Abstract
Cardiac biomarkers, such as cardiac troponin T (cTnT), brain natriuretic peptide (BNP), and N-terminal-pro-BNP (NT-pro-BNP), are commonly used to diagnose acute coronary syndrome and congestive heart failure exacerbation in symptomatic patients. Levels of these biomarkers are frequently chronically elevated in asymptomatic patients with ESRD who are receiving maintenance dialysis. Other imaging biomarkers commonly encountered in nephrologists’ clinical practice, such as coronary artery calcium measured by computed tomography, left ventricular hypertrophy, and carotid intima-media thickness, are also frequently abnormal in asymptomatic patients with ESRD. This article critically reviews the limited observational data on associations between cTnT, BNP, NT-pro-BNP, coronary artery calcium, left ventricular hypertrophy, and carotid intima-media thickness with cardiovascular events and death in non–dialysis-dependent patients with CKD. Although sufficient evidence suggests that these biomarkers may be used for prognostication, the diagnostic utility of cTnT, BNP, and NT-pro-BNP remain challenging in patients with CKD. Decreased renal clearance may affect the plasma levels of these biomarkers, and upper reference limits were originally derived in patients without CKD. Until better data are available, higher cutoffs, or a rise in level compared with previous values, have been proposed to help distinguish acute myocardial infarction from chronic elevations of cTnT in symptomatic patients with CKD. Additionally, it is not known whether these biomarkers are modifiable and amenable to interventions that could change hard clinical outcomes in patients with CKD not yet undergoing long-term dialysis.
Keywords: chronic kidney disease, cardiovascular disease, coronary calcification, clinical epidemiology
Introduction
Cardiovascular disease (CVD) is the leading cause of death in patients with CKD and ESRD, accounting for up to 50% of all deaths (1). Cardiac biomarkers, such as cardiac troponin T (cTnT) and I (cTnI), brain natriuretic peptide (BNP), and N-terminal-pro-BNP (NT-pro-BNP), are commonly used for diagnosing acute myocardial infarction (AMI) and congestive heart failure (CHF) exacerbation. However, chronic elevations of cTnT are observed in 80%–90% of asymptomatic patients with advanced CKD and ESRD (2). cTnT has evolved into an important prognostic factor in dialysis-dependent patients with ESRD, as elevated levels are associated independently with adverse cardiovascular (CV) outcomes (3). Fewer data describe an association between elevated troponins and CVD in patients with non–dialysis-dependent CKD. Other commonly used circulating and imaging-based cardiac biomarkers are also associated with poor CV outcomes in asymptomatic patients with ESRD, but such associations are less clearly established in CKD.
The first aim of this review is to summarize studies that reported associations between traditional cardiac biomarkers, such as cTnT, BNP, NT-pro-BNP, left ventricular mass index (LVMI), coronary artery calcium (CAC) scores, carotid intima-media thickness (cIMT), and clinical outcomes in patients with CKD not yet undergoing maintenance dialysis in an attempt to highlight strengths and limitations of existing data for prognostication. The second aim is to review data that support the utility of these biomarkers for diagnostic purposes in the acute setting. These specific biomarkers were chosen because they are noninvasive tests commonly used in clinical practice. For each biomarker, a general description is given, followed by discussion of levels in CKD, association with outcomes, and, finally, the clinical utility in patients with CKD. Knowledge gaps are identified and areas for future research suggested.
Cardiac Troponin Levels in CKD
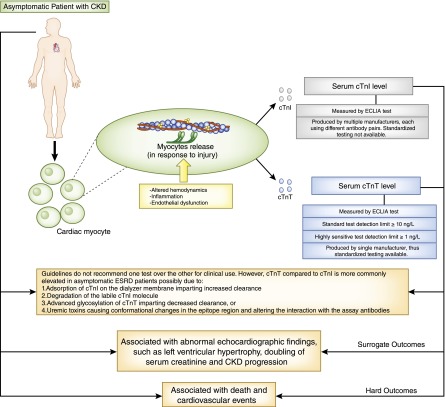
Both cTnI and cTnT are biomarkers of cardiac injury that can be measured with standard assays and high-sensitivity (hs) assays, which detect levels about 10-fold lower than the standard assay. However, the upper reference limits for cardiac troponins were originally derived in persons without CKD, and these biomarkers are elevated in up to 80% of patients with asymptomatic CKD and ESRD (2). Troponin elevation in this context does not necessarily indicate acute ischemia from coronary atherosclerosis but may be due to decreased renal clearance or chronic myocardial injury. The mechanisms for this are multifactorial and include myocardial strain from altered hemodynamics, inflammation, endothelial dysfunction, and subendocardial ischemia (3,4) (Figure 1). The effect of renal clearance on circulating troponin concentrations is uncertain (3). Previous literature suggested that cTnT levels, compared with cTnI levels, are more commonly elevated in asymptomatic patients with ESRD (5). Plausible mechanisms for differential elevations include adsorption of cTnI on the dialyzer membrane imparting increased clearance, degradation of the labile cTnI molecule, advanced glycosylation of cTnT imparting decreased clearance, or uremic toxins causing conformational changes in the epitope region and altering the interaction with the assay antibodies (3). Previous clinical data were heavily influenced by differing sensitivities of the cTnT and cTnI assays and are not relevant to contemporary clinical practice. Consensus guidelines, therefore, do not specify a preference for use of cTnI over cTnT in patients with CKD (4). cTnT and cTnI provide largely identical information, and selection between them is typically influenced by laboratory equipment and vendor selection. Unlike the cTnT assay produced by a single manufacturer, cTnI assays are produced by multiple manufacturers using different antibody pairs, and assays are not interchangeable across institutions and studies (6). We therefore chose to focus the following discussion on cTnT.
Figure 1.
Cardiac troponins in patients with CKD. In response to injury, including altered hemodynamics, inflammation, and endothelial dysfunction, cardiac myocytes release troponin T (cTnT) and troponin I (cTnI) into the circulation from the troponin I-troponin T-troponin C (troponin I-T-C) complex present on the thin filament of the contractile apparatus. Serum cTnI and cTnT levels are measured by electrochemiluminescence immunoassay (ECLIA). Compared with cTnI, cTnT assays are standardized. In addition, several reasons explain why serum cTnT, compared with cTnI levels, are elevated in asymptomatic patients with ESRD. In asymptomatic patients with CKD, cardiac troponin levels are associated with various surrogate and hard clinical outcomes.
Higher cutoffs than used in persons without CKD for the diagnosis of AMI were suggested in patients with CKD and ESRD. A cTnT cutoff of 350 ng/L (>10-fold higher than the recommended cutoff for general use) had the best sensitivity (95%) and specificity (97%) for AMI in 284 patients with ESRD presenting with chest pain (7). In 89 patients with asymptomatic CKD stages 3–5, the 95th percentile for hs-cTnT was 139 ng/L, >10-fold higher than that derived in the general population (8), with levels increasing across higher CKD stages. Another study reported that the specificity of a cutoff of >14.0 ng/L, as recommended for diagnosis of AMI in the general population, was much lower in those with an eGFR of ≤60 ml/min per 1.73 m2 (54%) versus >60 ml/min per 1.73 m2 (87%) (9). A higher cutoff of >43.2 ng/L had a much higher specificity (88%) in those with an eGFR≤60 ml/min per 1.73 m2. In addition to higher cutoffs, a rise in troponins compared with previous chronically elevated values, or a rise and/or fall using serial measurements, has been proposed to help distinguish AMI from chronic elevations of cTnT in patients with advanced CKD or ESRD (4,10,11).
Cardiac Troponins and Surrogate Outcomes
cTnT levels were associated with CV events and all-cause mortality in asymptomatic patients with ESRD (12). Although fewer data extend similar associations to patients with nondialysis CKD, several studies reported correlations between cTnT or hs-troponin T (hs-cTnT) with surrogate and hard outcomes (Table 1) (2,13–20). Cross-sectional studies revealed an association between higher cTnT and decreased eGFR, as well as measures of left ventricular hypertrophy (LVH). An analysis of the Chronic Renal Insufficiency Cohort (CRIC) reported detectable hs-cTnT (≥3 ng/L) among 81% (Table 1) (2). hs-cTnT was associated with higher LVMI across all LVMI categories, and lower ejection fraction, mainly in the lowest category (≤35%) (2). In another cross-sectional report from CRIC, the highest quartile of hs-cTnT (>24 ng/L) compared with undetectable levels was associated with the presence of LVH and left ventricular systolic dysfunction (LVSD) (21). Among asymptomatic outpatients with CAD and eGFR<60 ml/min per 1.73 m2, elevated hs-cTnT was associated with higher LVMI, lower creatinine- and cystatin-based eGFRs, and higher urine albumin-to-creatinine ratio (UACR) (Table 1) (17). Correlations between cTnT and eGFR were confirmed in British outpatients with atherosclerotic renovascular disease (16). A Japanese study of nondiabetic patients with CKD reported that those with echocardiographic evidence of left ventricular diastolic dysfunction (LVDD) had a significantly higher hs-cTnT level than those without (19).
Table 1.
Studies reporting associations of cardiac troponin T and high-sensitivity cardiac troponin T with outcomes in CKD
| Study (Reference) | Patients (n) | Study Design | Sample | Outcomes |
|---|---|---|---|---|
| Dubin et al. (2) | 2464 CRIC | Cross-sectional | Asymptomatic outpatients, eGFR 20–70 ml/min per 1.73 m2 | hs-cTnT independently associated with lower eGFR; aOR, 2.83 (95% CI, 2.41 to 3.33) for eGFR<30 ml/min per 1.73 m2 versus >60 ml/min per 1.73 m2, higher LVMI, and lower LVEF |
| Mishra et al. (21) | 3243 CRIC | Cross-sectional | Asymptomatic outpatients with eGFR<60 ml/min per 1.73 m2 and CAD | hs-cTnT>24 pg/ml versus undetectable independently associated with LVH (aOR, 2.43 [95% CI, 1.44 to 4.09]) and LVSD (aOR, 1.4 [95% CI, 1.2 to 1.7]), but not LVDD |
| deFilippi et al. (17) | 148 (50% diabetic) | Cross-sectional | Outpatients from United States with known CAD and eGFR<60 ml/min per 1.73 m2 | hs-cTnT independently associated with LVMI, decreased GFR, and increased UACR |
| Kitagawa et al. (19) | 93 (nondiabetic) | Cross-sectional | Japanese inpatients with nondiabetic CKD stages 1–5 | hs-cTnT≥9 pg/ml and BNP≥20 pg/ml were best cutoffs for severe LVDD |
| Abbas et al. (13) | 222 | Longitudinal | Asymptomatic British outpatients with CKD stages 3–5 | Detectable versus undetectable cTnT conferred increased risk for all-cause mortality (uOR, 3.47 [95% CI, 1.27 to 10.39]) (n=23) |
| Goicoechea et al. (14) | 176 | Longitudinal | Asymptomatic Spanish outpatients; n=128 with creatinine clearance <60 ml/min | Detectable versus undetectable cTnT increased hazard of CV event (uHR, 12.3 [95% CI, 4.91 to 31.02]) (n=21) |
| Chrysochou et al. (16) | 82 | Longitudinal | Asymptomatic British outpatients with ARVD at single center | cTnT independently associated with all-cause mortality (uHR, 3.9 [95% CI, 1.8 to 8.5]) |
| Scheven et al. (15) | 8121 PREVEND | Longitudinal | Asymptomatic Dutch outpatients; 18% with CKD (UACR>30 mg/g or eGFR<60 ml/min per 1.73 m2) | hs-cTnT independently associated with CV events (adjusted for eGFR, albuminuria, and CV risk factors) (aHR, 1.18 [95% CI not given; P=0.03]) |
| Hasegawa et al. (18) | 442 | Longitudinal | Asymptomatic Japanese outpatients with eGFR<60 ml/min per 1.73 m2 | hs-cTnT≥33 versus ≤9 pg/ml conferred increased risk for CV events (aHR, 6.18 [95% CI, 1.38 to 27.7]) (n=63) |
CRIC, Chronic Renal Insufficiency Cohort; PREVEND, Prevention of Renal and Vascular End Stage Diseases; CAD, coronary artery disease; ARVD, atheromatous renovascular disease; UACR, urinary albumin-to-creatinine ratio; hs-cTnT, highly sensitive cardiac troponin T; aOR, adjusted odds ratio; 95% CI, 95% confidence interval; LVMI, left ventricular mass index; LVEF, left ventricular ejection fraction; LVH, left ventricular hypertrophy; LVSD, left ventricular systolic dysfunction; LVDD, left ventricular diastolic dysfunction; BNP, brain natriuretic peptide; uOR, unadjusted odds ratio; CV, cardiovascular; uHR, unadjusted hazard ratio; aHR, adjusted hazard ratio.
Cardiac Troponins and Hard Outcomes
Limited prospective data are available regarding the association of cTnT with CV outcomes in nondialysis patients with CKD. In a British study, cTnT was detectable (≥10 ng/L) in 43% of asymptomatic patients with CKD stages 3–5 (13). Detectable cTnT was associated with increased all-cause mortality at 19 months (Table 1) (13). Similar results for the association of cTnT with increased CV events were reported by Goicoechea et al. in Spanish patients with creatinine clearance <60 ml/min (Table 1) (14). Given low event rates, however, these studies were limited by lack of multivariable analysis and adjustment for confounders (13,14,16). More recently, reports from larger cohorts showed an independent association between hs-cTnT and CV events among patients with CKD in adjusted analyses (Table 1) (15,18).
Clinical Utility of Cardiac Troponins in CKD
In summary, because troponin upper reference limits were originally derived in non-CKD samples, knowledge gaps exist in establishing consensus regarding appropriate diagnostic cutoff values in patients with CKD, as well as the required magnitude of the threshold of change in serial values. The updated consensus definition of AMI requires a rise and/or fall in serial levels, with at least one value above the 99th percentile of the upper reference limit, in addition to appropriate electrocardiographic changes, imaging consistent with myocardial damage, or new regional wall abnormalities (4). However, it does not specify different thresholds for defining AMI in patients with CKD. Nonetheless, it seems reasonable to consider higher threshold values in patients with CKD or rely more heavily on assessment of serial changes to confirm AMI diagnosis. There are no recommendations to support a specific threshold of change in patients with CKD, although recent data in 19 patients with ESRD support the use of a ≥20% change for hs-cTnT (10,11) a value that exceeds analytical variation alone (6). For prognostic purposes, it appears that detectable compared with undetectable troponins portend higher risk for future death and CV events. Future research needs to ascertain whether further work-up or intervention is warranted when clinicians find a detectable troponin in asymptomatic patients with CKD.
BNP and NT-pro-BNP in CKD
NT-pro-BNP and BNP are commonly tested in symptomatic patients suspected of acute CHF exacerbation. In one study, they were elevated in 56% of asymptomatic patients with CKD (22). Pre-pro-BNP is synthesized within the cardiac myocytes in response to ventricular wall stress and stretch (23). After removal of a signaling peptide within the cytosol, pro-BNP is further cleaved into the inactive form (NT-pro-BNP) and the active hormone (BNP) at the time of release from the myocyte or in the circulation (Figure 2). NT-pro-BNP is more stable, with a longer half-life, and may be a better biomarker for chronic volume expansion or stress than is BNP (23). Reduced renal function decreases the fractional plasma clearance of both BNP and NT-pro-BNP, and studies reported correlations between graded elevations in these peptides and declining eGFR or advancing CKD stages (Table 2) (22,24–32). The clearance of NT-pro-BNP is predominantly renal, while BNP is also degraded systemically (Figure 2) (23). This may explain the observed correlation of reduced eGFR to a greater extent with NT-pro-BNP than with BNP (23,24), and the increased ratio of NT-pro-BNP/BNP with advancing CKD stages (30), a finding not borne out by all studies. One study reported an equal dependence on renal clearances for both peptides, although most participants had a GFR≥30 ml/min per 1.73 m2 (33), suggesting that clearance may be similar for both until renal function deteriorates to advanced stages.
Figure 2.
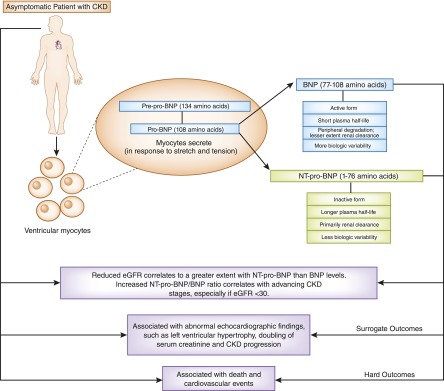
Brain natriuretic peptides in patients with CKD. In response to increased stretch or tension, left ventricular myocytes release brain natriuretic peptide (BNP) and N-terminal-pro-BNP (NT-pro-BNP) from precursors. BNP is an active molecule with a short plasma half-life and is degraded in the circulation by enzymatic action. NT-pro-BNP is the inactive form of BNP, with a longer half-life. It is primarily cleared by the kidneys. Reduced eGFR correlates to a greater extent with elevated plasma NT-pro-BNP than to BNP levels. Increased NT-pro-BNP/BNP ratio correlates with advancing CKD stages, especially if the eGFR is <30 ml/min per 1.73 m2. However, both BNP and NT-pro-BNP are associated with surrogate and hard clinical outcomes in asymptomatic patients with CKD.
Table 2.
Studies reporting associations of brain natriuretic protein and N-terminal B-type natriuretic peptide with outcomes in CKD
| Study (Reference) | Patients (n) | Study Design | Sample | Outcomes |
|---|---|---|---|---|
| Yasuda et al. (29) | 485 | Longitudinal | Japanese patients from single center with CKD stages 3–5 | Elevated BNP>86 pg/ml associated with doubling of creatinine or ESRD |
| Spanaus et al. (30) | 177 | Longitudinal | Central European nondiabetic outpatients with CKD | NT-pro-BNP, but not BNP, associated with doubling of creatinine or ESRD |
| Vickery et al. (24) | 213 | Cross-sectional | Predialysis CKD stages 3–5 | LVMI positively correlated with BNP and NT-pro-BNP (r=0.34 and 0.39) |
| Lee et al. (31) | 256 | Cross-sectional | Korean predialysis CKD, outpatients or from emergency department | NT-pro-BNP associated with LVSD independent of eGFR; higher NT-pro-BNP cutoffs for worse CKD stages: 2165 pg/ml in CKD 3, 4740 pg/ml in CKD 4, and 15,892 pg/ml in CKD 5 |
| Yang et al. (32) | 207 | Cross-sectional | Chinese outpatients with hypertension | LVDD and NT-pro-BNP associated with worse eGFR and UACR; LVDD positively correlated with Log-NT-pro-BNP (r=0.54) |
| Mishra et al. (20) | 3232 CRIC | Cross-sectional | Patients with CKD and without heart failure | Highest versus lowest quartile of NT-pro-BNP associated with LVH and LVSD (aOR, 2.7 [95% CI, 1.8 to 4.0] and 2.7 [95% CI, 1.7 to 4.5]) |
| Khan et al. (34) | 54 | Cross-sectional | Outpatients with predialysis CKD | Both NT-pro-BNP and BNP correlated with LVH and CAD |
| Bruch et al. (27) | 142 | Longitudinal | Stable German outpatients with CHF | Elevated NT-pro-BNP levels independently associated with NYHA class and inversely associated with eGFR and LVEF |
| Astor et al. (35) | 994 AASK | Longitudinal | African Americans with eGFR 20–65 ml/min per 1.73m2 and hypertension | Elevated versus undetectable NT-pro-BNP associated with CV events (aHR, 4.0 [95% CI, 2.1 to 7.6]) |
| Horii et al. (26) | 1083 | Longitudinal | Japanese patients with CKD stages 1–5 and CV disease on cardiac catheterization; ACS and acute CHF were excluded | BNP and NT-pro-BNP associated with death and CV composite; composite event AUC 0.720 for NT-pro-BNP and 0.666 for BNP; cutoffs for composite: CKD 1–3: BNP 91 pg/ml, NT-pro-BNP 260 pg/ml; CKD 4–5: BNP 157 pg/ml, NT-pro-BNP 5112 pg/ml |
| deFilippi et al. (22) | 207 | Cross-sectional | VA outpatients with predialysis CKD stages 1–5 | NT-pro-BNP >490 pg/ml independently associated with prior CAD events (n=67; AUC of 0.69) |
| Fu et al. (36) | 999 | Longitudinal | Chinese patients with CAD age >60 yr; 358 with CKD (eGFR<60 ml/min per 1.73 m2) | NT-pro-BNP associated with all-cause death if eGFR<60 ml/min per 1.73 m2 (aHR, 1.54 [95% CI, 1.32 to 1.80]) |
| Bruch et al. (37) | 341 | Longitudinal | German outpatients with stable CHF, 183 with CKD | Elevated NT-pro-BNP independently associated with CV events (including death) in patients with stable CHF with and without CKD; cutoff for both, 1474 pg/ml |
| Tarnow et al. (38) | 386 | Longitudinal | Danish outpatients with type 1 diabetes, 198 with diabetic nephropathy | Elevated NT-pro-BNP independently associated with death in patients with diabetic nephropathy (aRR, 2.49 [95% CI, 1.22 to 5.08]) |
| Anwaruddin et al. (40) | 599 | Cross-sectional | Dyspneic patients suspected of having CHF presenting to urban emergency department, 207 with CKD (eGFR<60 ml/min per 1.73 m2) | Elevated NT-pro-BNP and eGFR inversely correlated; NT-pro-BNP independently associated with 60-day mortality in patients with and without CKD |
| Oterdoom et al. (39) | 3840 | Longitudinal | Dutch outpatients, 606 renal transplant recipients | NT-pro-BNP, decreased eGFR, and hypertension medication use associated with death in both renal transplant and nontransplant patients |
AASK, African American Study of Kidney Disease and Hypertension; CHF. congestive heart failure; ACS, acute coronary syndrom; VA, Veterans Affairs; NT-pro-BNP, N-terminal B-type natriuretic peptide; 95% CI, 95% confidence interval; NYHA, New York Heart Association; aHR, adjusted hazard ratio; AUC, area under the curve; aRR, adjusted relative risk.
BNP and Surrogate Outcomes in CKD
Elevated levels of both BNP and NT-pro-BNP are associated with abnormal echocardiographic findings in patients with CKD (Table 2) (20,24,31,32,34). Among those with eGFR<60 ml/min per 1.73 m2, LVMI positively correlated with BNP and NT-pro-BNP levels (24). NT-pro-BNP was independently associated with presence of LVSD in patients with CKD (31) (Table 2). Higher gradations in NT-pro-BNP cutoffs to detect LVSD were reported for increasing CKD stages (31). A Chinese study showed that LVDD positively correlated with Log-NT-pro-BNP (32) (Table 2). In the CRIC study, the highest compared with lowest quartile of NT-pro-BNP was associated with a 3-fold higher odds of LVH and LVSD (20) (Table 2).
BNP and Hard Outcomes in CKD
BNP and NT-pro-BNP are also associated with hard outcomes in CKD (Table 2). In a Japanese study, both BNP and NT-pro-BNP were associated with death and the composite of death and CV events. On the basis of the areas under the curve, the authors concluded that NT-pro-BNP may be a superior marker to BNP for composite events in patients with CKD stages 4 and 5 (versus stages 1–3), although a formal statistical test was not used to determine whether the curves significantly differed (26). Among the African American Study of Kidney Disease and Hypertension cohort, those with elevated NT-pro-BNP had a four times higher hazard for CV events than those with undetectable levels (Table 2) (35). The association was significantly stronger in those with than without proteinuria (interaction P=0.05) (35). In Chinese patients with known CAD, NT-pro-BNP was associated with all-cause death if eGFR was <60 ml/min per 1.73 m2 (36). In addition, the NT-pro-BNP cutoff associated with mortality was higher in patients with CKD (2584 pg/ml) than in persons without CKD (370 pg/ml) (36). Several other studies reported similar associations between NT-pro-BNP, CV events, and all-cause death (Table 2) (37–39).
Clinical Utility of NT-pro-BNP and BNP in CKD
To summarize, NT-pro-BNP and BNP can be used for prognostication in patients with CKD because elevated levels are associated with both adverse surrogate and hard outcomes in this population. However, most studies included asymptomatic samples, and clinicians are still left with the important question of how to best interpret elevated BNP and NT-pro-BNP levels for acute CHF diagnosis in symptomatic patients. A study of patients presenting with dyspnea revealed that NT-pro-BNP may be a useful diagnostic test for CHF in patients with and without CKD, although the diagnostic cutoff was higher in those with eGFR<60 ml/min per 1.73 m2 (>1200 pg/ml) than in those with eGFR≥60 ml/min per 1.73 m2 (>450 pg/ml if age <50 years; >900 pg/ml if age ≥50 years) (40). More prospective, well controlled studies are needed to confirm these findings.
CAC in CKD
CAC as measured by computed tomography is a noninvasive measurement of the burden of coronary atherosclerosis. Patients with CKD have higher CAC scores compared with age-matched controls without CKD, and patients with CKD without baseline calcification exhibit higher incidence rates of developing future de novo CAC (41,42). Cross-sectional analyses have reported a graded relationship between lower eGFR and increasing CAC (41). These associations were attenuated after adjustment for traditional CV risk factors, such as diabetes, but remained statistically significant for patients with an eGFR<30 ml/min per 1.73 m2 (42). It is not entirely clear whether a decline in eGFR plays a mechanistic role for developing de novo CAC and CAC progression. Interestingly, several analyses reported higher baseline CAC and CAC progression to be associated with eGFR decline and worsening proteinuria (43–45) (Table 3). A plausible explanation may be that the progression of CAC and CKD are collinear because of the presence of similar risk factors for both disease processes.
Table 3.
Studies reporting association of coronary artery calcium with outcomes in CKD
| Study (Reference) | Patients (n) | Study Design | Sample | Outcomes |
|---|---|---|---|---|
| Chang et al. (43) | 279 | Longitudinal | Korean middle-aged outpatients with CKD, excluding those with CAD | CAC was associated with annual decrease in eGFR, even after adjustment for age, sex, baseline eGFR, albumin, and UPCR |
| Garland et al. (44) | 125 | Longitudinal | Consecutive Canadian outpatients from single center with CKD stages 3–5 | Log-transformed CAC correlated with decline in eGFR of ≥5% (r=0.22); higher baseline CAC associated with higher odds of decline in eGFR ≥5% at 1 yr |
| Maahs et al. (45) | 1066 | Longitudinal | CACTI study cohort with asymptomatic CVD | Worse UACR and eGFR associated with CAC progression |
| Russo et al. (46) | 341, 60 diabetic | Longitudinal | Single-center Italian inpatients and outpatients with CKD stages 2–5 and well controlled hypertension | CAC prevalence higher in patients with diabetes versus nondiabetic patients; patients with diabetes with CKD had higher annualized percentage increase in CAC and CV events |
| Russo et al. (50) | 181 | Longitudinal | Italian CKD stages 2–5 outpatients without symptomatic CVD | Compared with patients with baseline CAC score ≤100 AU, those with a score >100 AU had a higher hazard of CV death or MI (aHR, 4.11 [95% CI, 1.77 to 9.57]) (29 events) |
| Chiu et al. (47) | 225, all diabetic | Longitudinal | Proteinuric patients with mean UPCR of 2.7 and eGFR of 52 ml/min per 1.73 m2; 70% Latino | Those with highest (compared with lowest) quartile baseline CAC had a higher hazard of all-cause death (aHR, 2.61 [95% CI, 1.23 to 5.54]) (54 events) |
| Nguyen et al. (51) | 281, 42 diabetic | Longitudinal | Belgian kidney transplant recipients (98% white) from single center | Baseline CAC score was associated with CV composite (aHR, 1.40 [95% CI, 1.12 to 1.75]) (31 events, 8 CV deaths) |
CAC, coronary artery calcium; CACTI, Coronary Artery Calcification in Type 1 Diabetes Mellitus; UPCR, urinary protein-to-creatinine ratio; AU, Agatston units; MI, myocardial infarction; aHR, adjusted hazard ratio; 95% CI, 95% confidence interval.
Both traditional and nontraditional CV risk factors are associated with the presence and severity of CAC in patients with CKD who are not undergoing dialysis. Traditional factors explored included advanced age, white race, male sex, higher body mass index, and diabetes mellitus, in particular (46–48). A retrospective study of patients with stages 2–5 CKD with well controlled BP reported higher prevalence of CAC in patients with diabetes than in nondiabetic patients (77% versus 33%) (Table 3) (46), and another study found faster progression in CAC among diabetic patients with CKD than in those without diabetes (48). We previously reported in a multiethnic, population-based asymptomatic cohort that three nontraditional risk factors—calcium-phosphorus product, homocysteine, and osteoprotegerin—were independently associated with high CAC scores, and diminished the magnitude of the association between the presence of CKD and elevated CAC, suggesting that they may play mechanistic roles in the development of CAC (49). Others reported similar associations between elevated serum phosphorus and CAC in patients with CKD (48).
CAC and Clinical Outcomes
Fewer data are available on unfavorable clinical implications of CAC in predialysis CKD versus ESRD samples. The few observational studies reporting associations of CAC with adverse outcomes are limited by low event rates, limited follow-up, or ethnic homogeneity (Table 3) (46,47,50,51). A study of a predominantly Latino diabetic cohort reported that those in the highest compared with the lowest quartile of baseline CAC had a higher hazard of all-cause mortality at 39 months (47). During a 25-month follow-up, there was four times higher risk of CV death or AMI among outpatients with stages 2–5 CKD and baseline CAC scores >100 Agatston units (AU) compared with ≤100 AU (50). Finally, in renal transplant recipients, CAC score assessed at the inception of the cohort was associated with the composite of CV death, AMI, stroke, transient ischemic attack, and revascularization at 2.3 years (Table 3) (51). However, models were overadjusted for the few events in the last two studies (50,51).
Clinical Utility of CAC in CKD
CAC is being used as a screening test to assess risk of future CV events in patients without CKD who are at intermediate CV risk because it may add to the prognostic utility of the Framingham Risk Score (52,53). Asymptomatic persons without CKD and without CAC have a very low risk of CV events, whereas those with scores >400 AU have elevated risk similar to that in patients with diabetes or peripheral vascular disease (54). Studies in patients without CKD reported a strong correlation between CAC and total atherosclerotic plaque burden at the individual level (r=0.90) (55). Although current guidelines do not recommend the routine use of CAC for risk stratification, they do recommend its use to inform treatment decision-making in patients without CKD if a risk-based treatment decision is uncertain after quantitative risk assessment using traditional CV risk factors (56). However, it is too early to recommend the standard use of CAC for risk stratification in patients with CKD because it remains unclear whether such calcific lesions in a coronary artery segment increase or decrease biomechanical stability of atherosclerotic plaques in CKD (57). Similarly, it is not known whether increased CAC or its progression truly plays a mechanistic role in the development of future CV events or is merely a surrogate for other CV risk factors in patients with CKD. Finally, there are not enough data to show that CAC is a modifiable risk factor in CKD. For example, it is not known whether the reduction of calcium or phosphate using various binders persistently influences regression of CAC in CKD or whether CAC regression translates to improved outcomes (58).
LV Mass or LV Dysfunction in CKD
LVH and abnormal LV function, based on echocardiographic parameters, are highly prevalent among patients with CKD who initiate dialysis. According to a Canadian cohort, 74% have LVH, 36% have LV dilation, and 15% have LVSD (59). Higher baseline LVMI is associated with severity of CKD as well as progression, but it is not clear whether this is independent of high BP.
In a cross-sectional study of diabetic patients, severity of CKD stage paralleled increases in LVMI and decreases in LV ejection fraction (LVEF) (Table 4) (60). Patients with CKD stages 3–5 and LVH had lower eGFR and greater proteinuria than patients without LVH, as well as a weak inverse correlation between LVMI and eGFR (61). However, in multivariable models that included systolic BP and body mass index, eGFR was not independently associated with LVH (61). Another cross-sectional study did report a correlation between urinary protein-to-creatinine ratio and LVMI, independent of systolic BP, although a similar correlation was not observed with eGFR (62). These studies were limited by lack of controls without CKD. Interestingly, there were higher LV mass and greater degree of LVDD, but no difference in LVEF, among patients with CKD compared with age- and sex-matched controls according to univariate analyses (63). However, pulse pressure was significantly higher in patients with CKD than in controls, which could account for the observed differences (63). Finally, three prospective longitudinal studies reported changes in LV geometry to independently correlate with eGFR decline and progression to ESRD (Table 4) (64–66).
Table 4.
Studies reporting associations of left ventricular mass and function with outcomes in CKD
| Study (Reference) | Patients (n) | Study Design | Sample | Outcomes |
|---|---|---|---|---|
| Chen et al. (60) | 285 | Cross-sectional, all diabetic | Taiwanese outpatients | Stepwise increases in LVMI and decreases in LVEF corresponded to higher CKD stages |
| Nitta et al. (61) | 1185 | Cross-sectional, 41% diabetic | Japanese outpatients with CKD stages 3–5 | Echocardiography-based LVMI correlated with eGFR (r=0.18); patients with LVH had lower GFR and more proteinuria compared with those without |
| McQuarrie et al. (62) | 49 | Cross-sectional | British outpatients with CKD stages 2–4 | Log-PCR correlated with LVMI by cMRI (r=0.52); proteinuria explained 23% of LVMI variance |
| Chen et al. (64) | 415 | Longitudinal, 53% diabetic | Taiwanese outpatients with CKD stages 3–5 | cLVH measured by echocardiography associated with progression to ESRD (aHR, 2.03 [95% CI, 1.00 to 4.10]) |
| Chen et al. (65) | 540 | Longitudinal, 50% diabetic | Taiwanese outpatients with CKD stages 3–5 | Those with higher uric acid and LVMI had higher hazard of progression to dialysis and higher odds of rapid decline in eGFR (aHR, 1.83 [95% CI, 1.01 to 3.33]; aOR, 2.23 [95% CI, 1.06 to 4.70]) |
| Park et al. (66) | 3866 MESA | Longitudinal, 11% diabetic | eGFR>60 ml/min per 1.73 m2 | During a median follow-up of 4.8 yr, each SD higher LV concentricity was associated with a 9% and 8% decline in eGFRcr and eGFRcys |
| Silberberg et al. (59) | 91 | Longitudinal | Canadian patients from single center with incident ESRD | Those with highest versus lowest quintile of LVMI at baseline experienced higher hazards of all-cause mortality and CV mortality (aHR, 2.9 [95% CI, 1.3 to 6.9] and 2.7 [95% CI, 0.9 to 8.2]) |
| Chen et al. (67) | 505 | Longitudinal, 56% diabetic | Taiwanese outpatients with CKD stages 3–5 | Every g/m2 increase in LVMI and LVEF<55% versus ≥55% were associated with increased CV events (aHR, 1.006 [95% CI, 1.002 to 1.010] and 2.01 [95% CI, 1.01 to 3.74]) |
MESA, Multi-Ethnic Study of Atherosclerosis; cMRI, cardiac magnetic resonance imaging; cLVH, concentric LVH; aHR, adjusted hazard ratio; 95% CI, confidence interval; aOR, adjusted odds ratio; eGFRcr, eGFR calculated using serum creatinine; eGFRcys, eGFR calculated using cystatin C.
LV Mass, LV Dysfunction, and Clinical Outcomes
LVMI was independently associated with increased all-cause and CV mortality in patients initiating dialysis in a prospective study, even after adjustment for age, CAD, diabetes mellitus, and systolic BP (59). These findings were extended to outpatients with CKD stages 3–5, in whom higher LVMI and LVEF <55% versus ≥55% at baseline were associated with CV events, including death, AMI, sustained ventricular arrhythmia, hospitalization for unstable angina, congestive heart failure, transient ischemic attack, or stroke at 26 months (Table 4) (67).
Clinical Utility of LV Mass or LV Dysfunction in CKD
Although these data suggest that LVH is associated with CKD progression and CV events, elevated SBP and pulse pressure, which are highly prevalent in this patient population, may be major confounders in these analyses. In addition, lack of well controlled prospective studies limit the utility of echocardiographic parameters in predicting outcomes in clinical practice. Future studies need to analyze how changes in LV mass and function may be used to prognosticate hard clinical outcomes.
cIMT in CKD
The cIMT has become a frequently studied sonographic marker of early atherosclerotic changes in vessels. The thickening of the intima-media complex not only reflects a local vessel change in the carotid but could indicate a systemic change in all vessel walls. It may also predict future risk for CV events. The ease and safety of this imaging study allow its use as a potential new biomarker for systemic atherosclerosis in high-risk patient populations, such as predialysis patients with CKD. Several studies, mostly cross-sectional, suggested that cIMT measurements were elevated in CKD individuals, as reviewed later in this article (Table 5).
Table 5.
Studies reporting associations of carotid intima-media thickness with outcomes in CKD
| Study (Reference) | Patients (n) | Study Design | Sample | Outcomesa |
|---|---|---|---|---|
| Zoungas et al. (68) | 159 CKD, 159 control | Case-control | Australian outpatients with SCr≥0.40 mmol/L versus controls | Mean cIMT in CKD, 0.89±0.17 mm versus 0.73±0.13 mm in controls (P<0.05) |
| Lemos et al. (69) | 122 | Cross-sectional | Brazilian nondiabetic outpatients with CKD stages 2–5 | cIMT, 0.62±0.19 (eGFR<60 ml/min per 1.73 m2) versus 0.53±0.10 mm (eGFR>60 ml/min per 1.73 m2) (P=0.03) |
| Tanaka et al. (70) | 1003 | Cross-sectional | Japanese outpatients with and without CKD, nondialysis | In multivariable regression analysis, lower eGFR correlated with mean maximum cIMT after adjustment for age and sex (r=−0.104; P<0.001) |
| Aggarwal et al. (71) | 60 | Longitudinal | Indian outpatients with CKD stages 1–5, including ESRD | cIMT in left CCA at baseline: 0.5±0.08 mm in CKD stages 1–2, 0.7±0.10 mm in CKD stages 3–4, and 0.8±0.16 mm in CKD stage 5 (P<0.001) |
| Zhou et al. (72) | 227 | Cross-sectional | Chinese outpatients with CKD, nondialysis | CKD stage associated with increased cIMT: CKD stages 1–2, 0.64±0.18 mm; CKD stage 3, 0.74±0.25 mm; CKD stage 4, 0.81±0.25 mm; CKD stage 5, 0.86±0.20 (P<0.01) |
| Marcos et al. (76) | 117 | Longitudinal | Brazilian outpatients with CKD stages 2–4 | Patients with cIMT>0.6 mm had lower eGFR (P=0.01) ; no association with CV events or death |
| Szeto et al. (73) | 203 | Longitudinal | Chinese outpatients with CKD stages 3–4 | Event-free survival from CV death, nonfatal MI or stroke, unstable angina admission, PCI, CHF, or TIA was 94.4%, 89.8%, 77.7%, and 65.9% for cIMT quartiles 1, 2, 3, and 4, respectively (log-rank test P<0.01) |
| Desbien et al. (74) | 3364 | Longitudinal | German outpatients with and without CKD | Each 1-ml/min per 1.73 m2 decrease in creatinine clearance (aHR, 1.04 [95% CI, 1.02 to 1.23]) or 0.1-mm increase in cIMT (aHR, 1.15 [95% CI, 1.11 to 1.93]) was associated with fatal and nonfatal vascular events |
| Kim et al. (75) | 182 | Longitudinal | Korean nondiabetic, asymptomatic outpatients with eGFR<60 ml/min per 1.73 m2 | Carotid plaque associated with fatal or nonfatal ACS or stroke (OR, 7.80 [95% CI, 1.45 to 45.97]), but cIMT not significant in multivariable analysis |
| Adeseun et al. (77) | 220 CRIC | Cross-sectional | CKD outpatients with GFR 20–70 ml/min per 1.73 m2 | CAC, carotid plaque, or cIMT ability to discriminate prevalent CVD was not significant (c-statistics = 0.67, 0.64, and 0.61, respectively) (P>0.05) |
| Matsushita et al. (78) | 6553 MESA | Longitudinal | Outpatients without CVD, 1284 with CKD | CAC (aHR, 1.69 [95% CI, 1.45 to 1.97]) performed better than cIMT (aHR, 1.12 [95% CI, 1.00 to 1.25]) for prediction of CHD and heart failure in CKD |
MESA, Multi-Ethnic Study of Atherosclerosis; CRIC, Chronic Renal Insufficiency Cohort; SCr, serum creatinine; cIMT, carotid intima-media thickness; CCA, common carotid artery; PCI, percutaneous coronary intervention; TIA, transient ischemic attack; aHR, adjusted hazard ratio; 95% CI, 95% confidence interval; OR, odds ratio; aHR, adjusted hazard ratio; CHD, coronary heart disease.
Values expressed with a plus/minus sign are the mean±SD.
In a case-control study, case-patients with a serum creatinine ≥0.40 mmol/L had significantly higher cIMT than controls (Table 5) (68). Among patients with CKD stages 3–5, cIMT measurements were significantly higher if eGFR was <60 ml/min per 1.73 m2 than >60 ml/min per 1.73 m2 (69). Another study reported a weak but statistically significant correlation between lower eGFR and higher mean maximum wall thickness measured along 12 carotid segments, after adjustment for age and sex (70). Two studies revealed small but statistically significant stepwise increases in cIMT measurements with higher CKD stages (Table 5) (71,72).
cIMT and Clinical Outcomes
There are conflicting data on whether cIMT is associated with death or CV events in predialysis patients with CKD. A Chinese study of 203 patients with stages 3 or 4 CKD reported a statistically significant trend for higher adverse CV events for increasing cIMT quartiles (Table 5) (73). In a longitudinal study of 3364 outpatients with and without CKD, lower creatinine clearance and higher cIMT were associated with fatal and nonfatal vascular events (74). However, in a study of nondiabetic outpatients with eGFR<60 ml/min per 1.73 m2, carotid plaque burden but not cIMT was associated with fatal or nonfatal acute coronary syndrome (ACS) or stroke (75). Similarly, Marcos et al. did not show a significant association between the severity of cIMT and CV events or death (76). cIMT could not be used to reliably discriminate prevalent CVD in a group of outpatients with CKD (77). Finally, a recent analysis of the Multi-Ethnic Study of Atherosclerosis cohort revealed that CAC was superior to cIMT for CVD prediction in patients with and those without CKD (Table 5) (78).
Clinical Utility of cIMT in CKD
Although studies suggested that cIMT measurements are higher in patients with CKD than in those without CKD, the differences were small and of unclear clinical relevance. In addition, observed increases in cIMT with decreasing eGFR or advancing CKD stages could be confounded by other traditional risk factors that cause CKD progression, such as uncontrolled hypertension or diabetes. At present, cIMT has not proven to be a reliable predictor of hard outcomes in predialysis patients with CKD. Currently, the standardization of cIMT measurement is a major challenge, and it is not routinely recommended in clinical practice for risk assessment in the general population, let alone patients with CKD (56). Further research needs to delineate whether cIMT can be reliably measured in patients with CKD and used as a screening test for CV risk stratification in this patient population.
Conclusion
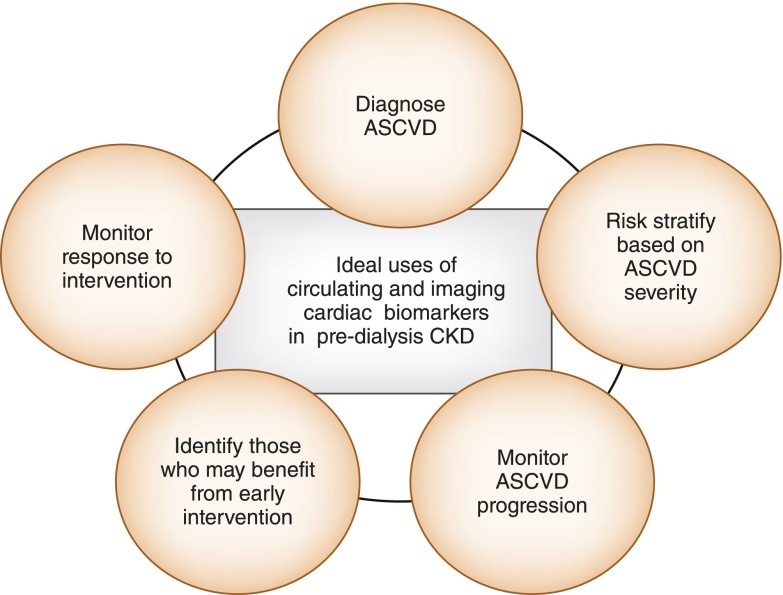
In summary, Figure 3 outlines the potential uses of an ideal circulating and imaging cardiac biomarker, which should be similar in patients with predialysis CKD. However, given current knowledge gaps, more data need to become available before all of these markers can be reliably used in this patient population. Observational studies reporting associations between cTnT and NT-pro-BNP and decline in eGFR in nondialysis patients with CKD may be confounded by decreased renal clearance of these biomarkers in the setting of advanced CKD. The same traditional and nontraditional factors associated with CAC are likely also correlated with CKD progression. Although the evidence presented suggests that these biomarkers may be used to predict future CV events in asymptomatic patients with CKD, future studies need to confirm reliable cutoffs for the utility of these biomarkers as diagnostic tests in patients presenting with symptoms concerning for ACS or acute CHF. In addition, it remains unclear whether cardiac biomarkers such as cTnT, NT-pro-BNP, BNP, CAC, and cIMT in asymptomatic patients with CKD are modifiable and amenable to interventions to reduce future CV risk. Further studies are needed to inform whether better risk stratification scores that include novel in addition to traditional biomarkers should be developed to quantify CV risk in patients with CKD.
Figure 3.
Utility of cardiac biomarkers in patients with CKD. Ideally, cardiac biomarkers should be useful in diagnosing atherosclerotic cardiovascular disease (ASCVD), risk-stratifying patients according to disease severity, monitoring ASCVD progression, identifying patients who may benefit from early intervention, and/or monitoring response to such interventions. The potential utility of circulating and imaging cardiac biomarkers in patients with CKD is similar. It includes one or many of the features of an ideal biomarker in relation with ASCVD.
Disclosures
J.A.de L. has received grant support and consulting income from Roche Diagnostics and Abbott Diagnostics.
Acknowledgments
This work is supported in part by the University of Texas Southwestern Medical Center O’Brien Kidney Research Core Center (P30-DK079328). N.J. is supported by a grant from the American Heart Association Clinical Research Program (12CRP11830004). S.S.H. receives support from a Veterans Affairs MERIT grant (CX000217) and a grant from the National Institute of Diabetes and Digestive and Kidney Diseases (R01-DK085512).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the American Heart Association, the National Institutes of Health, or the Department of Veterans Affairs.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.U.S. Renal Data System : USRDS 2010 Annual Report: Atlas of End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2010 [Google Scholar]
- 2.Dubin RF, Li Y, He J, Jaar BG, Kallem R, Lash JP, Makos G, Rosas SE, Soliman EZ, Townsend RR, Yang W, Go AS, Keane M, Defilippi C, Mishra R, Wolf M, Shlipak MG, CRIC Study Investigators : Predictors of high sensitivity cardiac troponin T in chronic kidney disease patients: A cross-sectional study in the chronic renal insufficiency cohort (CRIC). BMC Nephrol 14: 229, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jain N, Hedayati SS: How should clinicians interpret cardiac troponin values in patients with ESRD? Semin Dial 24: 398–400, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, Thygesen K, Alpert JS, White HD, Jaffe AS, Katus HA, Apple FS, Lindahl B, Morrow DA, Chaitman BR, Clemmensen PM, Johanson P, Hod H, Underwood R, Bax JJ, Bonow JJ, Pinto F, Gibbons RJ, Fox KA, Atar D, Newby LK, Galvani M, Hamm CW, Uretsky BF, Steg PG, Wijns W, Bassand JP, Menasche P, Ravkilde J, Ohman EM, Antman EM, Wallentin LC, Armstrong PW, Simoons ML, Januzzi JL, Nieminen MS, Gheorghiade M, Filippatos G, Luepker RV, Fortmann SP, Rosamond WD, Levy D, Wood D, Smith SC, Hu D, Lopez-Sendon JL, Robertson RM, Weaver D, Tendera M, Bove AA, Parkhomenko AN, Vasilieva EJ, Mendis S, Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck-Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S, Morais J, Aguiar C, Almahmeed W, Arnar DO, Barili F, Bloch KD, Bolger AF, Botker HE, Bozkurt B, Bugiardini R, Cannon C, de Lemos J, Eberli FR, Escobar E, Hlatky M, James S, Kern KB, Moliterno DJ, Mueller C, Neskovic AN, Pieske BM, Schulman SP, Storey RF, Taubert KA, Vranckx P, Wagner DR, Joint ESC/ACCF/AHA/WHF Task Force for Universal Definition of Myocardial Infarction. Authors/Task Force Members Chairpersons. Biomarker Subcommittee. ECG Subcommittee. Imaging Subcommittee. Classification Subcommittee. Intervention Subcommittee. Trials & Registries Subcommittee. Trials & Registries Subcommittee. Trials & Registries Subcommittee. Trials & Registries Subcommittee. ESC Committee for Practice Guidelines (CPG) Document Reviewers : Third universal definition of myocardial infarction. J Am Coll Cardiol 60: 1581–1598, 2012. 22958960 [Google Scholar]
- 5.Apple FS, Murakami MM, Pearce LA, Herzog CA: Predictive value of cardiac troponin I and T for subsequent death in end-stage renal disease. Circulation 106: 2941–2945, 2002 [DOI] [PubMed] [Google Scholar]
- 6.de Lemos JA: Increasingly sensitive assays for cardiac troponins: A review. JAMA 309: 2262–2269, 2013 [DOI] [PubMed] [Google Scholar]
- 7.Ryu DR, Park JT, Chung JH, Song EM, Roh SH, Lee JM, An HR, Yu M, Pyun WB, Shin GJ, Kim SJ, Kang DH, Choi KB: A more appropriate cardiac troponin T level that can predict outcomes in end-stage renal disease patients with acute coronary syndrome. Yonsei Med J 52: 595–602, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chotivanawan T, Krittayaphong R: Normal range of serum highly-sensitive troponin-T in patients with chronic kidney disease stage 3-5. J Med Assoc Thai 95[Suppl 2]: S127–S132, 2012 [PubMed] [Google Scholar]
- 9.Chenevier-Gobeaux C, Meune C, Freund Y, Wahbi K, Claessens YE, Doumenc B, Zuily S, Riou B, Ray P: Influence of age and renal function on high-sensitivity cardiac troponin T diagnostic accuracy for the diagnosis of acute myocardial infarction. Am J Cardiol 111: 1701–1707, 2013 [DOI] [PubMed] [Google Scholar]
- 10.Khalili H, de Lemos JA: What constitutes a relevant change in high-sensitivity troponin values over serial measurement? Clin Chem 60: 803–805, 2014 [DOI] [PubMed] [Google Scholar]
- 11.Aakre KM, Røraas T, Petersen PH, Svarstad E, Sellevoll H, Skadberg Ø, Sæle K, Sandberg S: Weekly and 90-minute biological variations in cardiac troponin T and cardiac troponin I in hemodialysis patients and healthy controls. Clin Chem 60: 838–847, 2014 [DOI] [PubMed] [Google Scholar]
- 12.Dierkes J, Domröse U, Westphal S, Ambrosch A, Bosselmann HP, Neumann KH, Luley C: Cardiac troponin T predicts mortality in patients with end-stage renal disease. Circulation 102: 1964–1969, 2000 [DOI] [PubMed] [Google Scholar]
- 13.Abbas NA, John RI, Webb MC, Kempson ME, Potter AN, Price CP, Vickery S, Lamb EJ: Cardiac troponins and renal function in nondialysis patients with chronic kidney disease. Clin Chem 51: 2059–2066, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Goicoechea M, Garca de Vinuesa S, Gómez-Campderá F, Gutierrez MJ, Blanco P, Amann R, Luño J: Clinical significance of cardiac troponin T levels in chronic kidney disease patients: Predictive value for cardiovascular risk. Am J Kidney Dis 43: 846–853, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Scheven L, de Jong PE, Hillege HL, Lambers Heerspink HJ, van Pelt LJ, Kootstra JE, Bakker SJ, Gansevoort RT, PREVEND study group : High-sensitive troponin T and N-terminal pro-B type natriuretic peptide are associated with cardiovascular events despite the cross-sectional association with albuminuria and glomerular filtration rate. Eur Heart J 33: 2272–2281, 2012 [DOI] [PubMed] [Google Scholar]
- 16.Chrysochou C, Manzoor S, Wright J, Roberts SA, Wood G, McDowell G, Kalra PA: Role of renal function and cardiac biomarkers (NT-proBNP and Troponin) in determining mortality and cardiac outcome in atheromatous renovascular disease. Kidney Blood Press Res 32: 373–379, 2009 [DOI] [PubMed] [Google Scholar]
- 17.deFilippi C, Seliger SL, Kelley W, Duh SH, Hise M, Christenson RH, Wolf M, Gaggin H, Januzzi J: Interpreting cardiac troponin results from high-sensitivity assays in chronic kidney disease without acute coronary syndrome. Clin Chem 58: 1342–1351, 2012 [DOI] [PubMed] [Google Scholar]
- 18.Hasegawa M, Ishii J, Kitagawa F, Kanayama K, Takahashi H, Ozaki Y, Yuzawa Y: Prognostic value of highly sensitive troponin T on cardiac events in patients with chronic kidney disease not on dialysis. Heart Vessels 28: 473–479, 2013 [DOI] [PubMed] [Google Scholar]
- 19.Kitagawa M, Sugiyama H, Morinaga H, Inoue T, Takiue K, Kikumoto Y, Uchida HA, Kitamura S, Maeshima Y, Toh N, Nakamura K, Ito H, Makino H: Serum high-sensitivity cardiac troponin T is a significant biomarker of left-ventricular diastolic dysfunction in subjects with non-diabetic chronic kidney disease. Nephron Extra 1: 166–177, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mishra RK, Li Y, Ricardo AC, Yang W, Keane M, Cuevas M, Christenson R, deFilippi C, Chen J, He J, Kallem RR, Raj DS, Schelling JR, Wright J, Go AS, Shlipak MG, Chronic Renal Insufficiency Cohort Investigators : Association of N-terminal pro-B-type natriuretic peptide with left ventricular structure and function in chronic kidney disease (from the Chronic Renal Insufficiency Cohort [CRIC]). Am J Cardiol 111: 432–438, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mishra RK, Li Y, DeFilippi C, Fischer MJ, Yang W, Keane M, Chen J, He J, Kallem R, Horwitz EJ, Rafey M, Raj DS, Go AS, Shlipak MG, CRIC Study Investigators : Association of cardiac troponin T with left ventricular structure and function in CKD. Am J Kidney Dis 61: 701–709, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DeFilippi CR, Fink JC, Nass CM, Chen H, Christenson R: N-terminal pro-B-type natriuretic peptide for predicting coronary disease and left ventricular hypertrophy in asymptomatic CKD not requiring dialysis. Am J Kidney Dis 46: 35–44, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Panteghini M, Clerico A: Understanding the clinical biochemistry of N-terminal pro-B-type natriuretic peptide: The prerequisite for its optimal clinical use. Clin Lab 50: 325–331, 2004 [PubMed] [Google Scholar]
- 24.Vickery S, Price CP, John RI, Abbas NA, Webb MC, Kempson ME, Lamb EJ: B-type natriuretic peptide (BNP) and amino-terminal proBNP in patients with CKD: Relationship to renal function and left ventricular hypertrophy. Am J Kidney Dis 46: 610–620, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Yi S, Contreras G, Miller ER, Appel LJ, Astor BC: Correlates of N-terminal prohormone brain natriuretic peptides in African Americans with hypertensive chronic kidney disease: the African American Study of Kidney Disease and Hypertension. Am J Nephrol 29: 292–298, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horii M, Matsumoto T, Uemura S, Sugawara Y, Takitsume A, Ueda T, Nakagawa H, Nishida T, Soeda T, Okayama S, Somekawa S, Ishigami K, Takeda Y, Kawata H, Kawakami R, Saito Y: Prognostic value of B-type natriuretic peptide and its amino-terminal proBNP fragment for cardiovascular events with stratification by renal function. J Cardiol 61: 410–416, 2013 [DOI] [PubMed] [Google Scholar]
- 27.Bruch C, Reinecke H, Stypmann J, Rothenburger M, Schmid C, Breithardt G, Wichter T, Gradaus R: N-terminal pro-brain natriuretic peptide, kidney disease and outcome in patients with chronic heart failure. J Heart Lung Transplant 25: 1135–1141, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Bodlaj G, Hubmann R, Saleh K, Biesenbach G, Pohanka E, Stojakovic T, Berg J: Serum levels of N-terminal pro-B-type natriuretic peptide are associated with allograft function in recipients of renal transplants. Wien Klin Wochenschr 121: 631–637, 2009 [DOI] [PubMed] [Google Scholar]
- 29.Yasuda K, Kimura T, Sasaki K, Obi Y, Iio K, Yamato M, Rakugi H, Isaka Y, Hayashi T: Plasma B-type natriuretic peptide level predicts kidney prognosis in patients with predialysis chronic kidney disease. Nephrol Dial Transplant 27: 3885–3891, 2012 [DOI] [PubMed] [Google Scholar]
- 30.Spanaus KS, Kronenberg F, Ritz E, Schlapbach R, Fliser D, Hersberger M, Kollerits B, König P, von Eckardstein A, Mild-to-Moderate Kidney Disease Study Group : B-type natriuretic peptide concentrations predict the progression of nondiabetic chronic kidney disease: the Mild-to-Moderate Kidney Disease Study. Clin Chem 53: 1264–1272, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Lee JE, Choi SY, Huh W, Park SW, Kim DJ, Oh HY, Kim YG: N-terminal pro-brain natriuretic peptide levels predict left ventricular systolic function in patients with chronic kidney disease. J Korean Med Sci 24[Suppl]: S63–S68, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang Y, Wang Y, Shi ZW, Zhu DL, Gao PJ: Association of E/E’ and NT-proBNP with renal function in patients with essential hypertension. PLoS ONE 8: e54513, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Kimmenade RR, Januzzi JL, Jr, Bakker JA, Houben AJ, Rennenberg R, Kroon AA, Crijns HJ, van Dieijen-Visser MP, de Leeuw PW, Pinto YM: Renal clearance of B-type natriuretic peptide and amino terminal pro-B-type natriuretic peptide a mechanistic study in hypertensive subjects. J Am Coll Cardiol 53: 884–890, 2009 [DOI] [PubMed] [Google Scholar]
- 34.Khan IA, Fink J, Nass C, Chen H, Christenson R, deFilippi CR: N-terminal pro-B-type natriuretic peptide and B-type natriuretic peptide for identifying coronary artery disease and left ventricular hypertrophy in ambulatory chronic kidney disease patients. Am J Cardiol 97: 1530–1534, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Astor BC, Yi S, Hiremath L, Corbin T, Pogue V, Wilkening B, Peterson G, Lewis J, Lash JP, Van Lente F, Gassman J, Wang X, Bakris G, Appel LJ, Contreras G: N-terminal prohormone brain natriuretic peptide as a predictor of cardiovascular disease and mortality in blacks with hypertensive kidney disease: The African American Study of Kidney Disease and Hypertension (AASK). Circulation 117: 1685–1692, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Fu S, Luo L, Ye P, Yi S, Liu Y, Zhu B, Wang L, Xiao T, Bai Y: The ability of NT-proBNP to detect chronic heart failure and predict all-cause mortality is higher in elderly Chinese coronary artery disease patients with chronic kidney disease. Clin Interv Aging 8: 409–417, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bruch C, Fischer C, Sindermann J, Stypmann J, Breithardt G, Gradaus R: Comparison of the prognostic usefulness of N-terminal pro-brain natriuretic Peptide in patients with heart failure with versus without chronic kidney disease. Am J Cardiol 102: 469–474, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Tarnow L, Gall MA, Hansen BV, Hovind P, Parving HH: Plasma N-terminal pro-B-type natriuretic peptide and mortality in type 2 diabetes. Diabetologia 49: 2256–2262, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Oterdoom LH, de Vries AP, van Ree RM, Gansevoort RT, van Son WJ, van der Heide JJ, Navis G, de Jong PE, Gans RO, Bakker SJ: N-terminal pro-B-type natriuretic peptide and mortality in renal transplant recipients versus the general population. Transplantation 87: 1562–1570, 2009 [DOI] [PubMed] [Google Scholar]
- 40.Anwaruddin S, Lloyd-Jones DM, Baggish A, Chen A, Krauser D, Tung R, Chae C, Januzzi JL, Jr: Renal function, congestive heart failure, and amino-terminal pro-brain natriuretic peptide measurement: Results from the ProBNP Investigation of Dyspnea in the Emergency Department (PRIDE) Study. J Am Coll Cardiol 47: 91–97, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Budoff MJ, Rader DJ, Reilly MP, Mohler ER, 3rd, Lash J, Yang W, Rosen L, Glenn M, Teal V, Feldman HI, CRIC Study Investigators : Relationship of estimated GFR and coronary artery calcification in the CRIC (Chronic Renal Insufficiency Cohort) Study. Am J Kidney Dis 58: 519–526, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lamprea-Montealegre JA, McClelland RL, Astor BC, Matsushita K, Shlipak M, de Boer IH, Szklo M: Chronic kidney disease, plasma lipoproteins, and coronary artery calcium incidence: The Multi-Ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol 33: 652–658, 2013 [DOI] [PubMed] [Google Scholar]
- 43.Chang JH, Sung JY, Nam HE, Jeong H, Jo MY, Hwang YH, Jung JY, Lee HH, Chung W, Sung YM, Kim S: Role of coronary artery calcification score on the decrease in GFR among subjects with CT coronary angiography. Clin Exp Hypertens 34: 24–30, 2012 [DOI] [PubMed] [Google Scholar]
- 44.Garland JS, Holden RM, Hopman WM, Gill SS, Nolan RL, Morton AR: Body mass index, coronary artery calcification, and kidney function decline in stage 3 to 5 chronic kidney disease patients. J Ren Nutr 23: 4–11, 2013 [DOI] [PubMed] [Google Scholar]
- 45.Maahs DM, Jalal D, Chonchol M, Johnson RJ, Rewers M, Snell-Bergeon JK: Impaired renal function further increases odds of 6-year coronary artery calcification progression in adults with type 1 diabetes: The CACTI study. Diabetes Care 36: 2607–2614, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Russo D, Morrone LF, Imbriaco M, Pota A, Russo L, Scognamiglio B, Sorrentino R: Coronary artery calcification and outcomes in diabetic patients with and without chronic kidney disease. Blood Purif 36: 17–20, 2013 [DOI] [PubMed] [Google Scholar]
- 47.Chiu YW, Adler SG, Budoff MJ, Takasu J, Ashai J, Mehrotra R: Coronary artery calcification and mortality in diabetic patients with proteinuria. Kidney Int 77: 1107–1114, 2010 [DOI] [PubMed] [Google Scholar]
- 48.Stavroulopoulos A, Porter CJ, Pointon K, Monaghan JM, Roe SD, Cassidy MJ: Evolution of coronary artery calcification in patients with chronic kidney disease Stages 3 and 4, with and without diabetes. Nephrol Dial Transplant 26: 2582–2589, 2011 [DOI] [PubMed] [Google Scholar]
- 49.Baber U, de Lemos JA, Khera A, McGuire DK, Omland T, Toto RD, Hedayati SS: Non-traditional risk factors predict coronary calcification in chronic kidney disease in a population-based cohort. Kidney Int 73: 615–621, 2008 [DOI] [PubMed] [Google Scholar]
- 50.Russo D, Corrao S, Battaglia Y, Andreucci M, Caiazza A, Carlomagno A, Lamberti M, Pezone N, Pota A, Russo L, Sacco M, Scognamiglio B: Progression of coronary artery calcification and cardiac events in patients with chronic renal disease not receiving dialysis. Kidney Int 80: 112–118, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nguyen PT, Henrard S, Coche E, Goffin E, Devuyst O, Jadoul M: Coronary artery calcification: A strong predictor of cardiovascular events in renal transplant recipients. Nephrol Dial Transplant 25: 3773–3778, 2010 [DOI] [PubMed] [Google Scholar]
- 52.D’Agostino RB, Sr, Grundy S, Sullivan LM, Wilson P, CHD Risk Prediction Group : Validation of the Framingham coronary heart disease prediction scores: Results of a multiple ethnic groups investigation. JAMA 286: 180–187, 2001 [DOI] [PubMed] [Google Scholar]
- 53.Budoff MJ, Achenbach S, Blumenthal RS, Carr JJ, Goldin JG, Greenland P, Guerci AD, Lima JA, Rader DJ, Rubin GD, Shaw LJ, Wiegers SE, American Heart Association Committee on Cardiovascular Imaging and Intervention. American Heart Association Council on Cardiovascular Radiology and Intervention. American Heart Association Committee on Cardiac Imaging, Council on Clinical Cardiology : Assessment of coronary artery disease by cardiac computed tomography: A scientific statement from the American Heart Association Committee on Cardiovascular Imaging and Intervention, Council on Cardiovascular Radiology and Intervention, and Committee on Cardiac Imaging, Council on Clinical Cardiology. Circulation 114: 1761–1791, 2006 [DOI] [PubMed] [Google Scholar]
- 54.Greenland P, Bonow RO, Brundage BH, Budoff MJ, Eisenberg MJ, Grundy SM, Lauer MS, Post WS, Raggi P, Redberg RF, Rodgers GP, Shaw LJ, Taylor AJ, Weintraub WS, Harrington RA, Abrams J, Anderson JL, Bates ER, Grines CL, Hlatky MA, Lichtenberg RC, Lindner JR, Pohost GM, Schofield RS, Shubrooks SJ, Jr, Stein JH, Tracy CM, Vogel RA, Wesley DJ, American College of Cardiology Foundation Clinical Expert Consensus Task Force (ACCF/AHA Writing Committee to Update the 2000 Expert Consensus Document on Electron Beam Computed Tomography) Society of Atherosclerosis Imaging and Prevention. Society of Cardiovascular Computed Tomography : ACCF/AHA 2007 clinical expert consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain: A report of the American College of Cardiology Foundation Clinical Expert Consensus Task Force (ACCF/AHA Writing Committee to Update the 2000 Expert Consensus Document on Electron Beam Computed Tomography). Circulation 115: 402–426, 2007 [DOI] [PubMed] [Google Scholar]
- 55.Rumberger JA, Sheedy PF, 3rd, Breen JF, Schwartz RS: Coronary calcium, as determined by electron beam computed tomography, and coronary disease on arteriogram. Effect of patient’s sex on diagnosis. Circulation 91: 1363–1367, 1995 [DOI] [PubMed] [Google Scholar]
- 56.Goff DC, Jr, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Sr, Gibbons R, Greenland P, Lackland DT, Levy D, O’Donnell CJ, Robinson JG, Schwartz JS, Shero ST, Smith SC, Jr, Sorlie P, Stone NJ, Wilson PW, American College of Cardiology/American Heart Association Task Force on Practice Guidelines : 2013 ACC/AHA guideline on the assessment of cardiovascular risk: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 63[25 Pt B]: 2935–2959, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Beckman JA, Ganz J, Creager MA, Ganz P, Kinlay S: Relationship of clinical presentation and calcification of culprit coronary artery stenoses. Arterioscler Thromb Vasc Biol 21: 1618–1622, 2001 [DOI] [PubMed] [Google Scholar]
- 58.McCullough PA, Chinnaiyan KM: Annual progression of coronary calcification in trials of preventive therapies: A systematic review. Arch Intern Med 169: 2064–2070, 2009 [DOI] [PubMed] [Google Scholar]
- 59.Silberberg JS, Barre PE, Prichard SS, Sniderman AD: Impact of left ventricular hypertrophy on survival in end-stage renal disease. Kidney Int 36: 286–290, 1989 [DOI] [PubMed] [Google Scholar]
- 60.Chen SC, Chang JM, Liu WC, Tsai YC, Tsai JC, Su HM, Hwang SJ, Chen HC: Stepwise increases in left ventricular mass index and decreases in left ventricular ejection fraction correspond with the stages of chronic kidney disease in diabetes patients. Exp Diabetes Res 2012: 789325, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nitta K, Iimuro S, Imai E, Matsuo S, Makino H, Akizawa T, Watanabe T, Ohashi Y, Hishida A: Risk factors for increased left ventricular hypertrophy in patients with chronic kidney disease. Clin Exp Nephrol 17: 730–742, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 62.McQuarrie EP, Patel RK, Mark PB, Delles C, Connell J, Dargie HJ, Steedman T, Jardine AG: Association between proteinuria and left ventricular mass index: A cardiac MRI study in patients with chronic kidney disease. Nephrol Dial Transplant 26: 933–938, 2011 [DOI] [PubMed] [Google Scholar]
- 63.Edwards NC, Ferro CJ, Townend JN, Steeds RP: Aortic distensibility and arterial-ventricular coupling in early chronic kidney disease: A pattern resembling heart failure with preserved ejection fraction. Heart 94: 1038–1043, 2008 [DOI] [PubMed] [Google Scholar]
- 64.Chen SC, Su HM, Hung CC, Chang JM, Liu WC, Tsai JC, Lin MY, Hwang SJ, Chen HC: Echocardiographic parameters are independently associated with rate of renal function decline and progression to dialysis in patients with chronic kidney disease. Clin J Am Soc Nephrol 6: 2750–2758, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen SC, Chang JM, Yeh SM, Su HM, Chen HC: Association of uric acid and left ventricular mass index with renal outcomes in chronic kidney disease. Am J Hypertens 26: 243–249, 2013 [DOI] [PubMed] [Google Scholar]
- 66.Park M, Shlipak MG, Katz R, Agarwal S, Ix JH, Hsu CY, Peralta CA: Subclinical cardiac abnormalities and kidney function decline: The multi-ethnic study of atherosclerosis. Clin J Am Soc Nephrol 7: 1137–1144, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen SC, Chang JM, Liu WC, Huang JC, Tsai JC, Lin MY, Su HM, Hwang SJ, Chen HC: Echocardiographic parameters are independently associated with increased cardiovascular events in patients with chronic kidney disease. Nephrol Dial Transplant 27: 1064–1070, 2012 [DOI] [PubMed] [Google Scholar]
- 68.Zoungas S, Cameron JD, Kerr PG, Wolfe R, Muske C, McNeil JJ, McGrath BP: Association of carotid intima-medial thickness and indices of arterial stiffness with cardiovascular disease outcomes in CKD. Am J Kidney Dis 50: 622–630, 2007 [DOI] [PubMed] [Google Scholar]
- 69.Lemos MM, Jancikic AD, Sanches FM, Christofalo DM, Ajzen SA, Carvalho AB, Draibe SA, Canziani ME: Intima-media thickness is associated with inflammation and traditional cardiovascular risk factors in non-dialysis-dependent patients with chronic kidney disease. Nephron Clin Pract 115: c189–c194, 2010 [DOI] [PubMed] [Google Scholar]
- 70.Tanaka M, Abe Y, Furukado S, Miwa K, Sakaguchi M, Sakoda S, Kitagawa K: Chronic kidney disease and carotid atherosclerosis. J Stroke Cerebrovasc Dis 21: 47–51, 2012 [DOI] [PubMed] [Google Scholar]
- 71.Aggarwal HK, Jain D, Lathar M, Yadav RK, Sawhney A: Lipoprotein-A and carotid intima media thickness as cardiovascular risk factors in patients of chronic kidney disease. Ren Fail 32: 647–652, 2010 [DOI] [PubMed] [Google Scholar]
- 72.Zhou W, Ni Z, Yu Z, Shi B, Wang Q: Brain natriuretic peptide is related to carotid plaques and predicts atherosclerosis in pre-dialysis patients with chronic kidney disease. Eur J Intern Med 23: 539–544, 2012 [DOI] [PubMed] [Google Scholar]
- 73.Szeto CC, Chow KM, Woo KS, Chook P, Ching-Ha Kwan B, Leung CB, Kam-Tao Li P: Carotid intima media thickness predicts cardiovascular diseases in Chinese predialysis patients with chronic kidney disease. J Am Soc Nephrol 18: 1966–1972, 2007 [DOI] [PubMed] [Google Scholar]
- 74.Desbien AM, Chonchol M, Gnahn H, Sander D: Kidney function and progression of carotid intima-media thickness in a community study. Am J Kidney Dis 51: 584–593, 2008 [DOI] [PubMed] [Google Scholar]
- 75.Kim JK, Song YR, Kim MG, Kim HJ, Kim SG: Clinical significance of subclinical carotid atherosclerosis and its relationship with echocardiographic parameters in non-diabetic chronic kidney disease patients. BMC Cardiovasc Disord 13: 96, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Marcos AG, Watanabe R, Lemos MM, Canziani ME: [Evaluation of intima-media thickness in patients with chronic kidney disease not on dialysis: a prospective study of 24 month]. J Bras Neurol 36: 35–41, 2014 [DOI] [PubMed] [Google Scholar]
- 77.Adeseun GA, Xie D, Wang X, Joffe MM, Mohler ER, 3rd, Townsend RR, Budoff M, Rosas SE: Carotid plaque, carotid intima-media thickness, and coronary calcification equally discriminate prevalent cardiovascular disease in kidney disease. Am J Nephrol 36: 342–347, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Matsushita K, Sang Y, Ballew SH, Shlipak M, Katz R, Rosas SE, Peralta CA, Woodward M, Kramer HJ, Jacobs DR, Sarnak MJ, Coresh J: Subclinical atherosclerosis measures for cardiovascular prediction in CKD [published online ahead of print August 21, 2014]. J Am Soc Nephrol 10.1681/ASN.2014020173 [DOI] [PMC free article] [PubMed] [Google Scholar]