Abstract
Urine differs greatly in ion and solute composition from plasma and contains harmful and noxious substances that must be stored for hours and then eliminated when it is socially convenient to do so. The urinary tract that handles this output is composed of a series of pressurizable muscular compartments separated by sphincteric structures. With neural input, these structures coordinate the delivery, collection, and, ultimately, expulsion of urine. Despite large osmotic and chemical gradients in this waste fluid, the bladder maintains a highly impermeable surface in the face of a physically demanding biomechanical environment, which mandates recurring cycles of surface area expansion and increased wall tension during filling, followed by rapid wall compression during voiding. Afferent neuronal inflow from mucosa and submucosa communicates sensory information about bladder fullness, and voiding is initiated consciously through coordinated central and spinal efferent outflow to the detrusor, trigonal internal sphincter, and external urethral sphincter after periods of relative quiescence. Provocative new findings suggest that in some cases, lower urinary tract symptoms, such as incontinence, urgency, frequency, overactivity, and pain may be viewed as a consequence of urothelial defects (either urothelial barrier breakdown or inappropriate signaling from urothelial cells to underlying sensory afferents and potentially interstitial cells). This review describes the physiologic and anatomic mechanisms by which urine is moved from the kidney to the bladder, stored, and then released. Relevant clinical examples of urinary tract dysfunction are also discussed.
Keywords: calcium, cell and transport physiology, cell signaling, urinary tract, epithelial
Anatomy of the Lower Urinary Tract
Urinary drainage from the kidneys occurs through the ureters (25–30 cm long in adults), which enter the bladder distally via a specialized region near the base of the bladder called the trigone. Unidirectional flow from the kidney pelvis into the ureter and from the ureter to the bladder is assisted by two constrictions at opposite ends: the ureteropelvic junction (UPJ) and the ureterovesical junction (UVJ), respectively. These unique fibromuscular structures, which are not strictly classic sphincters, provide antireflux protection in order to ensure that urine transport occurs in only one direction. The ureters consist of stratified layers composed of epithelium (the urothelium), lamina propria, and smooth muscle.
Ureteral smooth muscle cells are arranged in longitudinal, circular, and spiral bundles to facilitate peristaltic movement of urine toward the bladder. As urine is forced past the UPJ by calyceal and renal pelvis smooth muscular contraction, it enters the ureters as a bolus that travels down to the UVJ and is then extruded into the bladder. Distally, the ureters insert into the bladder at an oblique angle and traverse the muscle over a distance of approximately 1.5 cm. Beginning above the entry point to the detrusor, the ureter is sheathed by a layer of longitudinal smooth muscle. This sheath passes through the vesical wall and then diverges to merge with the deep trigone (1). The intravesical ureter forms a valve, which is important in the prevention of reflux. It also protects the kidney from retrograde exposure to the high pressures generated by the bladder at voiding and also from infections localized in the bladder. The physical relationship of the UVJ in terms of its length, diameter, and positioning relative to the trigone prevents retrograde flow. Damage to the trigone, congenital abnormalities, or trigonal muscular weakness are all primary causes of vesicoureteral reflux. The complexity of the UVJ makes it the most difficult anastomosis to perform in renal transplantation (2).
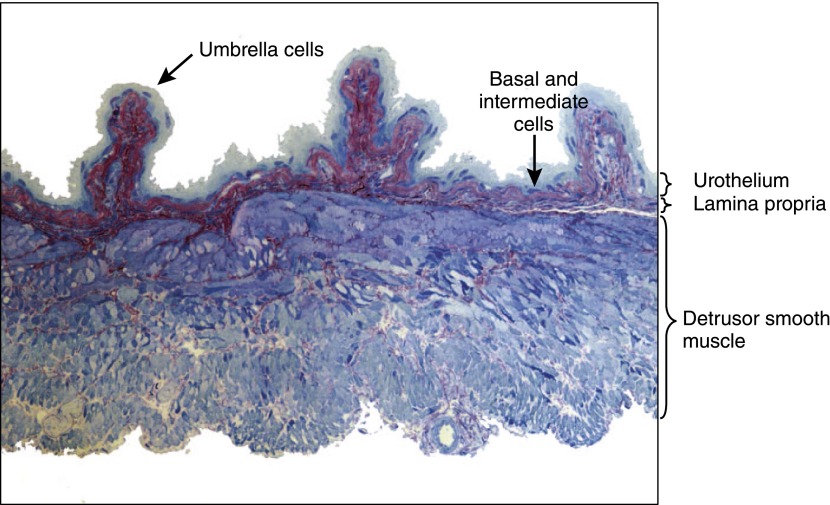
The bladder is a highly deformable muscular sac that has two primary functions: storage and expulsion. It features a layered structure similar to that of the ureters, with a highly impermeable urothelium, an intermediate vascularized lamina propria composed of connective tissue, several fibroblastic cell types, and a thick smooth muscle coat called the detrusor. Figure 1 illustrates the laminar nature of these layers and the surface topography of the bladder, which is highly folded and ridged when not fully stretched.
Figure 1.
Mouse bladder sectioned and counterstained with toluidine blue. The layers of the bladder and their relative dimensions are easily visualized by different degrees of red/blue coloration.
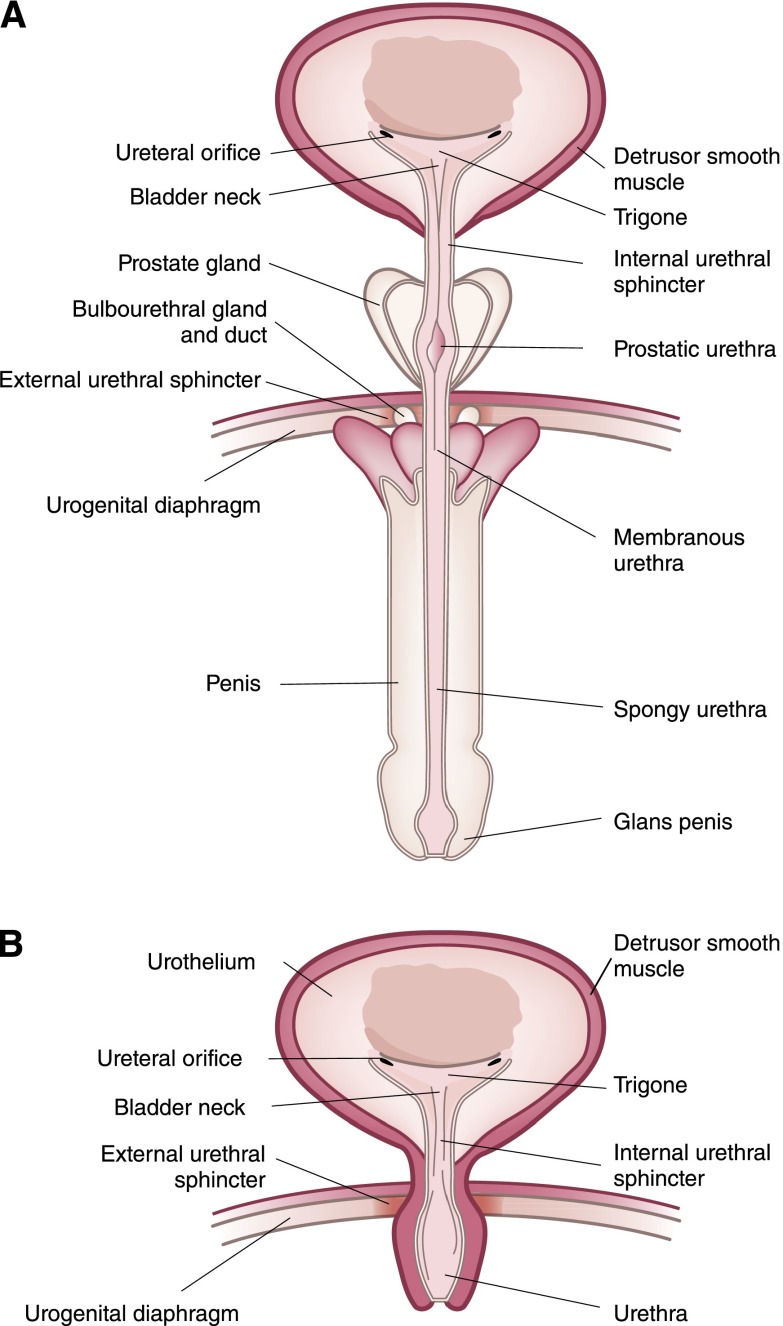
The urethra begins at the lower apex of the bladder neck and is formed of several layers of muscle. Figure 2 shows the anatomy of male and female urethrae. The urethra, on average, is 3.5–5 cm long in women, whereas it is approximately 20 cm long in men. The urethral tube is formed from an inner longitudinal smooth muscle, which, in turn, is surrounded by a thinner circular smooth muscle layer. Urinary continence is maintained in both sexes by an involuntary internal sphincter and a voluntary external urethral sphincter (EUS), sometimes called the rhabdosphincter.
Figure 2.
Lower urinary tract anatomy including bladder and urethra. (A) Human male and (B) female.
Physiology of the Ureters
Urine flow through the ureters occurs by peristalsis, which facilitates unidirectional flow. At normal urine production rates, contraction of the renal pelvis forces a bolus of urine into the ureter, upon which waves of contraction (20–80 cmH2O; 2–6 times/min) occur behind the bolus and force it distally into relaxed sections with baseline pressures of only 0–5 cmH2O. When urine production rates become particularly high, boluses can become larger and then merge and may ultimately become essentially a column of fluid. Upon expulsion of urine through the UVJ, the peristaltic wave dissipates.
Ureteral obstruction raises the intraluminal pressure above the obstruction and is usually accompanied by an increase in peristaltic frequency as well as changes in ureteral dimensions. In response to this distal pressure, the ureter becomes dilated and increases in length due to the retention of urine and tissue stretching. If the obstruction is not cleared, the contractions diminish, intraluminal pressures will subsequently diminish to almost baseline levels, and the ureter can ultimately decompensate, losing the ability to contract even if the obstruction is removed. Hence, the early response to calculi is an increase in peristaltic pressure, resulting in increased proximal pressure on the stone and simultaneous relaxation at, and distal to, the blockage. In tandem, these responses are designed to aid in stone passage. However, if this does not prove successful and blockage is maintained, irreversible damage can occur.
Smooth muscle contractions occur in response to increases in intracellular calcium (Ca2+); conversely, relaxation occurs when Ca2+ drops. This has been exploited therapeutically via the use of calcium channel blockers (e.g., nifedipine), which augment relaxation and thereby assist with stone passage, in an approach known as medical expulsive therapy (3,4). Smooth muscle contracts either as a response to neurotransmitters released from firing of motor neurons (neurogenic contractility) or as a result of local intrinsic network signaling originating within the muscle (myogenic or spontaneous contractility).
Myogenic Contractility
Electrical activity within the pyeloureters causes smooth muscle contractility at the kidney pelvis and results in coordinated peristalsis. This activity originates in a pacemaker cell network composed of atypical smooth muscle cells (SMCs) and interstitial cells of Cajal-like (ICC-like) cells. These pacemaker cells spontaneously generate electrical activity. Although the precise contribution of these two cell types is not yet defined in ureters, atypical SMCs appear to be the primary pacemakers at the UPJ (5–9). These cells exhibit spontaneous high-frequency Ca2+ spikes released from internal stores that propagate through gap junctions to induce transient membrane depolarization in typical SMCs. Depolarization activates an influx of Ca2+ through voltage-gated L-type calcium channels on the plasma membrane, which then initiates tonic cytosolic Ca2+ oscillations resulting in SMC contraction (10). The role of ICC-like cells is not clear because they form a separate, but interacting, electrically active network, which may be important in peristaltic contraction in distal regions of the ureter (8). Loss of ICC-like cells has been noted in studies of congenital UPJ obstruction (11–14). It is not clear, however, whether this reduction in cell number is a cause of the obstruction or a secondary consequence.
Neurogenic Contractility
Ureters are innervated directly by afferent and efferent fibers. However, peristalsis persists in the presence of tetrodotoxin, which blocks voltage-gated sodium channels and, hence, action potentials. Furthermore, ureters transplanted along with transplant kidneys, which lose their innervation, continue to exhibit peristalsis (15). This strongly suggests that neurogenic regulation is likely to be modulatory in nature (16). Neurotransmitter signaling pathways from both sympathetic and parasympathetic systems include adrenergic (adrenaline/noradrenaline), cholinergic (acetylcholine), and so-called nonadrenergic, noncholinergic effectors, such as ATP.
The sympathetic nervous system plays a prominent role in ureteral contractile function and both α- and β-adrenergic receptors (ARs) are present in human ureters with α-receptors presenting at higher density (17). α-ARs augment contractions and the clinically used α1-AR blocker tamsulosin can reduce or block human ureteric contraction (18). Likewise, the α1-AR blocker doxazosin also inhibits spontaneous contractions in human ureteral strips and both drugs have found clinical use in assisting ureteral stone passage through the relative relaxant effects (19,20). By contrast, β-ARs enhance ureteral wall relaxation. The β2 and β3 subtypes appear to be the predominant receptors mediating relaxation (21,22).
Bladder Accommodation and Expulsion of Urine
The bladder accommodates the flow of urine by maintaining a low intravesical pressure during filling. This compliance in the bladder wall allows volume accommodation and storage, but also ensures that the ureters are not forced to transport urine into a pressurized compartment, with the attendant risk of retrograde flow. Upon voiding, the bladder rapidly contracts and, in a few seconds, develops intravesical pressures of 50–60 cmH2O before voluntary sphincter opening allows expulsion of urine at flow rates of 20–30 ml/s. Both accommodation and expulsion of urine require coordinated communication between urothelium, afferent nerves, spinal and hypothalamic centers, efferent pathways, and detrusor and sphincter muscles.
Neural Pathways
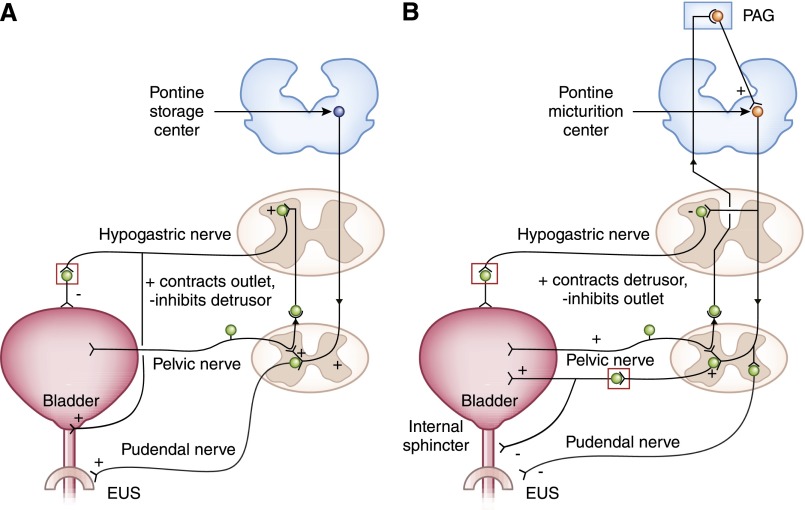
During infancy, and in patients who have suffered spinal cord injury, the bladder is capable of reflex emptying, but micturition is not under conscious control. The development of voiding control requires that this immature reflex contraction is inhibited. For infants, peripheral nervous system connections are ready for use at birth, but the coordination that needs to occur between the bladder and sphincter is thought to require maturation of central neural control. The three sets of peripheral nerves involved in bladder accommodation to filling are shown in Figure 3A and synapse onto different regions of the bladder and outlet. During filling, there is low-level activity from bladder afferent fibers that signal via the pelvic nerve and this, in turn, stimulates sympathetic outflow to the bladder neck and wall through the hypogastric nerve (Figure 3A). These fibers coordinately regulate negative innervation to the detrusor via β3-ARs and positive signals to α1-ARs in the internal smooth muscle sphincter (23) (Figure 4). There is also pudendal outflow, which keeps the EUS closed. These spinal reflex pathways coordinate relaxation of the detrusor and simultaneous contraction at the internal sphincter and EUS. During accommodation, neurons within the pontine storage center (also known as Barrington’s nucleus) in the brain are quiescent.
Figure 3.
Neural regulation of the storage/accommodation phase and the voiding phase. (A) Storage reflexes. During filling, there is low-level activity from bladder afferent fibers signaling distension via the pelvic nerve, which in turn stimulates sympathetic outflow to the bladder neck and wall via the hypogastric nerve. This sympathetic stimulation relaxes the detrusor and contracts the bladder neck at the internal sphincter. Afferent pelvic nerve impulses also stimulate the pudendal (somatic) outflow to the external sphincter causing contraction and maintenance of continence. (B) Voiding reflexes. Upon initiation of micturition, there is high-intensity afferent activity signaling wall tension, which activates the brainstem pontine micturition center. Spinobulbospinal reflex can be seen as an ascending signal from afferent pelvic nerve stimulation (left side), which passes through the periaqueductal gray matter before reaching the pontine micturition center and descending (right side) to elicit parasympathetic contraction of the detrusor, and somatic relaxation via the pudendal nerve. EUS, external urethral sphincter; PAG, periaqueductal gray matter. Modified from reference 102 as follows: 1) changes to the color and shape of spinal cord elements; 2) pontine storage center and pontine micturition center colored differently to indicate slightly different neuronal populations; 3) (A) new neural connection between the hypogastric nerve and the bladder outlet, with + and − signs to indicate contractile or relaxative signals, respectively; and (B) several new + and − signs, which were not included in the original.
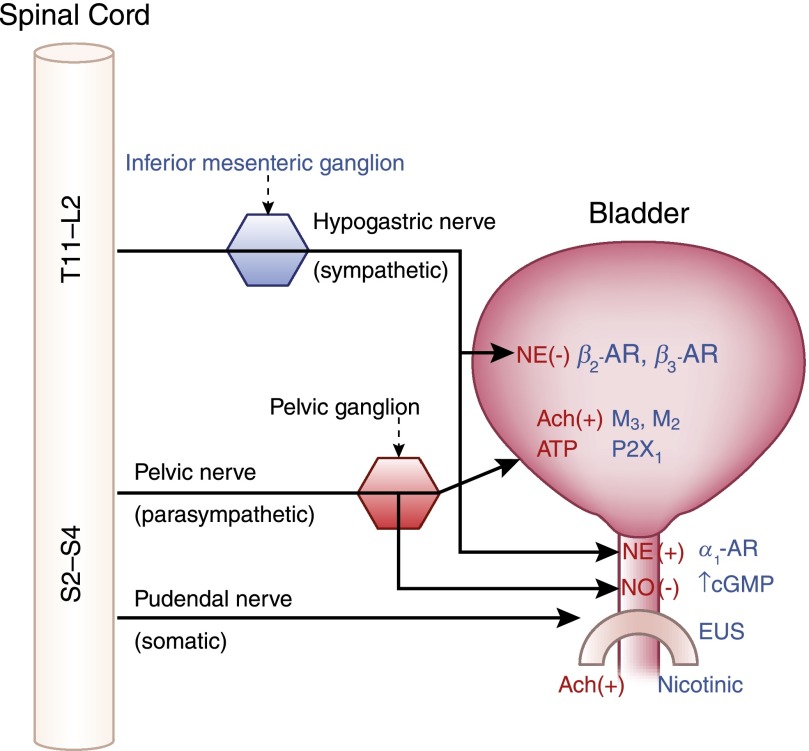
Figure 4.
Major efferent (motor) pathways of the bladder, neurotransmitters (red), receptors (blue), and sites of action. (+), contraction; (−), relaxation; Ach, acetylcholine; NE, norepinephrine; NO, nitric oxide; P2X1, purinergic receptor X1.
As the bladder becomes full, afferent firing increases and activates spinobulbospinal reflex pathways. At a critical level of bladder distention, the afferent activity arising from mechanoreceptors in the bladder wall switches the pontine micturition center (PMC) “on” and enhances its activity (Figure 3B). At this point, the accommodation center shuts “off” and ascending afferent input passes through the periaqueductal gray (PAG) relay center before reaching the PMC and elicits efferent outflow (24). Efferent innervation is supplied by the three major nerves shown in Figures 3B and 4, which are the pelvic, hypogastric, and pudendal nerves. The pelvic nerve emerges from sacral region S2–S4 of the spinal column and pelvic ganglion and provides positive (contractile) parasympathetic innervation to the detrusor smooth muscle and negative (or relaxative) innervation to the bladder neck by release of nitric oxide (NO) (Figures 3B and 4). The hypogastric nerve emerges from the inferior mesenteric ganglion after originating in the T11–L2 thoracolumbar segments and these sympathetic fibers are inhibited during micturition.
Somatic (conscious) innervation of the striated EUS is supplied by the pudendal nerve from the S2–S4 sacral region of the spinal column (25). When the bladder is filling, these muscles are under steady constant firing to maintain contraction (Figure 3A), but are inhibited by efferent signaling from the PAG during voiding. Striated muscle is composed of both fast twitch and slow twitch fibers and it is thought that slow twitch fibers that are slower to fatigue are likely responsible for maintenance of tone and the resting urethral pressure profile. The fast twitch fibers are available to come into play during transient events of increased abdominal pressure, such as during a cough. Any activities that cause abdominal compression, such as coughing or exercise, produce dramatic increases in the number of units firing with subsequent release of acetylcholine (26) (Figure 4).
Activation of the voiding reflex is under strict voluntary control in continent individuals and it requires complex integration of afferent signals along with conscious perceptions of how full one’s bladder is, combined with an appreciation of the social environment of the moment. The PAG is a key organizing center for several higher brain regions involved in micturition, including the prefrontal cortex with which it has strong connections. Functional imaging studies indicate that the frontal lobes are important in determining the appropriateness of behavioral reactions and cognitive responses to social situations. They receive and pass on sensory signals to areas involved with conscious perception. Furthermore, they communicate in reverse the results of that perception via the PAG directly as primary input to the PMC (27). Emphasizing the role of this cortical activity, patients with poor bladder control have been shown to exhibit weak prefrontal activity in response to bladder filling (28). Thus, in healthy people, signaling the need to “hang on,” if need be, arises from decisions about the appropriateness of the timing and results in neural suppression signals arriving at the PMC. The micturition switch is thereby maintained in the off position. Inputs at the PAG arrive from a number of connecting brain regions, including the thalamus, the insula, and the anterior cingulate cortex. In summary, they likely dictate how much attention is paid to bladder filling and what needs to be done about it.
Urothelium
The urothelium provides a continuous superficial lining that stretches from the edge of the renal pelvis to the end of the urethra and is formed of several cell layers that differ in their degree of differentiation. Undifferentiated basal cells line the basement membrane and were thought to replicate to permit the replacement of lost surface cells. However, a recent fate mapping study showed that intermediate cells, which are also relatively undifferentiated, contain the progenitor population for repopulating surface umbrella cells (29). In the relaxed bladder, the epithelial layers can be 4–8 cells deep; however, when the urothelium is distended, it becomes much thinner with fewer apparent cells in the cross-section. The umbrella cells sit on top and interface with the urine as a single layer of flagstone-shaped cells that have tight junctions and a highly impermeable apical plasma membrane. Because water, acid (H+), ammonia, urea, and carbon dioxide are normally highly permeable across biologic membranes, the apical membrane of the umbrella cell has a unique lipid and protein composition that makes it the least permeable epithelium in the human body (30–33).
Breakdown of the epithelial barrier can occur in a number of clinical settings including infection by uropathogenic Escherichia coli, radiation injury, carcinoma, and Hunner’s ulcers that are sometimes seen in interstitial cystitis. Interstitial cystitis, or painful bladder syndrome as it is now named, is a complex disease of undefined etiology, characterized by chronic lower urinary tract and/or pelvic pain that may be accompanied by dysuria, urgency, and frequency. Diagnosis is based on exclusionary criteria, such as absence of infection and carcinoma. Although recent research has emphasized the role of extrabladder factors (e.g., neurogenic) in pathogenesis and potential therapy, there remains a body of evidence implicating dysfunction of the urothelium in the course and severity of the disease. Plausible models in some patients at least, and in cats (34), suggest that damage to the urothelium, which is not balanced by successful repair, causes ongoing symptomatology.
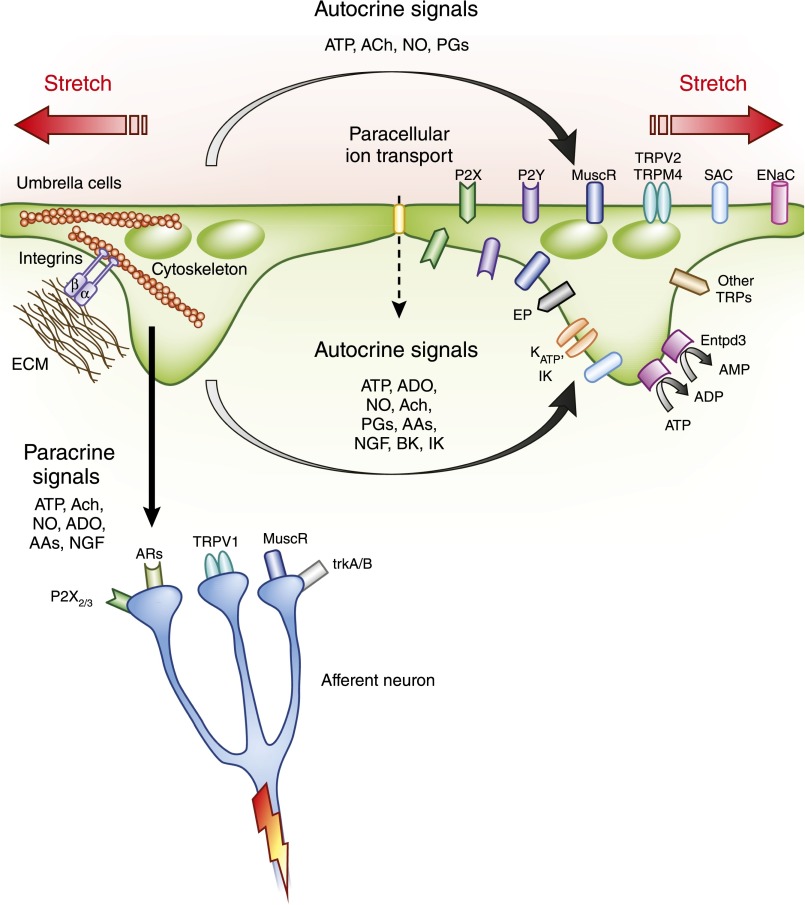
In addition to the passive protective functions that relate to structure, there is now recognition that the urothelium has sensory activity and communicates with other cell types to influence voiding. This conclusion, until recently, has relied on indirect evidence. For example, in response to physical and chemical stimuli, the urothelium releases potent mediators, including the neurotransmitters ATP, acetylcholine, and NO. These are thought to communicate information about the state of tissue stretch and aspects of the external environment to afferent neurons (35,36). An illustration of some of the mechanosensitive pathways activated by stretch is shown in Figure 5. In addition to autocrine effects, sensory afferents extending into and between cells of the urothelium are likely influenced by neurotransmitters released from urothelium.
Figure 5.
Mechanically induced signaling from the urothelium in response to membrane stretch. Release of a number of potent mediators occurs in response to urothelial stretch eliciting both autocrine- and paracrine-mediated effects. AA, adrenergic agonist; ADO, adenosine; AR, adrenergic receptor; BK, maxi K channel; EMC, extracellular matrix; ENaC, epithelial sodium channel; Entpd3, ectonucleoside triphosphate diphosphohydrolase 3; EP, PG receptor; NGF, nerve growth factor; P2Y, purinergic receptor Y; SAC, stretch-activated ion channel; trkA/B, tyrosine kinase receptors for nerve growth factor; TRP, transient receptor potential channel.
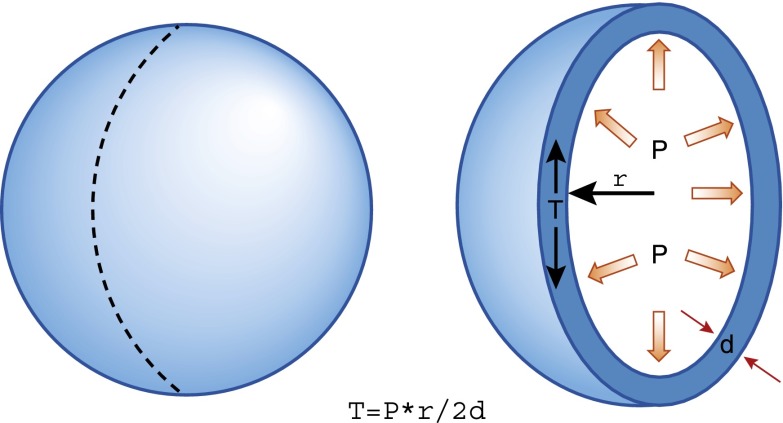
The urothelium experiences biomechanical forces and appears capable of sensing and responding to them. As the urine accumulates, the three-dimensional conformation of the urothelium changes from highly folded and involuted to smooth and spherical and is subject to mechanical tension that relates to the radius, wall thickness, and intravesical pressure according to LaPlace’s law (Figure 6). Whereas pressure is constant at all points within the bladder, the tension experienced at the different points in the bladder wall varies and is proportional to the radius of curvature (r) divided by the wall thickness. Wall tension, rather than hydrostatic pressure, is the relevant physical parameter that initiates mechanotransduction and, hence, activation of bladder afferents. In hypertrophic small capacity bladders, elevated wall tension occurs at much lower fill volumes and can induce ischemia and vesicoureteral reflux. Any remodeling of the bladder wall that occurs in response to outlet obstruction or spinal cord injury (denervation), for example, results in increased collagen content and a reduction in compliance (37,38). Wall tension increases in such clinical settings leading to altered mechanosensory signaling and a lower volume threshold for the onset of feelings of urgency.
Figure 6.
Illustration of LaPlace’s law relating wall tension to pressure, radius, and wall thickness. d, wall thickness; P, pressure; r, radius; T, tension.
The urothelium secretes ATP (39,40) and other macromolecules as wall tension increases, and high ATP concentrations contribute to nociception by binding purinergic receptor X2/3 (P2X2/3) on high threshold urothelial pain afferents. Thus, a question of considerable interest is whether the urothelium, through its ability to sense bladder wall stretch, is capable of regulating voiding and, if so, is it implicated in bladder dysfunction? As a way of addressing this, investigators have recently turned to conditional gene targeting approaches in mice that allow the expression or deletion of genes in specific cell types. Schnegelsberg et al. specifically overexpressed nerve growth factor in the urothelium of engineered mice (41). Mice producing excess urothelial nerve growth factor were shown to have nerve fiber hyperplasia, reduced bladder capacity, and increased nonvoiding bladder contractions, indicating that aberrant signaling originating in the urothelium can cause voiding dysfunction (41).
Our laboratory used the uroplakin promotor in an engineered mouse to direct expression of Cre recombinase purely in the urothelium. When this strain is crossed with a mouse that has loxP gene sequences added within or surrounding a gene of interest, the Cre recombinase will excise the DNA between the sites and recombine the ends. This strategy permits cell-specific gene deletion. Using this approach, we deleted β1-integrin from the urothelium in mice (42). Integrins connect the intracellular cytoskeleton with the extracellular matrix (see Figure 5) and our experiments revealed that mice lacking these cell surface receptors in the urothelium were incontinent and exhibited symptoms of overactive bladder (OAB) (42). Because integrins provide important molecular junctions for the transfer of information related to biomechanical stresses, these experiments provided direct evidence that the urothelium regulates voiding through its sensory and signaling functions and may prove to be important in certain bladder disorders.
Detrusor Smooth Muscle
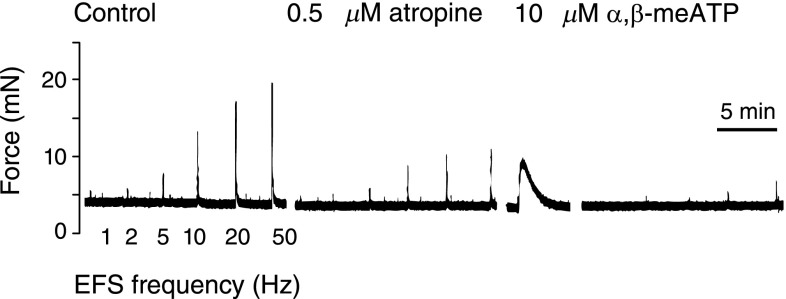
Detrusor contraction is primarily regulated by lumbosacral parasympathetic efferents (Figure 4) that release acetylcholine and ATP to initiate bladder contraction. This can be convincingly demonstrated in bladder strips (without urothelium) mounted in organ baths on isometric tension transducers. Figure 7 shows bladder muscle strips from mice contracting with successively greater force at higher electrical field frequencies due to neurotransmitter release. Inhibition of muscarinic and purinergic signaling with atropine and then α,β,methyl-ATP treatment reduces electrical field–stimulated contractions by approximately 90%, confirming the primacy of acetylcholine and ATP signals (43).
Figure 7.
Electrical field stimulation of mouse bladder muscle strips showing relative contribution of muscarinic and purinergic receptors to contraction force. Muscle strips were prepared by removing urothelium before mounting in organ baths. Increasing the frequency of electrical stimulation increases the muscle contractile force. Addition of atropine, a muscarinic receptor antagonist, diminishes force generation. The force can be further reduced by a purinergic receptor antagonist, α,β-methyl-ATP. Inhibition follows a transient stimulation and is the result of receptor desensitization. EFS, electrical field stimulation.
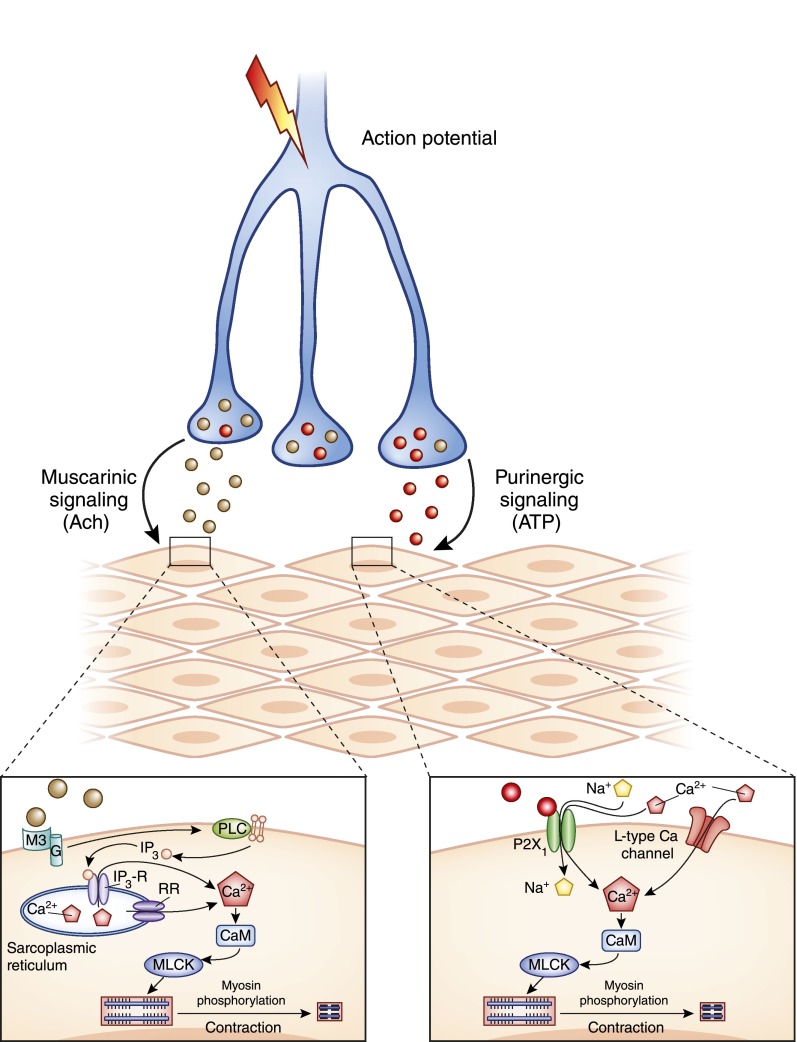
Bladder smooth muscle (BSM) is an excitable tissue, which means that it responds to electrical, chemical, or physical stimuli by a rapid depolarization of the membrane, creating an action potential that can be transmitted to other cells. Action potentials propagate from myocyte to myocyte through gap junctions and result in large-scale contraction. An action potential is the first step in a chain of events after muscarinic and/or purinergic stimulation of receptors, which result in raised intracellular Ca2+. Figure 8 shows several of the major receptor pathways activated in response to neurotransmitter release. The primary receptors for acetylcholine and ATP in BSM are the M2, M3, and P2X1 receptors. As the membrane depolarizes as a result of cation influx, subsequent activation of voltage-dependent, L-type Ca2+ channels leads to further Ca2+ influx and generation of an action potential that can propagate to other myocytes. High intracellular Ca2+ results in formation of calcium–calmodulin complexes, which, in turn, activate the myosin light chain kinase and lead to myosin phosphorylation, actomyosin crossbridge formation, and muscle cell contraction (44).
Figure 8.
Molecular mechanisms leading to bladder smooth muscle contraction. Parasympathetic neurons release primary neurotransmitters acetylcholine and ATP, which bind to the M3 muscarinic receptor and P2X1, respectively. This leads to several downstream effects, including membrane depolarization and increased cytosolic Ca2+ and ultimately myosin phosphorylation and myocyte contraction. CaM, calmodulin; G, G protein; IP3, inositol trisphosphate; IP3-R, inositol trisphosphate receptor; MLCK, myosin light chain kinase; PLC, phospholipase C; RR, ryanodine receptor.
Muscarinic Receptors.
M3 receptors are the primary contractile effector in detrusor, but M2 receptors are present at 3-fold higher expression density (45). M2 receptors appear to play a more subtle role in ‘recontracting’ BSM after relaxation (46,47). In the normal bladder, cholinergic contractility is dominant compared with purinergic pathways, and these receptors provide the mainstay target for therapies aimed at symptoms of urgency, incontinence, and OAB. Current anticholinergic drugs approved for OAB include oxybutynin, tolterodine, darifenacin, solifenacin, and trospium. All aim to dampen excess electrical activity leading to inappropriate detrusor contractility; however, their efficacy is questionable, and tolerability is poor because of the anticholinergic side effects.
Purinergic Receptors.
Purinergic receptors respond to nucleotides and nucleosides, like ATP, ADP, UTP, UDP, and adenosine. The primary purinergic receptor present on BSM is P2X; this has been confirmed pharmacologically by testing muscle strips for contractile responses with specific P2X1 agonists and antagonists by immunostaining (48) and from studies of P2X1 knockout mice (49). Recent work from our lab has confirmed the first functional evidence for a purinergic receptor Y (P2Y) in BSM–P2Y6 (50). P2Y6 appears to play a role in maintaining spontaneous bladder tone and, in doing so, facilitates the strength of P2X1-mediated contractions by almost 50%. This receptor is specifically activated by UDP. Ectonucleotidase, Entpd1 is present on BSM cell membranes and can degrade both ATP and UTP to ADP/UDP and, ultimately, to the monophosphorylated forms (51). Interestingly, BSM has all the enzymes required to convert ATP to adenosine, which is likely to be important because adenosine causes smooth muscle to relax. In this context, a number of studies have shown that caffeine can exacerbate lower urinary tract symptoms, including OAB (52–54). Although the mechanism remains unclear because caffeine has several cellular effects, it is known to inhibit adenosine receptors at the low doses found from drinking coffee and, thus, may inhibit detrusor relaxation (53). Intriguingly, purinergic signaling becomes more prominent in disease and in aging (55–57) and may emerge to play a dominant role in patients with partial outlet obstruction and interstitial cystitis, potentially accounting for up to 65% of the total contraction force (58).
ARs.
ARs in the bladder mediate both contractility and relaxation in response to sympathetic innervation, which primarily occurs at the bladder neck and urethral outlet (Figure 4). The major receptor isoforms present in the human bladder are α1 and β3; however, expression of α-isoforms as determined at the mRNA and protein levels is very low compared with β-receptors (23). The direct effects of α1-AR stimulation on smooth muscle contraction are rather weak, amounting to approximately 10%–40% of the contractile force that results from muscarinic stimulation or KCl depolarization. α1-Receptors, therefore, appear to play only a minor functional role in the detrusor, but feature prominently near the bladder outlet in terms of maintaining outlet resistance (59).
The β3-receptor is the predominant isoform in the human bladder and accounts for >95% of β-receptors. NE is released from the hypogastric nerves to activate these receptors (Figure 4). The canonical signaling pathway for β-ARs is through elevation of cAMP and generally results in smooth muscle relaxation. It appears likely that activation of the β3-receptor may involve interactions with other cellular signaling proteins, such as K channels (60). There appears to be good evidence for both cAMP-dependent, as well as independent, effects (61). Mirabegron (sold as Myrbetriq) is a β3-receptor agonist recently approved as a first-in-class agent designed to treat OAB, urgency, and leakage by promoting relaxation of BSM.
K Channels.
BSM expresses a rich variety of K channels, including large-conductance calcium-activated K (BK or maxi K), ATP-sensitive K (KATP), small-conductance K (SK), inwardly rectifying K (Kir), and voltage-activated K (Kv) channels. As noted above, the effects of β-AR stimulation appear to be at least partially mediated by activation of BK channels in rat, guinea pig, and human bladder (62–64). Overall, it appears that pharmacologic activation of virtually all K channels leads to smooth muscle relaxation as a result of K efflux hyperpolarizing the membrane potential (65–68).
NO.
In addition to the primary sympathetic and parasympathetic neurotransmitters, afferent and efferent nerves are known to secrete NO. The expression of NO synthase–containing nerves is highest in the outlet region of the bladder (69,70). Neuronal release of NO primarily elicits relaxation responses at the level of the neck and urethra to facilitate micturition (71). In the cytoplasm, it activates guanylate cyclase and thus increases cGMP, which then activates protein kinase G. In the clinical setting, this pathway has been targeted through use of phosphodiesterase inhibitors, such as sildenafil, which result in elevated levels of intracellular cGMP and enhanced relaxation of bladder neck and urethra (72). In one study, a group of healthy male volunteers, given a NO donor sublingually and studied urodynamically, exhibited lowered bladder outlet resistance characterized by reduced detrusor pressure during voiding and a lower maximal flow rate (73).
Bladder ICC: An Old Cell Newly Implicated in Lower Urinary Tract Symptoms
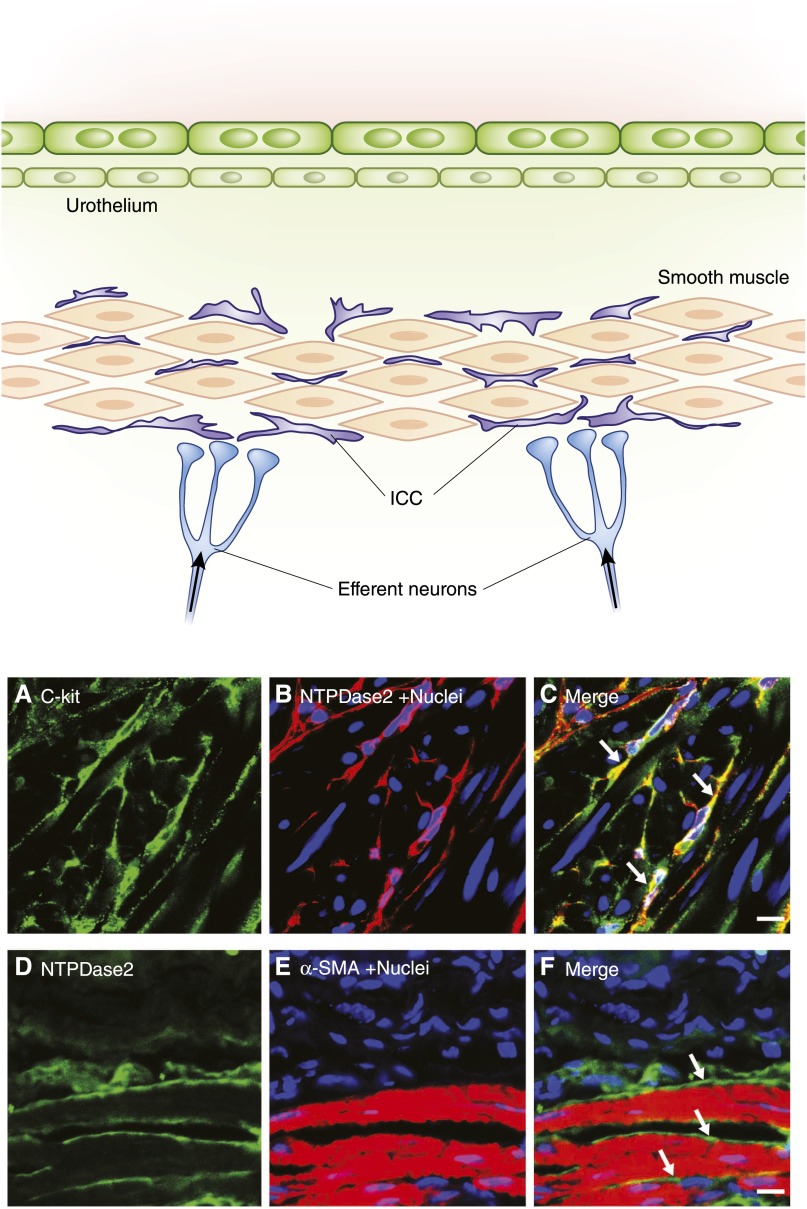
Embedded deep within the lamina propria and intercalated within the detrusor smooth muscle surrounding muscle bundles are a unique population of cells with stellate morphology. In the last decade, these have been identified as bladder ICCs (BICCs) and the emerging consensus is that they are likely to play an important role in modulating bladder motility (74–81). ICCs were first identified in the gastrointestinal tract where they function as pacemakers, neuron transducers, and mechanosensors to modulate smooth muscle motility (82–85), and more than a dozen gut disorders are linked to ICC dysfunction, including gastroparesis and intestinal pseudo-obstruction (86,87). In urinary bladder, much less is understood. We and others recently demonstrated that ICC markers are expressed in BICCs, including c-kit, anoctamin 1, gap junction protein connexin 43, and CD34 (88). These cells are closely associated with intramural nerves and BSM cells (89–91). The overlying schematic in Figure 9 illustrates the position they occupy in the bladder wall. Figure 9 also shows confocal images of BICCs with the top panels showing stellate-like morphology for cells staining positive with antibodies for c-kit (Figure 9A) and for the ATP breakdown enzyme NTPDase 2 (Figure 9B). These cells occur deep in BSM; Figure 9F shows that they are intimately associated with BSM (labeled with α-smooth muscle actin). BICCs are implicated in bladder diseases, such as megacystis microcolon intestinal hypoperistalsis syndrome, a lethal congenital disease in which infants have no ICCs (92,93). In patients with diabetes, decreased BICCs were observed and this was suggested to contribute to incontinence (74). Alterations to BICC function have also been reported in OAB and obstructed bladder in both humans and animal models (79,92,94–97). Increased expression of connexin 43 has been reported in patients with OAB, suggesting increased signal transduction between BICCs and BSM cells, potentially leading to motility problems (98,99). This cell is just beginning to attract attention as a possible contributor to bladder motility disorders but it is not currently known whether alterations in BICCs are primary or secondary to disease processes.
Figure 9.
Morphology and location of BICCs. The top panel provides a schematic of the bladder wall showing the location of ICCs adjacent to smooth muscle bundles and parasympathetic neurons. These stellate-shaped cells are also seen in the deep lamina propria. The lower panels show immunofluorescence staining of BICCs in 5-µm cryosections of BSM from mouse. (A) c-kit Staining. (B) Ectonucleotidase2 (NTPDase2) staining. (C) Merge showing colocalization (in yellow) of the two BICC markers (arrows). (D–F) images showing close apposition of BICC (arrows) and BSM cells as indicated by NTPDase2 and α-smooth muscle actin. α-SMA, α-smooth muscle actin; BICC, bladder interstitial cell of Cajal; BSM, bladder smooth muscle; ICC, interstitial cell of Cajal. Bar, 10 µm.
Urethral Contractile Physiology
The urethra is a distensible muscular structure composed of both smooth and striated muscle. Circular contractile elements are required for sphincteric action and longitudinal smooth muscle may assist by stabilizing the urethra and accentuating force developed by circumferential muscle. The periurethral striated muscle that forms part of the pelvic floor is separate from the intramural striated muscle but together they constitute the EUS. The region of highest urethral pressure occurs approximately 4 cm from the bladder neck in women and in the membranous urethra approximately 5 cm from the neck in men, and pressures of 100–120 cmH2O are considered normal.
While the bladder is filling, the urethral outlet remains closed and the EUS contracts with greater and greater frequency. This progressive increase in activity of the EUS in the face of bladder expansion is known as the guarding reflex. When the urinary bladder fills to approximately 200–300 ml, stretch receptors in the urinary bladder trigger a reflex arc. The signal stimulates the spinal cord, which responds with a parasympathetic impulse that relaxes the smooth muscle of the internal urethral sphincter and contracts the detrusor muscle. Urine does not flow, however, until a voluntary nerve impulse from the pudendal nerve relaxes the striated muscle of the EUS.
The most common clinical problem encountered with the urethra is blockage. Partial urethral obstruction is relatively common in men with aging, as a result of benign prostate hypertrophy, carcinoma, or calculi, and results in increased back pressure during micturition. During early stages of urethral restriction, which can take years to develop, the bladder undergoes compensatory hypertrophy in order to produce the greater contractile force required to force urine passed the restriction and keep residual urine volumes low (100). These patients present with voiding symptoms, which include urgency, frequency, slowing of the urinary stream, straining to void and nocturia all of which affect quality of life. The primary effects of urethral obstruction are found in the bladder and, if severe enough, will manifest as hydronephrosis and can threaten kidney function (101). Early stage treatment with α-antagonists, such as terazosin, doxazosin, tamsulosin, and alfuzosin, can provide some relief by inhibiting the α1-receptor on the smooth muscle within the prostate gland and the internal urethral sphincter; however, this antagonism does not affect the size of the prostate, and the latter requires treatment with a 5α-reductase inhibitor. α-Antagonists may cause postural hypotension because of the diffuse distribution of receptors along blood vessels. In addition, medical treatment may not always adequately treat symptoms and surgery is then required. If bladder outlet obstruction is left untreated, the bladder wall ultimately decompensates and becomes hyperactive with loss of functional capacity. Alteration in the contractility of the detrusor is accompanied by changes in the proteins that comprise the contractile apparatus and increases in markers of fibrosis such as collagen.
Recent research is beginning to shed light on the complex physiologic interactions between the urothelium, interstitium, smooth muscle, and nervous system. These interactions have highlighted a number of receptors, ion channels, and pathways as possible clinical targets for lower urinary tract symptoms, although the basic understanding of causes remains limited. Indeed, widespread use of the term lower urinary tract symptoms (or LUTS) to describe problems such as urgency, frequency, incontinence, and nocturia among others, indicates the shortcomings in our knowledge of causes and is deliberately nonspecific for that reason. Treatment options currently offered include education, behavioral modification, pelvic exercises, medication, botulinum toxin, catheterization, and surgery (often as a result of medication failure or symptom progression). The existence of multiple different guidelines indicates that current therapy of these disorders is empirical, often lacking in an evidence base, and is of inadequate benefit to patients. In clinical practice, the drugs in current use tend to target symptomology with an emphasis on control of smooth muscle motility. In general, these drugs have low efficacy. A major challenge, therefore, is to better understand the molecular and cellular physiology underlying normal functioning of the urinary tract, and greater efforts are needed here. Gaining deeper insights into basic physiology will surely lead to improvements in our understanding of the causes of voiding dysfunction and bladder pain syndromes and, thus, will suggest more rational and targeted therapeutic approaches.
Disclosures
None.
Acknowledgments
The author thanks Drs. Mark Zeidel and Melanie Hoenig for their generous assistance with suggestions and constructive criticism during the preparation of this review.
This work was supported by grants from the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases (Grants DK08399 and P20-DK097818). Its content is solely the responsibility of the author and does not necessarily represent the official view of the National Institutes of Health.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Tanagho EA, Pugh RC: The anatomy and function of the ureterovesical junction. Br J Urol 35: 151–165, 1963 [DOI] [PubMed] [Google Scholar]
- 2.Raman A, Lam S, Vasilaras A, Joseph D, Wong J, Sved P, Allen RD: Influence of ureteric anastomosis technique on urological complications after kidney transplantation. Transplant Proc 45: 1622–1624, 2013 [DOI] [PubMed] [Google Scholar]
- 3.Dellabella M, Milanese G, Muzzonigro G: Randomized trial of the efficacy of tamsulosin, nifedipine and phloroglucinol in medical expulsive therapy for distal ureteral calculi. J Urol 174: 167–172, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Ye Z, Yang H, Li H, Zhang X, Deng Y, Zeng G, Chen L, Cheng Y, Yang J, Mi Q, Zhang Y, Chen Z, Guo H, He W, Chen Z: A multicentre, prospective, randomized trial: Comparative efficacy of tamsulosin and nifedipine in medical expulsive therapy for distal ureteric stones with renal colic. BJU Int 108: 276–279, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Lang RJ, Hashitani H, Tonta MA, Bourke JL, Parkington HC, Suzuki H: Spontaneous electrical and Ca2+ signals in the mouse renal pelvis that drive pyeloureteric peristalsis. Clin Exp Pharmacol Physiol 37: 509–515, 2010 [DOI] [PubMed] [Google Scholar]
- 6.Lang RJ, Hashitani H, Tonta MA, Parkington HC, Suzuki H: Spontaneous electrical and Ca2+ signals in typical and atypical smooth muscle cells and interstitial cell of Cajal-like cells of mouse renal pelvis. J Physiol 583: 1049–1068, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lang RJ, Klemm MF: Interstitial cell of Cajal-like cells in the upper urinary tract. J Cell Mol Med 9: 543–556, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lang RJ, Tonta MA, Zoltkowski BZ, Meeker WF, Wendt I, Parkington HC: Pyeloureteric peristalsis: Role of atypical smooth muscle cells and interstitial cells of Cajal-like cells as pacemakers. J Physiol 576: 695–705, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lang RJ, Zoltkowski BZ, Hammer JM, Meeker WF, Wendt I: Electrical characterization of interstitial cells of Cajal-like cells and smooth muscle cells isolated from the mouse ureteropelvic junction. J Urol 177: 1573–1580, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Berridge MJ: Smooth muscle cell calcium activation mechanisms. J Physiol 586: 5047–5061, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koleda P, Apoznanski W, Wozniak Z, Rusiecki L, Szydelko T, Pilecki W, Polok M, Kalka D, Pupka A: Changes in interstitial cell of Cajal-like cells density in congenital ureteropelvic junction obstruction. Int Urol Nephrol 44: 7–12, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuvel M, Canguven O, Murtazaoglu M, Albayrak S: Distribution of Cajal like cells and innervation in intrinsic ureteropelvic junction obstruction. Arch Ital Urol Androl 83: 128–132, 2011 [PubMed] [Google Scholar]
- 13.Solari V, Piotrowska AP, Puri P: Altered expression of interstitial cells of Cajal in congenital ureteropelvic junction obstruction. J Urol 170: 2420–2422, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Yang X, Zhang Y, Hu J: The expression of Cajal cells at the obstruction site of congenital pelviureteric junction obstruction and quantitative image analysis. J Pediatr Surg 44: 2339–2342, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Levent A, Büyükafsar K: Expression of Rho-kinase (ROCK-1 and ROCK-2) and its substantial role in the contractile activity of the sheep ureter. Br J Pharmacol 143: 431–437, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee HW, Baak CH, Lee MY, Kim YC: Spontaneous contractions augmented by cholinergic and adrenergic systems in the human ureter. Korean J Physiol Pharmacol 15: 37–41, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malin JM, Jr, Deane RF, Boyarsky S: Characterisation of adrenergic receptors in human ureter. Br J Urol 42: 171–174, 1970 [DOI] [PubMed] [Google Scholar]
- 18.Rajpathy J, Aswathaman K, Sinha M, Subramani S, Gopalakrishnan G, Kekre NS: An in vitro study on human ureteric smooth muscle with the alpha1-adrenoceptor subtype blocker, tamsulosin. BJU Int 102: 1743–1745, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Nakada SY, Coyle TL, Ankem MK, Moon TD, Jerde TJ: Doxazosin relaxes ureteral smooth muscle and inhibits epinephrine-induced ureteral contractility in vitro. Urology 70: 817–821, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Al-Ansari A, Al-Naimi A, Alobaidy A, Assadiq K, Azmi MD, Shokeir AA: Efficacy of tamsulosin in the management of lower ureteral stones: A randomized double-blind placebo-controlled study of 100 patients. Urology 75: 4–7, 2010 [DOI] [PubMed] [Google Scholar]
- 21.Park YC, Tomiyama Y, Hayakawa K, Akahane M, Ajisawa Y, Miyatake R, Kiwamoto H, Sugiyama T, Kurita T: Existence of a beta3-adrenoceptro and its functional role in the human ureter. J Urol 164: 1364–1370, 2000 [PubMed] [Google Scholar]
- 22.Wanajo I, Tomiyama Y, Yamazaki Y, Kojima M, Shibata N: Pharmacological characterization of beta-adrenoceptor subtypes mediating relaxation in porcine isolated ureteral smooth muscle. J Urol 172: 1155–1159, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Michel MC, Vrydag W: Alpha1-, alpha2- and beta-adrenoceptors in the urinary bladder, urethra and prostate. Br J Pharmacol 147[Suppl 2]: S88–S119, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Groat WC, Yoshimura N: Afferent nerve regulation of bladder function in health and disease. Handb Exp Pharmacol 194: 91–138, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoshimura N, Kaiho Y, Miyazato M, Yunoki T, Tai C, Chancellor MB, Tyagi P: Therapeutic receptor targets for lower urinary tract dysfunction. Naunyn Schmiedebergs Arch Pharmacol 377: 437–448, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Stafford RE, Ashton-Miller JA, Sapsford R, Hodges PW: Activation of the striated urethral sphincter to maintain continence during dynamic tasks in healthy men. Neurourol Urodyn 31: 36–43, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fowler CJ, Griffiths D, de Groat WC: The neural control of micturition. Nat Rev Neurosci 9: 453–466, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Griffiths D, Derbyshire S, Stenger A, Resnick N: Brain control of normal and overactive bladder. J Urol 174: 1862–1867, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Gandhi D, Molotkov A, Batourina E, Schneider K, Dan H, Reiley M, Laufer E, Metzger D, Liang F, Liao Y, Sun TT, Aronow B, Rosen R, Mauney J, Adam R, Rosselot C, Van Batavia J, McMahon A, McMahon J, Guo JJ, Mendelsohn C: Retinoid signaling in progenitors controls specification and regeneration of the urothelium. Dev Cell 26: 469–482, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu XR, Kong XP, Pellicer A, Kreibich G, Sun TT: Uroplakins in urothelial biology, function, and disease. Kidney Int 75: 1153–1165, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu P, Deng FM, Liang FX, Hu CM, Auerbach AB, Shapiro E, Wu XR, Kachar B, Sun TT: Ablation of uroplakin III gene results in small urothelial plaques, urothelial leakage, and vesicoureteral reflux. J Cell Biol 151: 961–972, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu P, Meyers S, Liang FX, Deng FM, Kachar B, Zeidel ML, Sun TT: Role of membrane proteins in permeability barrier function: Uroplakin ablation elevates urothelial permeability. Am J Physiol Renal Physiol 283: F1200–F1207, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Min G, Zhou G, Schapira M, Sun TT, Kong XP: Structural basis of urothelial permeability barrier function as revealed by Cryo-EM studies of the 16 nm uroplakin particle. J Cell Sci 116: 4087–4094, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Lavelle JP, Meyers SA, Ruiz WG, Buffington CA, Zeidel ML, Apodaca G: Urothelial pathophysiological changes in feline interstitial cystitis: A human model. Am J Physiol Renal Physiol 278: F540–F553, 2000 [DOI] [PubMed] [Google Scholar]
- 35.Apodaca G: The uroepithelium: Not just a passive barrier. Traffic 5: 117–128, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Apodaca G, Balestreire E, Birder LA: The uroepithelial-associated sensory web. Kidney Int 72: 1057–1064, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Gosling JA: Modification of bladder structure in response to outflow obstruction and ageing. Eur Urol 32[Suppl 1]: 9–14, 1997 [PubMed] [Google Scholar]
- 38.Macarak EJ, Howard PS: The role of collagen in bladder filling. Adv Exp Med Biol 462: 215–223, discussion 225–233, 1999 [DOI] [PubMed] [Google Scholar]
- 39.Ferguson DR, Kennedy I, Burton TJ: ATP is released from rabbit urinary bladder epithelial cells by hydrostatic pressure changes—a possible sensory mechanism? J Physiol 505: 503–511, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lewis SA, Lewis JR: Kinetics of urothelial ATP release. Am J Physiol Renal Physiol 291: F332–F340, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Schnegelsberg B, Sun TT, Cain G, Bhattacharya A, Nunn PA, Ford AP, Vizzard MA, Cockayne DA: Overexpression of NGF in mouse urothelium leads to neuronal hyperinnervation, pelvic sensitivity, and changes in urinary bladder function. Am J Physiol Regul Integr Comp Physiol 298: R534–R547, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kanasaki K, Yu W, von Bodungen M, Larigakis JD, Kanasaki M, Ayala de la Pena F, Kalluri R, Hill WG: Loss of β1-integrin from urothelium results in overactive bladder and incontinence in mice: A mechanosensory rather than structural phenotype. FASEB J 27: 1950–1961, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iacovou JW, Hill SJ, Birmingham AT: Agonist-induced contraction and accumulation of inositol phosphates in the guinea-pig detrusor: Evidence that muscarinic and purinergic receptors raise intracellular calcium by different mechanisms. J Urol 144: 775–779, 1990 [DOI] [PubMed] [Google Scholar]
- 44.Ding HL, Ryder JW, Stull JT, Kamm KE: Signaling processes for initiating smooth muscle contraction upon neural stimulation. J Biol Chem 284: 15541–15548, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hegde SS: Muscarinic receptors in the bladder: From basic research to therapeutics. Br J Pharmacol 147[Suppl 2]: S80–S87, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Uchiyama T, Chess-Williams R: Muscarinic receptor subtypes of the bladder and gastrointestinal tract. J Smooth Muscle Res 40: 237–247, 2004 [DOI] [PubMed] [Google Scholar]
- 47.Zhang X, DiSanto ME: Rho-kinase, a common final path of various contractile bladder and ureter stimuli. Handb Exp Pharmacol 202: 543–568, 2011 [DOI] [PubMed] [Google Scholar]
- 48.Lee HY, Bardini M, Burnstock G: Distribution of P2X receptors in the urinary bladder and the ureter of the rat. J Urol 163: 2002–2007, 2000 [PubMed] [Google Scholar]
- 49.Vial C, Evans RJ: P2X receptor expression in mouse urinary bladder and the requirement of P2X(1) receptors for functional P2X receptor responses in the mouse urinary bladder smooth muscle. Br J Pharmacol 131: 1489–1495, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu W, Sun X, Robson SC, Hill WG: Extracellular UDP enhances P2X-mediated bladder smooth muscle contractility via P2Y6 activation of the phospholipase C/inositol trisphosphate pathway. FASEB J 27: 1895–1903, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yu W, Robson SC, Hill WG: Expression and distribution of ectonucleotidases in mouse urinary bladder. PLoS ONE 6: e18704, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maserejian NN, Wager CG, Giovannucci EL, Curto TM, McVary KT, McKinlay JB: Intake of caffeinated, carbonated, or citrus beverage types and development of lower urinary tract symptoms in men and women. Am J Epidemiol 177: 1399–1410, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lohsiriwat S, Hirunsai M, Chaiyaprasithi B: Effect of caffeine on bladder function in patients with overactive bladder symptoms. Urol Ann 3: 14–18, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kershen R, Mann-Gow T, Yared J, Stromberg I, Zvara P: Caffeine ingestion causes detrusor overactivity and afferent nerve excitation in mice. J Urol 188: 1986–1992, 2012 [DOI] [PubMed] [Google Scholar]
- 55.Yoshida M, Miyamae K, Iwashita H, Otani M, Inadome A: Management of detrusor dysfunction in the elderly: Changes in acetylcholine and adenosine triphosphate release during aging. Urology 63[Suppl 1]: 17–23, 2004 [DOI] [PubMed] [Google Scholar]
- 56.Yoshida M, Homma Y, Inadome A, Yono M, Seshita H, Miyamoto Y, Murakami S, Kawabe K, Ueda S: Age-related changes in cholinergic and purinergic neurotransmission in human isolated bladder smooth muscles. Exp Gerontol 36: 99–109, 2001 [DOI] [PubMed] [Google Scholar]
- 57.Sjögren C, Andersson KE, Husted S, Mattiasson A, Moller-Madsen B: Atropine resistance of transmurally stimulated isolated human bladder muscle. J Urol 128: 1368–1371, 1982 [DOI] [PubMed] [Google Scholar]
- 58.Andersson KE, Arner A: Urinary bladder contraction and relaxation: Physiology and pathophysiology. Physiol Rev 84: 935–986, 2004 [DOI] [PubMed] [Google Scholar]
- 59.Michel MC, Barendrecht MM: Physiological and pathological regulation of the autonomic control of urinary bladder contractility. Pharmacol Ther 117: 297–312, 2008 [DOI] [PubMed] [Google Scholar]
- 60.Frazier EP, Mathy MJ, Peters SL, Michel MC: Does cyclic AMP mediate rat urinary bladder relaxation by isoproterenol? J Pharmacol Exp Ther 313: 260–267, 2005 [DOI] [PubMed] [Google Scholar]
- 61.Frazier EP, Peters SL, Braverman AS, Ruggieri MR, Sr, Michel MC: Signal transduction underlying the control of urinary bladder smooth muscle tone by muscarinic receptors and beta-adrenoceptors. Naunyn Schmiedebergs Arch Pharmacol 377: 449–462, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hristov KL, Chen M, Kellett WF, Rovner ES, Petkov GV: Large-conductance voltage- and Ca2+-activated K+ channels regulate human detrusor smooth muscle function. Am J Physiol Cell Physiol 301: C903–C912, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hristov KL, Cui X, Brown SM, Liu L, Kellett WF, Petkov GV: Stimulation of beta3-adrenoceptors relaxes rat urinary bladder smooth muscle via activation of the large-conductance Ca2+-activated K+ channels. Am J Physiol Cell Physiol 295: C1344–C1353, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Petkov GV, Nelson MT: Differential regulation of Ca2+-activated K+ channels by beta-adrenoceptors in guinea pig urinary bladder smooth muscle. Am J Physiol Cell Physiol 288: C1255–C1263, 2005 [DOI] [PubMed] [Google Scholar]
- 65.Afeli SA, Rovner ES, Petkov GV: SK but not IK channels regulate human detrusor smooth muscle spontaneous and nerve-evoked contractions. Am J Physiol Renal Physiol 303: F559–F568, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen M, Kellett WF, Petkov GV: Voltage-gated K(+) channels sensitive to stromatoxin-1 regulate myogenic and neurogenic contractions of rat urinary bladder smooth muscle. Am J Physiol Regul Integr Comp Physiol 299: R177–R184, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hristov KL, Chen M, Afeli SA, Cheng Q, Rovner ES, Petkov GV: Expression and function of K(V)2-containing channels in human urinary bladder smooth muscle. Am J Physiol Cell Physiol 302: C1599–C1608, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Petkov GV, Heppner TJ, Bonev AD, Herrera GM, Nelson MT: Low levels of K(ATP) channel activation decrease excitability and contractility of urinary bladder. Am J Physiol Regul Integr Comp Physiol 280: R1427–R1433, 2001 [DOI] [PubMed] [Google Scholar]
- 69.Persson K, Alm P, Johansson K, Larsson B, Andersson KE: Nitric oxide synthase in pig lower urinary tract: Immunohistochemistry, NADPH diaphorase histochemistry and functional effects. Br J Pharmacol 110: 521–530, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dixon JS, Jen PY: Development of nerves containing nitric oxide synthase in the human male urogenital organs. Br J Urol 76: 719–725, 1995 [DOI] [PubMed] [Google Scholar]
- 71.Hedlund P: Nitric oxide/cGMP-mediated effects in the outflow region of the lower urinary tract—is there a basis for pharmacological targeting of cGMP? World J Urol 23: 362–367, 2005 [DOI] [PubMed] [Google Scholar]
- 72.Uckert S, Oelke M: Phosphodiesterase (PDE) inhibitors in the treatment of lower urinary tract dysfunction. Br J Clin Pharmacol 72: 197–204, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Müntener M, Schurch B, Wefer B, Reitz A: Systemic nitric oxide augmentation leads to a rapid decrease of the bladder outlet resistance in healthy men. Eur Urol 50: 112–117, discussion 117–118, 2006 [DOI] [PubMed] [Google Scholar]
- 74.Canda AE: Diabetes might adversely affect expression and function of interstitial cells in the urinary bladder and urethra in humans: A new mechanism in the development of diabetic lower urinary dysfunction? Med Hypotheses 76: 632–634, 2011 [DOI] [PubMed] [Google Scholar]
- 75.Drake M: Interstitial cells of cajal in the human normal urinary bladder and in the bladder of patients with megacystis-microcolon intestinal hypoperistalsis syndrome. BJU Int 94: 1402, 2004 [DOI] [PubMed] [Google Scholar]
- 76.Hashitani H, Yanai Y, Suzuki H: Role of interstitial cells and gap junctions in the transmission of spontaneous Ca2+ signals in detrusor smooth muscles of the guinea-pig urinary bladder. J Physiol 559: 567–581, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Johnston L, Carson C, Lyons AD, Davidson RA, McCloskey KD: Cholinergic-induced Ca2+ signaling in interstitial cells of Cajal from the guinea pig bladder. Am J Physiol Renal Physiol 294: F645–F655, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Johnston L, Cunningham RM, Young JS, Fry CH, McMurray G, Eccles R, McCloskey KD: Altered distribution of interstitial cells and innervation in the rat urinary bladder following spinal cord injury. J Cell Mol Med 16: 1533–1543, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kim SO, Song SH, Ahn KY, Kwon DD: Distribution of interstitial cells of cajal in menopausal rat urinary bladder showing detrusor overactivity. Int Neurourol J 14: 48–53, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kubota Y, Kojima Y, Shibata Y, Imura M, Sasaki S, Kohri K: Role of KIT-positive interstitial cells of cajal in the urinary bladder and possible therapeutic target for overactive bladder. Adv Urol 2011: 816342, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.McCloskey KD: Interstitial cells in the urinary bladder—localization and function. Neurourol Urodyn 29: 82–87, 2010 [DOI] [PubMed] [Google Scholar]
- 82.Won KJ, Sanders KM, Ward SM: Interstitial cells of Cajal mediate mechanosensitive responses in the stomach. Proc Natl Acad Sci U S A 102: 14913–14918, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Choi KM, Gibbons SJ, Roeder JL, Lurken MS, Zhu J, Wouters MM, Miller SM, Szurszewski JH, Farrugia G: Regulation of interstitial cells of Cajal in the mouse gastric body by neuronal nitric oxide. Neurogastroenterol Motil 19: 585–595, 2007 [DOI] [PubMed] [Google Scholar]
- 84.Ward SM, Beckett EA, Wang X, Baker F, Khoyi M, Sanders KM: Interstitial cells of Cajal mediate cholinergic neurotransmission from enteric motor neurons. J Neurosci 20: 1393–1403, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nakayama S, Kajioka S, Goto K, Takaki M, Liu HN: Calcium-associated mechanisms in gut pacemaker activity. J Cell Mol Med 11: 958–968, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Streutker CJ, Huizinga JD, Driman DK, Riddell RH: Interstitial cells of Cajal in health and disease. Part I: normal ICC structure and function with associated motility disorders. Histopathology 50: 176–189, 2007 [DOI] [PubMed] [Google Scholar]
- 87.Mostafa RM, Moustafa YM, Hamdy H: Interstitial cells of Cajal, the Maestro in health and disease. World J Gastroenterol 16: 3239–3248, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yu W, Zeidel ML, Hill WG: Cellular expression profile for interstitial cells of cajal in bladder - a cell often misidentified as myocyte or myofibroblast. PLoS ONE 7: e48897, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lagou M, De Vente J, Kirkwood TB, Hedlund P, Andersson KE, Gillespie JI, Drake MJ: Location of interstitial cells and neurotransmitters in the mouse bladder. BJU Int 97: 1332–1337, 2006 [DOI] [PubMed] [Google Scholar]
- 90.Johnston L, Woolsey S, Cunningham RM, O’Kane H, Duggan B, Keane P, McCloskey KD: Morphological expression of KIT positive interstitial cells of Cajal in human bladder. J Urol 184: 370–377, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Davidson RA, McCloskey KD: Morphology and localization of interstitial cells in the guinea pig bladder: Structural relationships with smooth muscle and neurons. J Urol 173: 1385–1390, 2005 [DOI] [PubMed] [Google Scholar]
- 92.Piaseczna Piotrowska A, Rolle U, Solari V, Puri P: Interstitial cells of Cajal in the human normal urinary bladder and in the bladder of patients with megacystis-microcolon intestinal hypoperistalsis syndrome. BJU Int 94: 143–146, 2004 [DOI] [PubMed] [Google Scholar]
- 93.Rolle U, Puri P: Structural basis of voiding dysfunction in megacystis microcolon intestinal hypoperistalsis syndrome. J Pediatr Urol 2: 277–284, 2006 [DOI] [PubMed] [Google Scholar]
- 94.Okada S, Kojima Y, Kubota Y, Mizuno K, Sasaki S, Kohri K: Attenuation of bladder overactivity in KIT mutant rats. BJU Int 108: E97–E103, 2011 [DOI] [PubMed] [Google Scholar]
- 95.McCloskey KD, Anderson UA, Davidson RA, Bayguinov YR, Sanders KM, Ward SM: Comparison of mechanical and electrical activity and interstitial cells of Cajal in urinary bladders from wild-type and W/Wv mice. Br J Pharmacol 156: 273–283, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Biers SM, Reynard JM, Doore T, Brading AF: The functional effects of a c-kit tyrosine inhibitor on guinea-pig and human detrusor. BJU Int 97: 612–616, 2006 [DOI] [PubMed] [Google Scholar]
- 97.Hashitani H: Interaction between interstitial cells and smooth muscles in the lower urinary tract and penis. J Physiol 576: 707–714, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sui GP, Rothery S, Dupont E, Fry CH, Severs NJ: Gap junctions and connexin expression in human suburothelial interstitial cells. BJU Int 90: 118–129, 2002 [DOI] [PubMed] [Google Scholar]
- 99.Fry CH, Sui GP, Severs NJ, Wu C: Spontaneous activity and electrical coupling in human detrusor smooth muscle: Implications for detrusor overactivity? Urology 63[Suppl 1]: 3–10, 2004 [DOI] [PubMed] [Google Scholar]
- 100.Zderic SA, Chacko S: Alterations in the contractile phenotype of the bladder: Lessons for understanding physiological and pathological remodelling of smooth muscle. J Cell Mol Med 16: 203–217, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zeidel ML: Obstructive uropathy. In: Goldman's Cecil Medicine, edited by Goldman L, Schafer AI, 24th Ed., Philadelphia, Elsevier, 2011, pp 776–780 [Google Scholar]
- 102.Yoshimura N, Chancellor MB: Physiology and pharmacology of the bladder and urethra. In: Campbell-Walsh Urology, 10th Ed., Philadelphia, Elsevier Saunders, 2012, pp 1786–1833.e17 [Google Scholar]