Abstract
Background and objectives
In Canada, approximately 17% of patients use an arteriovenous access (fistula or arteriovenous graft) at commencement of hemodialysis, despite guideline recommendations promoting its timely creation and use. It is unclear if this low pattern of use is attributable to the lack of surgical creation or a high nonuse rate.
Design, setting, participants, & measurements
Using large health care databases in Ontario, Canada, a population-based cohort of adult patients (≥18 years old) who initiated hemodialysis as their first form of RRT between 2001 and 2010 was studied. The aims were to (1) estimate the proportion of patients who had an arteriovenous access created before starting hemodialysis and the proportion who successfully used it at hemodialysis start, (2) test for secular trends in arteriovenous access creation, and (3) estimate the effect of late nephrology referral and patient characteristics on arteriovenous access creation.
Results
There were 17,183 patients on incident hemodialysis. The mean age was 65.8 years, 60% were men, and 40% were referred late to a nephrologist; 27% of patients (4556 of 17,183) had one or more arteriovenous accesses created, and the median time between arteriovenous access creation and hemodialysis start was 184 days. When late referrals were excluded, 39% of patients (4007 of 10,291) had one or more arteriovenous accesses created, and 27% of patients (2724 of 10,291) used the arteriovenous access. Since 2001, there has been a decline in arteriovenous access creation before hemodialysis initiation. Women, higher numbers of comorbidities, and rural residence were consistently associated with lower rates of arteriovenous access creation. These results persisted even after removing patients with <6 months nephrology care or who had AKI 6 months before starting hemodialysis.
Conclusions
In Canada, arteriovenous access creation before hemodialysis initiation is low, even among patients followed by a nephrologist. Better understanding of the barriers and influencers of arteriovenous access creation is needed to inform both clinical care and guidelines.
Keywords: vascular access, hemodialysis access, hemodialysis, arteriovenous access, epidemiology and outcomes
Introduction
Successful use of an arteriovenous (AV) access (fistula or AV graft) is associated with lower complications and costs, improved quality of life, and superior vascular access and patient survival compared with catheters (1–5). In Canada, although 60%–70% of patients with incident end stage kidney disease (ESKD) see a nephrologist ≥12 months before starting hemodialysis (6), <20% initiate hemodialysis using an AV access (16% fistula and 1% AV graft in 2011), which is lower than most jurisdictions (7,8). This suboptimal pattern of AV access use is not solely attributable to patient characteristics (8,9), and a gap between guideline recommendations and clinical practice clearly exists (10). Although there has been significant work identifying variables associated with vascular access use, there are few data on variables associated with AV access creation. It is unclear if the low use of an AV access is attributable to AV access not being created or created AV accesses not being useable for hemodialysis (e.g., failed to mature). Therefore, we aimed to (1) estimate the proportion of patients who had an AV access created before starting hemodialysis and the proportion who successfully used it at hemodialysis start, (2) examine secular trends in AV access creation, and (3) estimate the effect of late nephrology referral and patient characteristics on AV access creation.
Materials and Methods
Design and Setting
We conducted a retrospective population-based cohort study of patients who initiated hemodialysis for ESKD as their first form of RRT between January 1, 2001, and December 31, 2010. We used linked health care administrative databases in the province of Ontario, Canada. Ontario has approximately 13.5 million residents with universal access to health care and physician services. There are 26 regional programs that oversee 97 dialysis facilities, which in 2012, cared for more than 10,000 patients on incident and prevalent hemodialysis (11). We conducted this observational study according to published guidelines (Supplemental Material, Item 1) (12) using a prespecified protocol that was approved by the privacy office at Sunnybrook Health Sciences Centre (Toronto, Ontario, Canada).
Data Sources
We ascertained type of vascular access used at hemodialysis start, patient demographics and comorbidities, and date of AV access creation using records from four linked databases (Canadian Organ Replacement Register [CORR], Ontario Health Insurance Plan, Discharge Abstract Database, and Registered Persons Database). We have previously described these databases to study vascular access and hemodialysis initiation outcomes (13–16) (Supplemental Material, Item 2). To increase the sensitivity, we captured patient comorbidities in two ways. First, we used CORR, which collects patient information at the first dialysis session, and second, we used physician fee-for-service claims and hospital records (International Classification of Diseases 9/10 codes) in the 5 years before hemodialysis start (details of codes are in Supplemental Table 1).
Patients
We studied adult patients initiating hemodialysis who were registered in the national ESKD registry (CORR). The 5 years before hemodialysis start served as the index date for our study. We looked forward from the index date to capture AV access creations before initiating hemodialysis. We used a 5-year look-back window, because it is unlikely that an AV access was created >5 years before hemodialysis initiation.
Outcomes
The primary outcome was AV access creation before hemodialysis start. All AV access creations were identified using reliable provincial physician billing codes R827, R840, and R851 (sensitivity of 84.5% and specificity of 97.2%) (15,17). Our secondary outcome was use of an AV access at the time of hemodialysis initiation.
Data Analyses
We compared baseline characteristics between patients who did and did not have at least one AV access creation using standardized differences (18,19). This method describes differences between group means or proportions relative to the pooled SD, where a standardized difference >10% is accepted as a meaningful difference (18). Prespecified subgroup analysis was conducted for patients with and without late referral to a nephrologist.
To calculate the adjusted odds ratio for AV access creation, we used a multivariable generalized estimating equation for correlated data within a dialysis facility with a logit link and an exchangeable correlation matrix. We adjusted for age, sex, body mass index, race, rural residence, late nephrology referral (defined as first seen by a nephrologist <3 months before hemodialysis start), type of facility where patient initiated hemodialysis therapy, year of hemodialysis start, primary etiology of ESKD, last predialysis laboratory values, and comorbidities or ESKD-modified comorbid index (a modified Charlson–weighted score on the basis of the history of the following comorbidities: congestive heart failure [CHF], peripheral vascular disease [PVD], cerebral vascular disease, dementia, chronic lung disease, rheumatologic comorbidities, peptic ulcer disease, diabetes, diabetes with complications, moderate/severe liver disease, metastatic disease, leukemia, and lymphoma) (20). Because all patients initiated hemodialysis as their initial ESKD therapy, censoring for loss to follow-up, death, preemptive kidney transplantation, or receipt of other dialysis modality was not required.
All missing information on late nephrology referral (11% missing data), predialysis laboratory values (albumin=20%, hemoglobin=12%, and creatinine=10%), and body mass index (9%) were imputed using the multiple imputation Markov chain Monte Carlo method (21). Complete variables chosen to impute missing data were age, sex, race, ESKD-modified comorbid index (20), year of hemodialysis, body mass index, eGFR at the time of hemodialysis initiation, hemoglobin, albumin, and late referral to a nephrologist. Four additional analyses were performed to test the robustness of our findings. (1) We redefined late nephrology referral as no nephrology care within the 6 months before hemodialysis start. (2) We removed patients who had AKI in the 6 months before hemodialysis start. (3) We removed patients who may be considered too ill to have or benefit from an AV access creation (patients with an ESKD comorbidity index ≥7 were excluded). We repeated this analysis excluding patients with an ESKD comorbidity index ≥5, and (4) we conducted a complete case analysis excluding those with missing data (full details in Supplemental Material, Item 3, Methods). We used SAS, version 9.2 (SAS Institute, Cary, North Carolina) to conduct all statistical analyses.
Results
Patient Population
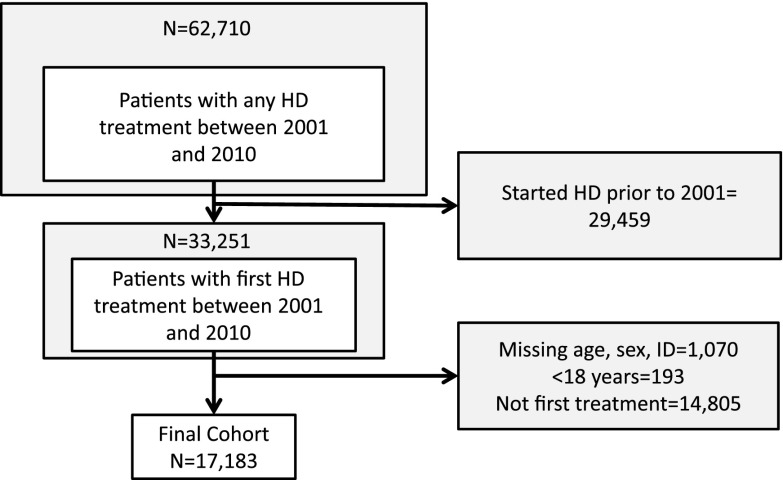
Between January 1, 2001, and December 31, 2010, 17,183 adult patients started hemodialysis as their first form of RRT (Figure 1). The mean age was 65.8 years (SD=15.1), with 60% being ≥65 years old, and 60% of patients were men. Patients with history of diabetes was 47%, CHF was 47%, PVD was 22%, and coronary artery disease 60%. Thirteen percent of all patients resided in a rural area. Over the study period, the proportion of patients who had a late referral decreased from 48% in 2001 to 36% in 2010 (P value <0.001). Baseline characteristics of patients with and without an AV access creation are presented in Table 1. Patient characteristics by dialysis era are shown in Table 2. With the exception of diabetes and coronary artery disease, the patient characteristics and presence of comorbidities were similar across year of enrollment.
Figure 1.
Cohort selection. HD, hemodialysis; ID, identification.
Table 1.
Baseline characteristics of patients who initiated hemodialysis for ESKD
| Variable | All Patients | No Arteriovenous Access Creation | One or More Arteriovenous Access Creations | Standardized Difference (%)a |
|---|---|---|---|---|
| Total number, N | 17,183 | 12,627 | 4556 | |
| Age (yr) at hemodialysis start, N (%) | ||||
| Mean (SD) | 65.8 (15.1) | 65.7 (15.5) | 65.9 (13.9) | 1 |
| <65 | 6928 (40) | 5094 (40) | 1834 (40) | <1 |
| 65–74 | 4476 (26) | 3179 (25) | 1297 (29) | 7 |
| 75–84 | 4711 (27) | 3487 (28 | 1224 (27) | 2 |
| >84 | 1068 (6) | 867 (7) | 201 (4) | 11 |
| Men, N (%) | 10,269 (60) | 7339 (58) | 2930 (64) | 13 |
| Race, N (%) | ||||
| Asian | 914 (5) | 554 (5) | 249 (5) | 1 |
| Black | 774 (5) | 569 (5) | 205 (5) | <1 |
| Other | 1866 (11) | 1389 (11) | 477 (10) | 1 |
| Unknown | 740 (4) | 524 (4) | 216 (5) | 2 |
| White | 12,889 (75) | 9480 (75) | 3409 (75) | 3 |
| Acute dialysis start,b N (%) | 7655 (45) | 6576 (52) | 1079 (24) | 60 |
| Evidence of AKI within 6 mo of hemodialysis start, N (%) | 4809 (28) | 4337 (34) | 472 (10) | 60 |
| Primary etiology of ESKD diagnosis, N (%) | ||||
| GN | 1636 (9) | 1151 (9) | 485 (11) | 5 |
| Diabetes | 6187 (36) | 4291 (34) | 1896 (42) | 16 |
| Renal vascular disease | 3610 (21) | 2652 (21) | 958 (21) | <1 |
| Polycystic kidney disease | 544 (3) | 213 (2) | 331 (7) | 27 |
| Other | 2893 (17) | 2447 (19) | 446 (10) | 27 |
| Unknown | 2313 (14) | 1873 (15) | 440 (10) | 16 |
| Comorbidities, N (%) | ||||
| Diabetes mellitus | 8030 (47) | 5763 (46) | 2267 (50) | 8 |
| CHF | 8118 (47) | 6249 (49) | 1869 (41) | 17 |
| Coronary artery disease | 10,334 (60) | 7547 (60) | 2787 (61) | 3 |
| Coronary artery bypass graft/percutaneous coronary intervention | 2699 (16) | 2042 (16) | 657 (14) | 5 |
| Peripheral vascular disease | 3816 (22) | 2602 (21) | 1214 (27) | 14 |
| Cerebrovascular disease | 2865 (17) | 2189 (17) | 676 (15) | 7 |
| Leukemia | 1291 (8) | 1078 (9) | 213 (5) | 16 |
| Lymphoma | 118 (1) | 103 (1) | 15 (0) | 6 |
| Other cancerc | 7470 (43) | 6423 (51) | 1047 (23) | 60 |
| Lung disease (chronic obstructive pulmonary disease) | 3098 (18) | 2401 (19) | 697 (15) | 9 |
| Dementia | 1284 (7) | 1015 (8) | 269 (6) | 8 |
| Hypertension | 15,571 (91) | 11,276 (89) | 4295 (94) | 18 |
| Other serious illnessd | 1850 (11) | 1540 (12) | 310 (7) | 18 |
| Smoking | 2079 (12) | 1630 (13) | 449 (10) | 9 |
| ESKD comorbidity index,e N (%) | ||||
| 0 | 2001 (12) | 1424 (11) | 577 (13) | 4 |
| 1 | 513 (3) | 348 (3) | 165 (4) | 5 |
| 2 | 1677 (10) | 1189 (9) | 488 (11) | 4 |
| 3 | 1538 (9) | 1108 (9) | 430 (9) | 2 |
| 4 | 1725 (10) | 1294 (10) | 431 (9) | 3 |
| 5 | 2091 (12) | 1491 (12) | 600 (13) | 4 |
| 6 | 1509 (9) | 1101 (9) | 408 (9) | 1 |
| 7+ | 6129 (36) | 4672 (37) | 1457 (32) | 11 |
| Year of hemodialysis start, N (%) | ||||
| 2001–2003 | 4724 (27) | 3207 (25) | 1517 (33) | 17 |
| 2004–2006 | 5164 (31) | 3749 (30) | 1415 (31) | 3 |
| 2007–2010 | 7295 (42) | 5671 (45) | 1624 (36) | 9 |
| Location of dialysis treatments, N (%) | ||||
| Acute care hospital | 16,859 (98) | 12,479 (99) | 4380 (96) | 17 |
| Community center | 261 (2) | 129 (1) | 132 (3) | 14 |
| Home hemodialysis | 63 (<1) | 19 (<1) | 44 (1) | 11 |
| Assistance/care with dialysis treatments, N (%) | ||||
| Total care | 17,094 (99) | 12,592 (100) | 4502 (99) | 11 |
| Limited self-care | 25 (<1) | 15 (<1) | 10 (<1) | 3 |
| Total self-care | 64 (<1) | 20 (<1) | 44 (1) | 11 |
| Laboratory valuef | ||||
| Albumin mean (SD), g/dl | 3.2 (0.7) | 3.1 (0.7) | 3.4 (0.6) | 46 |
| Hemoglobin mean (SD), g/dl | 10.0 (1.74) | 9.8 (1.73) | 10.6 (1.63) | 48 |
| eGFRg (ml/min per 1.73 m2) median (quartile 1, quartile 3) | 9 (6, 12) | 9 (6, 12) | 9 (7, 12) | 6 |
| Late referral,h N (%) | 6892 (40) | 6343 (50) | 549 (12) | 89 |
| Early initiation,i N (%) | 6015 (35) | 4464 (35) | 1551 (34) | 3 |
| Rural residence, N (%) | 2197 (13) | 1170 (14) | 427 (9) | 15 |
CHF, congestive heart failure.
Standardized differences describe differences between group means or proportions relative to the pooled SD. A standardized difference >10% is accepted as a meaningful difference (18).
Having a dialysis treatment as an inpatient in the 2 weeks before hemodialysis start.
Other cancer included all other types of cancer (excluding skin cancer but including melanoma).
Other serious illness that may shorten life expectancy <5 years.
The ESKD comorbidity index is a modified Charlson–weighted score on the basis of the history of the following comorbidities: congestive heart failure (CHF), peripheral vascular disease, cerebral vascular disease, dementia, chronic lung disease, rheumatologic comorbidities, peptic ulcer disease, diabetes, diabetes with complications, moderate/severe liver disease, metastatic disease, leukemia, and lymphoma (20).
Last laboratory values before starting hemodialysis.
eGFR calculated using the creatinine level at the last laboratory evaluation before hemodialysis start and the Modification of Diet in Renal Disease formula.
Late referral defined as first seen by a nephrologist <90 days before initiation of dialysis.
Early initiation defined as eGFR>10.5 ml/min per 1.73 m2 at the start of dialysis.
Table 2.
Characteristics by year of hemodialysis start
| Variable | 2001–2003 | 2004–2006 | 2007–2010 |
|---|---|---|---|
| N | 4724 | 5164 | 7295 |
| Age (yr) at hemodialysis start, N (%) | |||
| Mean (SD) | 65.6 (14.8) | 65.8 (15.3) | 65.9 (15.1) |
| <65 | 1878 (40) | 2076 (40) | 2974 (41) |
| 65–74 | 1323 (28) | 1315 (25) | 1838 (25) |
| 75–84 | 1287 (27%) | 1454 (28) | 1970 (27) |
| >84 | 236 (5) | 319 (6) | 513 (7) |
| Men, N (%) | 2813 (60) | 3047 (59) | 4409 (60) |
| Race, N (%) | |||
| Asian | 248 (5) | 281 (5) | 385 (5) |
| Black | 197 (4) | 230 (4) | 347 (5) |
| Caucasian | 3664 (78) | 3897 (75) | 5328 (73) |
| Other | 398 (8) | 544 (11) | 924 (13) |
| Unknown | 217 (5) | 212 (4) | 311 (4) |
| Acute dialysis starta | 1955 (41) | 2409 (47) | 3291 (45) |
| Primary renal diagnosis, N (%) | |||
| GN | 505 (11) | 541 (10) | 590 (8) |
| Diabetes | 1652 (35) | 1839 (36) | 2696 (37) |
| Renal vascular disease | 1058 (22) | 1103 (21) | 1449 (20) |
| Polycystic kidney disease | 176 (4) | 176 (3) | 192 (3) |
| Other | 750 (16) | 874 (17) | 1269 (17) |
| Unknown | 583 (12) | 631 (12) | 1099 (15) |
| Comorbidities, N (%) | |||
| Diabetes mellitus | 2059 (44) | 2308 (45) | 3663 (50) |
| CHF | 2272 (48) | 2423 (47) | 3423 (47) |
| Coronary artery disease | 2935 (62) | 3150 (61) | 4249 (58) |
| Coronary artery bypass graft/percutaneous coronary intervention | 638 (14) | 787 (15) | 1274 (17) |
| Peripheral vascular disease | 1205 (26) | 1120 (22) | 1491 (20) |
| Cerebrovascular disease | 768 (16) | 866 (17) | 2018 (28) |
| Leukemia | 353 (7) | 373 (7) | 565 (8) |
| Lymphoma | 36 (1) | 36 (1) | 46 (1) |
| Other cancerb | 1138 (24) | 1314 (25) | 2018 (28) |
| Lung disease (chronic obstructive pulmonary disease) | 942 (20) | 855 (17) | 1301 (18) |
| Dementia | 319 (7) | 353 (7) | 612 (8) |
| Hypertension | 4295 (91) | 4668 (90) | 6608 (91) |
| Other serious illnessc | 363 (8) | 455 (9) | 1032 (14) |
| Smoking | 597 (13) | 580 (11) | 902 (12) |
| ESKD comorbidity index,d N (%) | |||
| 0 | 572 (12) | 631 (12) | 678 (9) |
| 1 | 151 (3) | 126 (2) | 236 (3) |
| 2 | 486 (10) | 542 (10) | 649 (9) |
| 3 | 390 (8) | 445 (9) | 703 (10) |
| 4 | 487 (10) | 551 (11) | 687 (9) |
| 5 | 555 (12) | 651 (13) | 885 (12) |
| 6 | 397 (8) | 479 (9) | 633 (9) |
| 7+ | 1686 (36) | 1739 (34) | 2704 (37) |
| Laboratory valuee | |||
| Albumin mean (SD), g/L | 3.21 (0.66) | 3.16 (0.70) | 3.15 (0.72) |
| Hemoglobin mean (SD), g/L | 10.14 (1.77) | 10.11 (1.75) | 9.85 (1.68) |
| eGFRf (ml/min per 1.73 m2) median (quartile 1, quartile 3) | 8 (6, 11) | 9 (6, 12) | 9 (7, 13) |
| Late referral,g N (%) | 2090 (44) | 2096 (41) | 2690 (37) |
| Rural, N (%) | 628 (13) | 658 (13) | 911 (12) |
Having a dialysis treatment as an inpatient in the 2 weeks before hemodialysis start.
Other cancer included all other types of cancer (excluding skin cancer but including melanoma).
Other serious illness that may shorten life expectancy <5 years.
The ESKD comorbidity index is a modified Charlson–weighted score on the basis of the history of the following comorbidities: CHF, peripheral vascular disease, cerebral vascular disease, dementia, chronic lung disease, rheumatologic comorbidities, peptic ulcer disease, diabetes, diabetes with complications, moderate/severe liver disease, metastatic disease, leukemia, and lymphoma (20).
Last laboratory values before starting hemodialysis.
eGFR calculated using the creatinine level at the last laboratory evaluation before hemodialysis start and the Modification of Diet in Renal Disease formula.
Late referral defined as first seen by a nephrologist <90 days before initiation of dialysis.
In our total cohort, 27% of patients had an AV access created, and 17% used an AV access for their initial hemodialysis treatments. Among the patients with one or more AV access creations, 65% (n=2977) used the AV access, and 33% (n=1490) used a catheter at the start of hemodialysis; 2% (n=89) of patients had missing information. There was no change in AV access use in patients with only one AV access creation (n=3589).
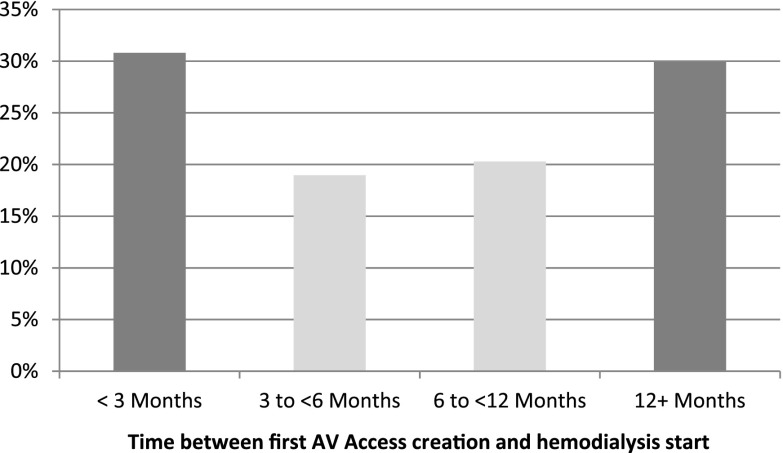
The proportions of patients who had one, two, or three or more AV accesses created were 22%, 3%, and 1%, respectively. The proportion of patients with an AV access creation before dialysis start declined from 32% in 2001–2003 to 27% in 2004–2007 to 22% in 2008–2010 (P value <0.001). The median time between AV access creation and hemodialysis start was 184 days (25th percentile: 73 days; 75th percentile: 439 days). The distribution of time between the first AV access creation and the first day of hemodialysis is depicted in Figure 2. When late-referral patients were excluded (n=10,291), 39% of the remaining cohort had an AV access creation prehemodialysis initiation, and 27% used an AV access at hemodialysis initiation. In the late-referral cohort (n=6892), 8% had an AV access creation before hemodialysis initiation, and 4% used an AV access at hemodialysis initiation. Table 3 summarizes the number of AV accesses created according to patients with AV access use at hemodialysis start, catheter use at hemodialysis start, missing vascular access information, and early and late referral to a nephrologist.
Figure 2.
The light gray bars are considered optimal timing of fistula creation according to vascular access guidelines (1–3). AV, arteriovenous.
Table 3.
Proportion of patients with arteriovenous access creations before hemodialysis initiation
| Group | N | Patients with Number of Arteriovenous Access Creations (%) | |||
|---|---|---|---|---|---|
| 0 | 1 | 2 | ≥3 | ||
| All patients | 17,183 | 73 | 22 | 3 | 1 |
| Patients who started hemodialysis using arteriovenous access | 2977 | 0 | 84 | 13 | 2 |
| Patients who started hemodialysis using a catheter | 13,672 | 89 | 9 | 1 | 0 |
| Patients who had missing vascular access information at hemodialysis start | 534 | 83 | 15 | 2 | 0 |
| Early referrala | 10,291 | 61 | 33 | 5 | 1 |
| Late referralb | 6892 | 92 | 7 | 1 | 0 |
Arteriovenous access refers to an arteriovenous fistula or graft.
Patients who have been referred to a nephrologist at least 90 days before hemodialysis start.
Patients who have been referred to a nephrologist <90 days before dialysis start.
Factors Associated with AV Access Creation
The results of the primary analysis are presented in Table 4. A higher likelihood of AV access creation was associated with sex, history of hypertension, and PVD. Patients were less likely to have an AV access creation if they had a history of coronary artery disease, CHF, cerebrovascular disease, leukemia, lymphoma, dementia, smoking, or other serious life-threatening illnesses. Timing of hemodialysis initiation, a rural residence, and late nephrology referral were also associated with a lower likelihood of AV access creation. Patients with a late nephrology referral (8% versus 39% with early referral) were the least likely to have an AV access creation.
Table 4.
Multivariable analysis examining covariates associated with a higher likelihood of arteriovenous access creation before the initiation of hemodialysis (adjusting for correlated outcomes within each dialysis facility)
| Variable/Level | Adjusted Odds Ratio | 95% Confidence Interval |
|---|---|---|
| Age group (yr) | ||
| <65 | 1.00 | Reference |
| 65–74 | 1.25 | 1.13 to 1.39 |
| 75–84 | 1.18 | 1.06 to 1.31 |
| 85+ | 0.84 | 0.70 to 1.02 |
| Men | 1.30 | 1.20 to 1.41 |
| Race | ||
| Asian | 0.89 | 0.75 to 1.06 |
| Black | 0.81 | 0.67 to 0.98 |
| Other | 0.90 | 0.79 to 1.03 |
| Unknown | 0.98 | 0.80 to 1.20 |
| White | 1.00 | Reference |
| Body mass index, kg/m2 | ||
| <18.5 | 0.87 | 0.68 to 1.11 |
| 18.5–24.9 | 1.00 | Reference |
| 25–29.9 | 1.20 | 1.08 to 1.34 |
| ≥30 | 1.36 | 1.23 to 1.52 |
| Year of dialysis start | ||
| 2001–2003 | 1.00 | Reference |
| 2004–2006 | 0.74 | 0.67 to 0.81 |
| 2007–2010 | 0.53 | 0.49 to 0.59 |
| Laboratory valuesa | ||
| Hemoglobin, g/L | 1.16 | 1.12 to 1.19 |
| Albumin, g/L | 1.54 | 1.42 to 1.67 |
| Primary etiology of ESKD diagnosis | ||
| Diabetes | 1.10 | 0.91 to 1.32 |
| GN | 1.00 | Reference |
| Polycystic kidney disease | 2.27 | 1.81 to 2.86 |
| Renal vascular disease | 0.86 | 0.74 to 1.00 |
| Other | 0.74 | 0.62 to 0.87 |
| Unknown | 0.75 | 0.63 to 0.89 |
| Comorbidity | ||
| Peripheral vascular disease | 1.40 | 1.27 to 1.54 |
| Coronary artery diseaseb | 0.85 | 0.77 to 0.94 |
| CHF | 0.68 | 0.62 to 0.75 |
| Cerebrovascular disease | 0.81 | 0.72 to 0.90 |
| Diabetes | 0.92 | 0.80 to 1.06 |
| Leukemia | 0.77 | 0.64 to 0.91 |
| Lymphoma | 0.73 | 0.40 to 1.36 |
| Other cancerc | 0.99 | 0.90 to 1.09 |
| Gastric ulcer | 0.95 | 0.82 to 1.10 |
| Liver disease | 0.90 | 0.77 to 1.05 |
| Dementia | 0.79 | 0.67 to 0.92 |
| Hypertension | 1.36 | 1.15 to 1.60 |
| Lung disease (COPD) | 0.90 | 0.80 to 1.00 |
| Smokers | 0.83 | 0.73 to 0.95 |
| Other serious life-threatening diseased | 0.73 | 0.63 to 0.84 |
| Late referrale | 0.18 | 0.15 to 0.20 |
| Rural resident | 0.77 | 0.67 to 0.87 |
COPD, chronic obstructive pulmonary disease.
Last laboratory values before starting hemodialysis.
Coronary artery disease was determined from the presence of a history of at least one of the following: coronary artery bypass grafting, previous myocardial infarction, or previous angina.
Other cancer included all other types of cancer (excluding skin cancer but including melanoma).
Other serious illness that may shorten life expectancy <5 years.
Late referral defined as first seen by a nephrologist <90 days before initiation of dialysis.
Prespecified Subgroup Analyses
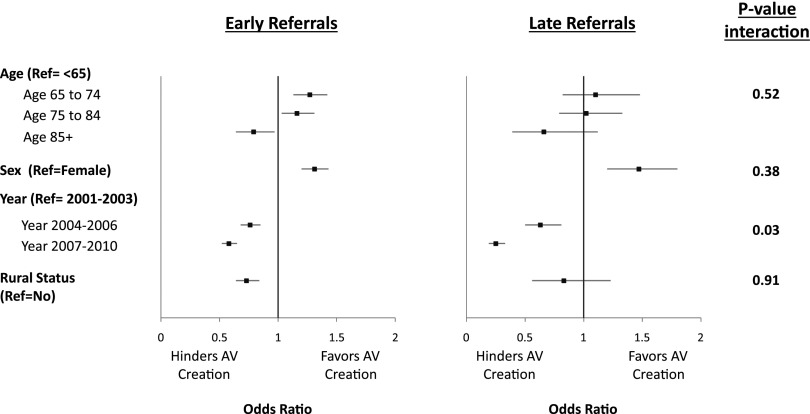
Only year of hemodialysis start modified the likelihood of having an AV access creation among the patients with early and late nephrology care. The likelihood of AV access creation declined more for patients with a late referral compared with patients with >3 months of nephrology follow-up. Figure 3 depicts the likelihood of AV access creation in the prespecified subgroup analyses separated by nephrology referral. With the exception of age group, sex, and rural status, which had numeric differences, all other covariates were similar among the two subgroups.
Figure 3.
Subgroup analysis for patients with early and late referrals. The models were adjusted for age, sex, race, body mass index, year of dialysis start, laboratory values (serum albumin and hemoglobin), eGFR, primary reason for renal diagnosis, ESKD comorbidity index, smoking status, and residence in a rural area. Ref, reference.
Sensitivity Analyses
We conducted four additional analyses to test the robustness of our findings. Our results were consistent across all four analyses (Supplemental Material, Item 3, Results).
Discussion
In Canada, AV access use at hemodialysis start is low compared with other jurisdictions (22). We identified that both lack of creation and a high nonuse rate contributed to the low rate of AV access use in Ontario. We found that approximately one quarter of incident patients had an AV access created before starting hemodialysis and that approximately two thirds of these patients used an AV access at hemodialysis start. As expected, nephrology care >3 months is an important influencer for AV access creation. AV access creation rates remained low, even after accounting for patient comorbidities and removing patients with <6 months of nephrology care, patients who had AKI in the 6 months before hemodialysis, and those who may be considered too ill to benefit from an AV access creation (sensitivity analyses). Additionally, we found that the likelihood of patients having an AV access created decreased over time, with the lowest rates of AV access creation in the more recent years.
In a single-center Canadian study, Weber et al. (23) examined fistula use in 125 patients with at least one fistula creation. Hemodialysis was started by 74% (n=93) of patients, and 73% of these patients started hemodialysis using a fistula. Fistula use was slightly higher than the observed 67% AV access use in our cohort. The proportion of patients with a second fistula creation was 17% in the study by Weber et al. (23) compared with 13% in our cohort.
The timely creation of an AV access in patients not yet on hemodialysis requires consideration of factors, such as patient preferences, life expectancy, likelihood of needing hemodialysis, timing of hemodialysis start, AV access eligibility, and risk of complications. In addition, Canadians face challenging system-wide barriers to AV access creation, such as limited access to surgical services (6,24,25). Furthermore, because of the lack of validated standardized eligibility criteria for AV access creation, there exists large variation in vascular access use across hemodialysis programs (8,22,26). Vascular access use is not driven solely by patient characteristics, and the effects of center-specific variables influence patterns of use (27). Additionally, Canadian patients and health care providers have attitudes that are markedly different from the rest of the world, and it would seem that Canada has a culture that is more permissive of catheters (22). In surveys, we have found that this attitude is pervasive among patients, and nephrologists as well as vascular access surgeons had marked variations in their practices regarding AV access referral patterns and creation, respectively (26,28,29).
This variation in practice is supported by our results, which show that the timing of AV access creation is widely distributed, with the majority of AV access creations occurring outside the guideline’s recommended timeframe—only 39% of patients in our cohort had an AV access created within the recommended timelines (between 3 and 12 months before hemodialysis start) (1–3). Previous studies from Canada and Australia have found that incident fistula creations were created between an eGFR of 7 and 14 ml/min per 1.73 m2—below the recommended window of 15–30 ml/min per 1.73 m2 (2,10,23). Timing of AV access creation remains controversial in predialysis patients, with concerns that early access creations may lead to wasted or, more importantly, unnecessary procedures (25,30). However, current timing of AV access creation in Ontario is reassuring, with approximately 81% of patients with a predialysis AV access creation starting hemodialysis after 2 years of follow-up (15). Also, using Ontario data, Oliver et al. (15) estimated the risk of AV access nonuse, reporting that 8.5% of patients with an AV access creation died before starting hemodialysis and another 10.2% of patients never started hemodialysis after a 2-year follow-up.
In terms of predialysis nephrology care, we found that late referral significantly modified the likelihood of having an AV access created in the predialysis period. Not surprisingly, patients with late nephrology care were not only less likely to have an AV access created but also, less likely to use an AV access at hemodialysis initiation. These results intuitively make sense, because (1) late nephrology referral limits the planning and preparation time required for creating and facilitating an AV access for hemodialysis use, and (2) even among those with an AV access creation, there may not be adequate time for an AV access (specifically, a fistula) to mature before starting hemodialysis. However, even among patients with early referral, only 39% had an AV access creation. This low rate of AV access creation may be attributable to system barriers, delay in referral from nephrologists, and long surgical wait times. Mendelssohn et al. (6) reported long delays in appointments with vascular access surgeons as well as long wait times for surgical room booking and operating time in the Canadian system compared with Europe and the United States.
Interestingly, despite the 2006 Canadian Society of Nephrology’s endorsement for AV access use (1,31), between 2001 and 2010, we found a uniform decline in the proportion of patients with an AV access creation. This was evident even after excluding patients with late nephrology referral and those who had AKI in the 6 months before hemodialysis start. Our results are concordant with the AV access creation rates observed among prevalent patients (data not shown) and cannot be fully explained by the increase in the proportion of sicker patients. Even after adjusting for patient characteristics and excluding patients with ESKD comorbidity index ≥5, the likelihood of patients having an AV access creation decreased over time. This supports our previous work, which showed an increase in the proportion of catheter use and a decrease in AV access use in Canada (7,13).
Beyond the process of care, our findings support the need for a standardized, validated approach to patient selection and referral for AV access (32,33). There is a large body of research showing that previous history of PVD is a key variable that is associated with poor fistula maturation (9,34,35). Within our cohort, we found that, although PVD was associated with lower likelihood of AV access use (data not shown), it was correlated with increased AV access creation: 27% of patients with an AV access created had a previous history of PVD compared with 21% in the group of patients with no AV access creation. There are several interpretations of this finding. (1) The surgeon/nephrologist was aware of the high risk of failure in patients with PVD and that an AV access should be attempted predialysis, allowing time for either facilitation of a challenging access or the opportunity to create another one if it failed before hemodialysis start. (2) A knowledge gap exists regarding PVD. (3) Patients with PVD were otherwise healthier than those in the group with no AV access creation (not tested).
Our study has several limitations. First, given the observational nature of the administrative data, selection bias may exist given that patients with no AV access creation may be different from patients with one or more AV access creations beyond the data elements used for adjustment. Second, we could not distinguish between types of AV access created; however, given that AV graft use in Canada is <3%, we can safely assume that most of the AV access creations were fistulas. Third, other patient-level elements were missing in our datasets (e.g., condition of vasculature, vein diameter, patient attitudes, etc.), which may influence both vascular access creation and use. Fourth, large administrative databases can be limited by their data’s validity; however, we used data from three linked databases to increase the sensitivity of capturing the correct data elements of interest to provide reliable information. Fifth, several data elements were incomplete; however, we accounted for this by performing multiple data imputations. In sensitivity analyses, there were no differences in the direction or magnitude of the measure of effect when we used imputed versus nonimputed data. Sixth, we had no information on patients who were evaluated for AV access and thought to be nonsuitable. Seventh, we are missing an important portion (15) of patients who had an AV access created but never started hemodialysis and therefore, were not included in our study. Inclusion of these data in future studies is needed to understand the AV access nonuse rate among those patients who do not start hemodialysis. Nevertheless, we conducted multiple sensitivity analyses to ensure robustness and consistency of our findings, which made no change to our final results.
In conclusion, in Ontario, the rate of AV access creation among patients on incident hemodialysis, even those followed by a nephrologist, is low, contributing significantly to the low rate of incident AV access use. The rate of AV access creation is declining, despite stable patient characteristics. Better understanding of the barriers and influencers of AV access creation is needed to inform patient-centered care and clinical practice guidelines.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank research personnel and investigators in the Ontario Institute for Clinical Evaluative Sciences Kidney, Dialysis and Transplantation Program (ICES KDT) team for administrative, technical, and scientific support. Specifically, we thank Dr. Stephanie Dixon, lead analyst and biostatistician at ICES KDT, for her input with study design, cohort creation, and statistical analysis. We also thank the editors and reviewers of CJASN for their valuable input that significantly improved the quality of our work.
The Ontario Institute for Clinical Evaluative Sciences is a nonprofit research corporation funded by the Ontario Ministry of Health and Long-Term Care (MOHLTC). This project was conducted by the ICES KDT.
The opinions, results, and conclusions reported in this paper are those of the authors and independent from the funding sources. No endorsement by the MOHLTC is intended or should be inferred.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.06220614/-/DCSupplemental.
References
- 1.Jindal K, Chan CT, Deziel C, Hirsch D, Soroka SD, Tonelli M, Culleton BF, Canadian Society of Nephrology Committee for Clinical Practice Guidelines : Hemodialysis clinical practice guidelines for the Canadian Society of Nephrology. J Am Soc Nephrol 17[Suppl 1]: S1–S27, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Vascular Access 2006 Work Group : Clinical practice guidelines for vascular access. Am J Kidney Dis 48[Suppl 1]: S176–S247, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Tordoir J, Canaud B, Haage P, Konner K, Basci A, Fouque D, Kooman J, Martin-Malo A, Pedrini L, Pizzarelli F, Tattersall J, Vennegoor M, Wanner C, ter Wee P, Vanholder R: EBPG on vascular access. Nephrol Dial Transplant 22[Suppl 2]: ii88–ii117, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Ravani P, Palmer SC, Oliver MJ, Quinn RR, MacRae JM, Tai DJ, Pannu NI, Thomas C, Hemmelgarn BR, Craig JC, Manns B, Tonelli M, Strippoli GFM, James MT: Associations between hemodialysis access type and clinical outcomes: A systematic review. J Am Soc Nephrol 24: 465–473, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ravani P, Gillespie BW, Quinn RR, MacRae J, Manns B, Mendelssohn D, Tonelli M, Hemmelgarn B, James M, Pannu N, Robinson BM, Zhang X, Pisoni R: Temporal risk profile for infectious and noninfectious complications of hemodialysis access. J Am Soc Nephrol 24: 1668–1677, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mendelssohn DC, Ethier J, Elder SJ, Saran R, Port FK, Pisoni RL: Haemodialysis vascular access problems in Canada: Results from the Dialysis Outcomes and Practice Patterns Study (DOPPS II). Nephrol Dial Transplant 21: 721–728, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Lok CE, Sontrop JM, Tomlinson G, Rajan D, Cattral M, Oreopoulos G, Harris J, Moist L: Cumulative patency of contemporary fistulas versus grafts (2000-2010). Clin J Am Soc Nephrol 8: 810–818, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ethier J, Mendelssohn DC, Elder SJ, Hasegawa T, Akizawa T, Akiba T, Canaud BJ, Pisoni RL: Vascular access use and outcomes: An international perspective from the Dialysis Outcomes and Practice Patterns Study. Nephrol Dial Transplant 23: 3219–3226, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pisoni RL, Young EW, Dykstra DM, Greenwood RN, Hecking E, Gillespie B, Wolfe RA, Goodkin DA, Held PJ: Vascular access use in Europe and the United States: Results from the DOPPS. Kidney Int 61: 305–316, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Lopez-Vargas PA, Craig JC, Gallagher MP, Walker RG, Snelling PL, Pedagogos E, Gray NA, Divi MD, Gillies AH, Suranyi MG, Thein H, McDonald SP, Russell C, Polkinghorne KR: Barriers to timely arteriovenous fistula creation: A study of providers and patients. Am J Kidney Dis 57: 873–882, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Webster G, Wu J, Williams B, Ivis F, de Sa E, Hall N: Canadian Organ Replacement Register Annual Report: Treatment of End-Stage Organ Failure in Canada 2003–2012, Ottawa, Ontario, Canadian Institute for Health Information, 2014 [Google Scholar]
- 12.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, STROBE Initiative : The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J Clin Epidemiol 61: 344–349, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Moist LM, Trpeski L, Na Y, Lok CE: Increased hemodialysis catheter use in Canada and associated mortality risk: Data from the Canadian Organ Replacement Registry 2001-2004. Clin J Am Soc Nephrol 3: 1726–1732, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ellwood AD, Jassal SV, Suri RS, Clark WF, Na Y, Moist LM: Early dialysis initiation and rates and timing of withdrawal from dialysis in Canada. Clin J Am Soc Nephrol 8: 265–270, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oliver MJ, Quinn RR, Garg AX, Kim SJ, Wald R, Paterson JM: Likelihood of starting dialysis after incident fistula creation. Clin J Am Soc Nephrol 7: 466–471, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perl J, Wald R, McFarlane P, Bargman JM, Vonesh E, Na Y, Jassal SV, Moist L: Hemodialysis vascular access modifies the association between dialysis modality and survival. J Am Soc Nephrol 22: 1113–1121, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quinn RR, Laupacis A, Austin PC, Hux JE, Garg AX, Hemmelgarn BR, Oliver MJ: Using administrative datasets to study outcomes in dialysis patients: A validation study. Med Care 48: 745–750, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Austin PC: Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput 38: 1228–1234, 2009 [Google Scholar]
- 19.Mamdani M, Sykora K, Li P, Normand SL, Streiner DL, Austin PC, Rochon PA, Anderson GM: Reader’s guide to critical appraisal of cohort studies: 2. Assessing potential for confounding. BMJ 330: 960–962, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hemmelgarn BR, Manns BJ, Quan H, Ghali WA: Adapting the charlson comorbidity index for use in patients with ESRD. Am J Kidney Dis 42: 125–132, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Rubin DB: Multiple Imputation for Nonresponse in Surveys, Hoboken, NJ, John Wiley & Sons, Inc., 1987 [Google Scholar]
- 22.Fissell RB, Fuller DS, Morgenstern H, Gillespie BW, Mendelssohn DC, Rayner HC, Robinson BM, Schatell D, Kawanishi H, Pisoni RL: Hemodialysis patient preference for type of vascular access: Variation and predictors across countries in the DOPPS. J Vasc Access 14: 264–272, 2013 [DOI] [PubMed] [Google Scholar]
- 23.Weber CL, Djurdjev O, Levin A, Kiaii M: Outcomes of vascular access creation prior to dialysis: Building the case for early referral. ASAIO J 55: 355–360, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Hughes SA, Mendelssohn JG, Tobe SW, McFarlane PA, Mendelssohn DC: Factors associated with suboptimal initiation of dialysis despite early nephrologist referral. Nephrol Dial Transplant 28: 392–397, 2013 [DOI] [PubMed] [Google Scholar]
- 25.O’Hare AM, Bertenthal D, Walter LC, Garg AX, Covinsky K, Kaufman JS, Rodriguez RA, Allon M: When to refer patients with chronic kidney disease for vascular access surgery: Should age be a consideration? Kidney Int 71: 555–561, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Xi W, MacNab J, Lok CE, Lee TC, Maya ID, Mokrzycki MH, Moist LM: Who should be referred for a fistula? A survey of nephrologists. Nephrol Dial Transplant 25: 2644–2651, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tangri N, Moorthi R, Tighiouhart H, Meyer KB, Miskulin DC: Variation in fistula use across dialysis facilities: Is it explained by case-mix? Clin J Am Soc Nephrol 5: 307–313, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xi W, Harwood L, Diamant MJ, Brown JB, Gallo K, Sontrop JM, MacNab JJ, Moist LM: Patient attitudes towards the arteriovenous fistula: A qualitative study on vascular access decision making. Nephrol Dial Transplant 26: 3302–3308, 2011 [DOI] [PubMed] [Google Scholar]
- 29.Nica A, Lok CE, Harris J, Lee TC, Mokrzycki MH, Maya ID, Vazquez MA, Xi W, Moist LM, North American Vascular Access Consortium (NAVAC) : Understanding surgical preference and practice in hemodialysis vascular access creation. Semin Dial 26: 520–526, 2013 [DOI] [PubMed] [Google Scholar]
- 30.DeSilva RN, Patibandla BK, Vin Y, Narra A, Chawla V, Brown RS, Goldfarb-Rumyantzev AS: Fistula first is not always the best strategy for the elderly. J Am Soc Nephrol 24: 1297–1304, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mendelssohn DC, Beaulieu M, Kiaii M, Jindal K, Macrae JM, Kappel L, Miller L, Lok CE, Moist LM, Donnelly S, Oliver MJ, Chan C, Ehtier J, Delziel C, McKinnon M, Soroka SD, Hirsch D, Barrett B, Canadian Society of Nephrology (CSN) : Report of the canadian society of nephrology vascular access working group. Semin Dial 25: 22–25, 2012 [DOI] [PubMed] [Google Scholar]
- 32.Allon M, Lok CE: Dialysis fistula or graft: The role for randomized clinical trials. Clin J Am Soc Nephrol 5: 2348–2354, 2010 [DOI] [PubMed] [Google Scholar]
- 33.Hiremath S, Knoll G, Weinstein MC: Should the arteriovenous fistula be created before starting dialysis?: A decision analytic approach. PLoS ONE 6: e28453, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lok CE, Allon M, Moist L, Oliver MJ, Shah H, Zimmerman D: Risk equation determining unsuccessful cannulation events and failure to maturation in arteriovenous fistulas (REDUCE FTM I). J Am Soc Nephrol 17: 3204–3212, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Chan MR, Young HN, Becker YT, Yevzlin AS: Obesity as a predictor of vascular access outcomes: Analysis of the USRDS DMMS Wave II study. Semin Dial 21: 274–279, 2008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.