 | Reference | |
| Adult | 92 | 133.6±10.1 | −4.5±2.7 | 0.10 |
| Pediatric and adult | 43 | 134.5±11.7 | −4.0±2.8 | 0.16 |
| GFR evaluation method | 0.002 | |||
| Inulin clearance | 38 | 137.8±9.8 | Reference | |
| Isotopes, iohexol | 73 | 134.1±9.7 | −3.3±2.3 | 0.15 |
| Creatinine clearance | 25 | 137.6±13.4 | 0.6±3.3 | 0.87 |
| Formulas | 26 | 128.0±15.2 | −10.7±3.2 | 0.001 |
| Justification of cutoff | 0.05 | |||
| None | 51 | 135.0±12.6 | Reference | |
| Reference/previous work | 70 | 134.8±10.2 | −2.0±2.2 | 0.37 |
| Control group | 35 | 135.4±9.9 | −0.6±2.7 | 0.83 |
| Same sample | 6 | 122.7±23.6 | −13.9±5.0 | 0.006 |
β values represent mean adjusted difference (compared with the reference in each category).
Rounded values (multiples of 10) for GH threshold were reported in 47.6% of all papers. This percentage went up to 75.0% in studies giving no justification and no literature reference for the threshold choice, and it decreased to around 33% for studies that cited a reference, included a control group, or estimated threshold value on the basis of the same population sample.
Discussion
The main finding of our study is that GH threshold, when reported, varies between studies, ranging from 90.7 to 175 ml/min per 1.73 m2, although half of GH thresholds lie between 130 and 140 ml/min per 1.73 m2. Studies reporting a GH threshold often have no control group and do not adapt their GH threshold to the naturally declining renal function in the elderly. This could render some study conclusions unreliable and also make comparison between studies difficult.
We explored whether characteristics of the studies or the participants could explain the differences in reporting frequency and values of GH threshold. The commonly used methods to measure GFR can give very different values. We identified 13 different methods to evaluate GFR. Against the gold standard evaluation, which is inulin clearance, precision and accuracy of creatinine clearance, isotope clearance, and formulas greatly vary (17). This is especially true with high GFR values or high filtration fraction (18). Both the Modification of Diet in Renal Disease Study and the Cockcroft–Gault formulas systematically underestimate GFR, especially at high levels (>60 ml/min per 1.73 m2), which might particularly compromise their suitability in patients with incipient kidney disease and hyperfiltration (19). In contrast, the CKD Epidemiology Collaboration equation might be a superior surrogate marker of GFR in patients with hyperfiltration (20,21). Indeed, studies using formulas to estimate GFR had a significantly lower GH threshold than studies using any other GFR estimation method. On the other hand, diethylenetriaminepenta-acetate gives systematically slightly higher results (22). Cr-51 EDTA plasma clearance seems to be the most accurate method to assess GFR, besides inulin clearance (23). The interpretation of the results should consider the various performances of the GFR method used. If GH threshold is taken from the literature, the same GFR method should have been used in both the experimental group and the control group.
In addition to the choice of method for GFR measurement, body mass index is another important predictor of GH; obese individuals have a much higher GFR than their lean counterparts. Some authors demonstrated that adjustment of GFR to body surface area decreases the prevalence of participants with hyperfiltration (24,25). This should be taken into account in the development of future GH guidelines.
Thirty percent of studies had no control groups for defining the normal upper GFR level. Ideally, a control group with similar characteristics, in particular age and sex, should be included to define a GH threshold. If unable to have an age-adjusted control group, authors should at the minimum select from the literature a control group with characteristics similar to those of their participants and with similar GFR evaluation methods. Among the four studies reporting a GH threshold <2 SDs of the median (of this systematic review), three used their own control group, with age adjusted to their study population (26–28). These three studies were performed in Japan and Taiwan, and GFR was indexed for body surface area, which is lower in Asian population (29). On the other hand, four of five studies with GH threshold >2 SDs of the median had no control group or even references (30–33).
We observed a significant increase over time in the use of formulas to assess GFR. This, together with an increasing use of GH threshold with no justification/control group, could contribute to an increase in the uncertainty of the findings of future studies.
GH thresholds that were reported in pediatric studies were not significantly different from studies that included mixed-age or adult populations. This is surprising because GFR changes dramatically over time: After a sharp increase in the first 2 years of life (34), GFR stabilizes at 90–120 ml/min per 1.73 m2. From the third decade of life, GFR declines at approximately 0.75 ml/min per year at the beginning, reaching 3 ml/min per year in elderly persons age 70–110 years (35). It also varies, to a lesser extent, with sex (being lower in women) and race. Future studies should consider declining renal function in the elderly.
The meta-regression analysis showed that the level of GH threshold had no effect on the number of participants classified as hyperfiltering or not. Despite wide variability, this was not explained by the GH threshold. For half of studies, GH threshold was between 130 and 140 ml/min per 1.73 m2, and this might explain the negative finding.
GH threshold values were frequently rounded to the nearest zero. This rounding effect was significantly more common in studies without a control group. Zero end-digit preference happens in BP recording (36). This rounding effect leads to additional imprecision in the classification (and potentially management) of patients with GH.
The clinical implication of GH cutoff choice remains to be demonstrated. Recently, Eriksen et al. (37) unmasked an independent link between a high GFR and subclinical cardiovascular damage, independent of diabetes, opening the way for future epidemiologic and interventional studies. Indeed, several RCT are studying the consequences of GH, the indications for a specific treatment (such as angiotensin-converting enzyme inhibitors or bariatric surgery), and the evaluation of potential benefits (Supplemental Material 1). In addition, some authors have already suggested integrating multiple levels of GH (38) in the different CKD groups. However, to the best of our knowledge, no consensus guidelines currently established by the Kidney Disease Outcomes Quality Initiative (39), or other guidelines organizations, such as the National Guidelines Clearinghouse, National Institute for Health and Care Excellence, Canada’s database of clinical practice guidelines, the Scottish Intercollegiate Guidelines Network, and Guidelines International Network, define and integrate GH in their recommendations. Future studies should be conducted to determine the prognostic effect of GH on patient-relevant outcomes. We suggest that GH threshold be adjusted for age and sex, especially in studies including children or the elderly, groups with physiologically rapidly changing GFR. In studies including a population with a narrow age range, a reference to a previously published control group sharing the same characteristics (age; sex; race; and, ideally, methods for GFR measurement) can be used.
Our analysis had some limitations. Only studies retrieved using the words “hyperfiltration” or “glomerular hyperfiltration” were included. Studies of glomerular hyperfiltration that are not indexed in MEDLINE or Embase under these search terms may have been missed. However, after an extensive hand search of all references in review papers, we identified only 37 additional papers.
Although repeat creatinine or cystatin measurement and their subsequent clearances show great within-participant variability (40,41), no included studies assessed renal function more than once in any one patient, which could also lead to patient misclassification and therefore decrease the generalizability of our findings.
In conclusion, different methods to detect GH were found in the literature. In addition, reported reference values to define GH varied from 90 to 175 ml/min per 1.73 m2. However, most reported GH cutoffs ranged from 130 to 140 ml/min per 1.73 m2. Most studies did not report an appropriate age- and sex-adjusted control group. To avoid the limitations of a defined GH cutoff due to the absence of guidelines, association between GH and outcomes such as death, CKD, or microalbuminuria may be best studied on a continuous rather than dichotomous basis, as has been shown with hypertension (42) and microalbuminuria (43).
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Mrs. De Kaenel and all the staff from the medical library of the University of Lausanne for invaluable help, especially in providing all of the papers included in our research. We also thank Dr. Borowski, Mrs. Chatfield, Mrs. Samuelson, and Mrs. Ilic for Polish and Czech, Japanese, Russian and Bulgarian, and Serbo-Croatian translations, respectively. Finally, we also wish to thank the reviewers for their invaluable help and suggestions.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.03080314/-/DCSupplemental.
References
- 1.Meguid El Nahas A, Bello AK: Chronic kidney disease: The global challenge. Lancet 365: 331–340, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Ginter E, Simko V: Type 2 diabetes mellitus, pandemic in 21st century. Adv Exp Med Biol 771: 42–50, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Sasson AN, Cherney DZ: Renal hyperfiltration related to diabetes mellitus and obesity in human disease. World J Diabetes 3: 1–6, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang GK, Maahs DM, Perkins BA, Cherney DZ: Renal hyperfiltration and systemic blood pressure in patients with uncomplicated type 1 diabetes mellitus. PLoS ONE 8: e68908, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aygun B, Mortier NA, Smeltzer MP, Hankins JS, Ware RE: Glomerular hyperfiltration and albuminuria in children with sickle cell anemia. Pediatr Nephrol 26: 1285–1290, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palatini P, Dorigatti F, Saladini F, Benetti E, Mos L, Mazzer A, Zanata G, Garavelli G, Casiglia E: Factors associated with glomerular hyperfiltration in the early stage of hypertension. Am J Hypertens 25: 1011–1016, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Kuo CC, Wu VC, Tsai CW, Wu KD, Taiwan Primary Aldosteronism Investigation (TAIPAI) Study Group) : Relative kidney hyperfiltration in primary aldosteronism: A meta-analysis. J Renin Angiotensin Aldosterone Syst 12: 113–122, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Cornelis T, Odutayo A, Keunen J, Hladunewich M: The kidney in normal pregnancy and preeclampsia. Semin Nephrol 31: 4–14, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Helal I, Fick-Brosnahan GM, Reed-Gitomer B, Schrier RW: Glomerular hyperfiltration: Definitions, mechanisms and clinical implications. Nat Rev Nephrol 8: 293–300, 2012 [DOI] [PubMed] [Google Scholar]
- 10.Magee GM, Bilous RW, Cardwell CR, Hunter SJ, Kee F, Fogarty DG: Is hyperfiltration associated with the future risk of developing diabetic nephropathy? A meta-analysis. Diabetologia 52: 691–697, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Thomas MC, Moran JL, Harjutsalo V, Thorn L, Wadén J, Saraheimo M, Tolonen N, Leiviskä J, Jula A, Forsblom C, Groop PH, FinnDiane Study Group : Hyperfiltration in type 1 diabetes: Does it exist and does it matter for nephropathy? Diabetologia 55: 1505–1513, 2012 [DOI] [PubMed] [Google Scholar]
- 12.Nistrup Holmegaard S, Christoffersen H, Haase J: Albuminuria, intermittent hyperfiltration and salt wasting in patients with stroke: a pilot study. Scand J Clin Lab Invest 66: 437–449, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Raes A, Donckerwolcke R, Craen M, Hussein MC, Vande Walle J: Renal hemodynamic changes and renal functional reserve in children with type I diabetes mellitus. Pediatr Nephrol 22: 1903–1909, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Cachat F, Chehade H, Cauderay M, Combescure C, Girardin E: A systematic review of glomerular hyperfiltration assessment and definition in the medical literature. PROSPERO 2013:CRD42013005845. Available at http://www.crd.york.ac.uk/PROSPERO_REBRANDING/display_record.asp?ID=CRD42013005845 [DOI] [PMC free article] [PubMed]
- 15.Thompson SG, Higgins JPT: How should meta-regression analyses be undertaken and interpreted? Stat Med 21: 1559–1573, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Higgins JPT, Thompson SG: Quantifying heterogeneity in a meta-analysis. Stat Med 21: 1539–1558, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Delanaye P: How measuring glomerular filtration rate? Comparison of reference methods. In: Basic Nephrology and Acute Kidney Injury, Prof, edited by Sahay M, Rijeka, Croatia, InTech, 2012 [Google Scholar]
- 18.Huang SH, Sharma AP, Yasin A, Lindsay RM, Clark WF, Filler G: Hyperfiltration affects accuracy of creatinine eGFR measurement. Clin J Am Soc Nephrol 6: 274–280, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fontseré N, Salinas I, Bonal J, Bayés B, Riba J, Torres F, Rios J, Sanmartí A, Romero R: Are prediction equations for glomerular filtration rate useful for the long-term monitoring of type 2 diabetic patients? Nephrol Dial Transplant 21: 2152–2158, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Vučić Lovrenčić M, Radišić Biljak V, Božičević S, Prašek M, Pavković P, Knotek M: Estimating glomerular filtration rate (GFR) in diabetes: The performance of MDRD and CKD-EPI equations in patients with various degrees of albuminuria. Clin Biochem 45: 1694–1696, 2012 [DOI] [PubMed] [Google Scholar]
- 21.Stevens LA, Schmid CH, Greene T, Zhang YL, Beck GJ, Froissart M, Hamm LL, Lewis JB, Mauer M, Navis GJ, Steffes MW, Eggers PW, Coresh J, Levey AS: Comparative performance of the CKD Epidemiology Collaboration (CKD-EPI) and the Modification of Diet in Renal Disease (MDRD) Study equations for estimating GFR levels above 60 mL/min/1.73 m2. Am J Kidney Dis 56: 486–495, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rehling M, Møller ML, Thamdrup B, Lund JO, Trap-Jensen J: Simultaneous measurement of renal clearance and plasma clearance of 99mTc-labelled diethylenetriaminepenta-acetate, 51Cr-labelled ethylenediaminetetra-acetate and inulin in man. Clin Sci (Lond) 66: 613–619, 1984 [DOI] [PubMed] [Google Scholar]
- 23.Granerus G, Aurell M: Reference values for 51Cr-EDTA clearance as a measure of glomerular filtration rate. Scand J Clin Lab Invest 41: 611–616, 1981 [DOI] [PubMed] [Google Scholar]
- 24.Wuerzner G, Pruijm M, Maillard M, Bovet P, Renaud C, Burnier M, Bochud M: Marked association between obesity and glomerular hyperfiltration: A cross-sectional study in an African population. Am J Kidney Dis 56: 303–312, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Geddes CC, Woo YM, Brady S: Glomerular filtration rate—what is the rationale and justification of normalizing GFR for body surface area? Nephrol Dial Transplant 23: 4–6, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Ishizaka N, Ishizaka Y, Toda E, Shimomura H, Koike K, Seki G, Nagai R, Yamakado M: Association between cigarette smoking and chronic kidney disease in Japanese men. Hypertens Res 31: 485–492, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Wu VC, Yang SY, Lin JW, Cheng BW, Kuo CC, Tsai CT, Chu TS, Huang KH, Wang SM, Lin YH, Chiang CK, Chang HW, Lin CY, Lin LY, Chiu JS, Hu FC, Chueh SC, Ho YL, Liu KL, Lin SL, Yen RF, Wu KD, TAIPAI Study Group : Kidney impairment in primary aldosteronism. Clin Chim Acta 412: 1319–1325, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Sauriasari R, Sakano N, Wang DH, Takaki J, Takemoto K, Wang B, Sugiyama H, Sato Y, Takigawa T, Takahashi N, Kanbara S, Hitomi Y, Nakamura H, Ogino K: C-reactive protein is associated with cigarette smoking-induced hyperfiltration and proteinuria in an apparently healthy population. Hypertens Res 33: 1129–1136, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Delanaye P, Krzesinski JM: Indexing of renal function parameters by body surface area: intelligence or folly? Nephron Clin Pract 119: c289–c292, 2011 [DOI] [PubMed] [Google Scholar]
- 30.King L, MooSang M, Miller M, Reid M: Prevalence and predictors of microalbuminuria in Jamaican children with sickle cell disease. Arch Dis Child 96: 1135–1139, 2011 [DOI] [PubMed] [Google Scholar]
- 31.Hjorth L, Wiebe T, Karpman D: Hyperfiltration evaluated by glomerular filtration rate at diagnosis in children with cancer. Pediatr Blood Cancer 56: 762–766, 2011 [DOI] [PubMed] [Google Scholar]
- 32.Schell M, Lachaux A, Hadj-Aïssa A, Dubourg L, Mahmoud A, Boillot O, Saïd MH, Cochat P: Fading renal hyperfiltration in children following liver transplantation. Pediatr Transplant 5: 51–55, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Levy-Marchal C, Laborde K, Kindermans C, Dechaux M: Persisting glomerular hyperfiltration in short-term diabetic children without microalbuminuria. Acta Paediatr Scand 78: 712–716, 1989 [DOI] [PubMed] [Google Scholar]
- 34.Arant BS, Jr: Postnatal development of renal function during the first year of life. Pediatr Nephrol 1: 308–313, 1987 [DOI] [PubMed] [Google Scholar]
- 35.Abdelhafiz AH, Brown SH, Bello A, El Nahas M: Chronic kidney disease in older people: physiology, pathology or both? Nephron Clin Pract 116: c19–c24, 2010 [DOI] [PubMed] [Google Scholar]
- 36.Broad J, Wells S, Marshall R, Jackson R: Zero end-digit preference in recorded blood pressure and its impact on classification of patients for pharmacologic management in primary care - PREDICT-CVD-6. Br J Gen Pract 57: 897–903, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eriksen BO, Løchen ML, Arntzen KA, Bertelsen G, Eilertsen BA, von Hanno T, Herder M, Jenssen TG, Mathisen UD, Melsom T, Njølstad I, Solbu MD, Toft I, Mathiesen EB: Subclinical cardiovascular disease is associated with a high glomerular filtration rate in the nondiabetic general population. Kidney Int 86: 146–153, 2014 [DOI] [PubMed] [Google Scholar]
- 38.Sunder-Plassmann G, Hörl WH: A critical appraisal for definition of hyperfiltration. Am J Kidney Dis 43: 396–, author reply 396–397., 2004 [DOI] [PubMed] [Google Scholar]
- 39.[Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group] : KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 3: 1–150, 2013 [DOI] [PubMed] [Google Scholar]
- 40.Andersen TB, Erlandsen EJ, Frøkiaer J, Eskild-Jensen A, Brøchner-Mortensen J: Comparison of within- and between-subject variation of serum cystatin C and serum creatinine in children aged 2-13 years. Scand J Clin Lab Invest 70: 54–59, 2010 [DOI] [PubMed] [Google Scholar]
- 41.Sepahpanah F, Burns SP, McKnight B, Yang CC: Role of creatinine clearance as a screening test in persons with spinal cord injury. Arch Phys Med Rehabil 87: 524–528, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Forman JP, Brenner BM: ‘Hypertension’ and ‘microalbuminuria’: The bell tolls for thee. Kidney Int 69: 22–28, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Ruggenenti P, Remuzzi G: Time to abandon microalbuminuria? Kidney Int 70: 1214–1222, 2006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.