Abstract
Background and objectives
The role of reversibility of nontraditional risk factors, like inflammation and CKD-mineral bone disorder, in the reduction of cardiovascular risk after renal transplantation is still scarcely defined.
Design, setting, participants, and measurements
The longitudinal relationship between C-reactive protein, CKD-mineral bone disorder biomarkers, and intima media thickness was investigated in a series of 178 patients (age=32±10 years) with stage 5 CKD maintained on chronic dialysis who underwent echo-color Doppler studies of the carotid arteries before and after renal transplantation. Smokers and patients with diabetes were excluded from the study. In all patients, immunosuppression was performed by a standard regimen on the basis of calcineurin inhibitors. Healthy controls were specifically selected to match the age and sex distribution of the patients. Biochemical and intima media thickness assessments were repeated 6 months after transplantation.
Results
Before transplantation, intima media thickness in patients with stage 5 CKD on dialysis (average=0.9±0.2 mm) was higher (P<0.001) than in well matched healthy controls (0.6±0.1 mm) and reduced substantially (−22%; 95% confidence interval, −24% to −20%) after transplantation (P=0.001). GFR (multivariable-adjusted β=0.23; P<0.001), C-reactive protein (β=0.15; P<0.001), and fibroblast growth factor 23 (β=0.28; P<0.001) were the strongest independent correlates of intima media thickness before transplantation. Similarly, longitudinal changes in the same biomarkers were the sole independent correlates of simultaneous changes in intima media thickness (C-reactive protein: β=0.25; fibroblast growth factor 23: β=0.26; P<0.001 for both) after renal transplantation. The evolution of intima media thickness after transplantation was largely independent of classic risk factors, including BP, LDL cholesterol, and insulin resistance, as measured by homeostatic model assessment.
Conclusions
Intima media thickness improves after renal transplantation. Such an improvement associates with parallel changes in serum C-reactive protein and fibroblast growth factor 23. These observations are in keeping with the hypothesis that the decline in cardiovascular risk after transplantation, in part, depends on partial resolution of nontraditional cardiovascular risk factors, like inflammation and CKD-mineral bone disorder.
Keywords: renal transplantation, arteriosclerosis, chronic inflammation
Introduction
The risk of death and cardiovascular complications is about three to four times lower in patients with renal transplants than patients with coeval stage 5 CKD on dialysis awaiting renal transplantation (1). The decline in the risk for such events after transplantation occurs in the face of an increase in the prevalence of classic risk factors (2,3), and it is, therefore, mainly attributed to better control of CKD-related risk factors (4). Systemic inflammation as measured by C-reactive protein (CRP) is a powerful predictor of death (5) and progression of atherosclerosis in patients on dialysis (6). Biomarkers of CKD-mineral bone disorder (CKD-MBD)—including high serum phosphate and parathyroid hormone (PTH), low vitamin D, and high fibroblast growth factor 23 (FGF23)—are among the most indicted risk factors for the high rate of cardiovascular events in patients on dialysis (7,8). Both CRP (9) and CKD-MBD (10) improve after successful renal transplantation, and such an improvement may favorably affect vascular disease in these patients.
Current Kidney Disease Improving Global Outcomes guidelines (11) remark that there is a paucity of studies on the evolution of vascular disease shown by imaging techniques in patients on dialysis after renal transplantation. Carotid intima media thickness (IMT) is an intermediate phenotype of atherosclerosis and one of the strongest predictors of cardiovascular events, including myocardial infarction and stroke (12). Reduction in IMT over time entails a parallel decrease in the risk for cardiovascular events, and this parameter is considered as a valid surrogate end point by the Food and Drug Administration (13) and the European Medicines Agency (14). The validity of IMT as a cardiovascular risk biomarker in the CKD population is supported by studies in patients with predialysis CKD (15) and patients on dialysis (6,16). In the sole longitudinal study focusing on IMT, this parameter regressed in parallel with PTH levels after renal transplantation (17). Until now, there was no sufficiently large study investigating the evolution of IMT after transplantation face to face with ongoing changes in established biomarkers of inflammation and CKD-MBD and no studies providing information on FGF23, which is now held as the main vasculotoxic factor among CKD-MBD biomarkers (18).
Probing the evolution of arterial disease and parallel changes in pertinent risk factors after renal transplantation is of relevance in that such studies may help the interpretation of the substantial cardiovascular risk reduction brought about by restored renal function (1,19).With this background in mind, we studied the longitudinal association of changes in IMT in carotid arteries over time (before and after renal transplantation) with ongoing changes in classic risk factors and CRP and CKD-MBD biomarkers in a large incident series of patients who underwent successful renal transplantation.
Materials and Methods
Patients and Controls
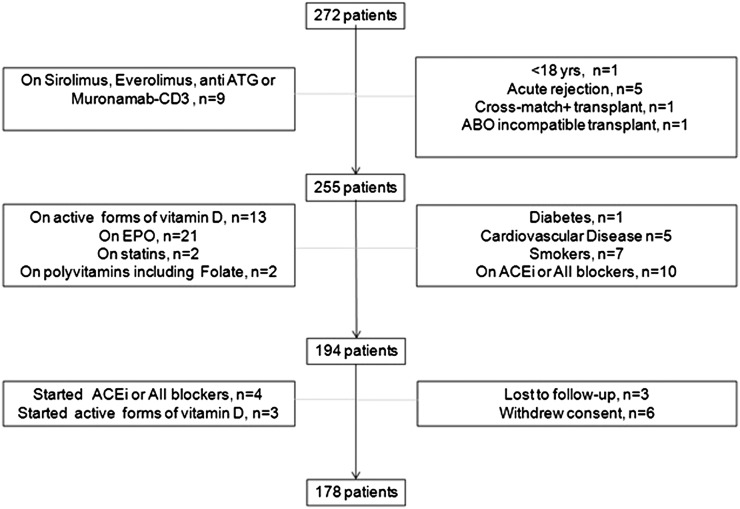
The study protocol was approved by the ethical committee of the Gulhane School of Medicine in Ankara, Turkey. All consecutive adult patients (n=272) who underwent a kidney transplant at our center between January of 2003 and January of 2012 were considered for this analysis. Some of these patients are part of an ongoing prospective study encompassing measurements of vascular function, which has been described in a separate publication (20). Because the scope of our study was that of investigating the reversibility of atherosclerosis (as measured by IMT) after restoration of renal function by renal transplantation and the functional relationship between the longitudinal changes in IMT and CKD-MBD biomarkers, by protocol, we excluded patients with risk factors that may distort the interpretation of carotid artery changes brought about by successful renal transplantation, including diabetes, background cardiovascular disease, smoking, rejection episodes, and use of mammalian target of rapamycin inhibitors, a class of drugs that may per se have a favorable effect on atherosclerosis (21,22). The flowchart of the selection process is shown in Figure 1. We selected all patients with uneventful clinical course (no rejection episodes). We excluded five patients on sirolimus or everolimus, because these drugs per se prevent intimal hyperplasia and have a different effect on vascular function compared with standard cyclosporin-based regimens (21,22) and four patients on a regimen including antithymocyte globulin (Muromonab-CD3), a drug that interferes in a complex manner with several components of the inflammation cascade (23). Five additional patients were excluded, because they had acute rejection episodes, and two patients were excluded because of positive cross-match transplant (n=1) or blood group system–incompatible transplant (n=1). Furthermore, we excluded patients with diabetes mellitus (n=1), patients with history of cardiovascular disease (electrocardiogram-documented angina, myocardial infarction documented by electrocardiogram, and biomarkers of myocardial necrosis, cerebral ischemia, or revascularization procedures; n=5), smokers (n=7), and those taking angiotensin-converting enzyme inhibitors or angiotensin receptor blockers (n=10), active forms of vitamin D (n=13), erythropoietin (n=21), statins (n=2), and supplementary vitamin pills, including folate (n=2). Three patients were lost to follow-up before the second study (i.e., the study after transplantation), six patients withdrew consent, and seven patients were excluded because of starting angiotensin-converting enzyme inhibitors/angiotensin receptor blockers (n=4) or active vitamin D compounds (n=3). As expected, the selected cohort (n=178) had a young mean age (32±9 years); 159 patients in this cohort before transplantation had been treated by hemodialysis, and 19 patients had been treated by peritoneal dialysis. Thirty-three patients were anuric before transplantation. Before transplantation, patients received standard treatment for CKD-MBD at our institution (i.e., phosphate binders: calcium acetate [n=62], calcium carbonate [n=34], or sevelamer [n=21]). Of 178 patients, 124 patients had live donor transplantation with at least one matched haplotype, whereas 54 patients had cadaveric transplantations. Phosphate binders were stopped in all patients after transplantation.
Figure 1.
Flowchart of patients enrolled in the study. ABO, blood group system; ACEi, angiotensin-converting enzyme inhibitor; ATG, antithymocyte globulin; EPO, erythropoietin.
In all patients, immunosuppression was performed by a standard regimen on the basis of calcineurin inhibitors (cyclosporin in 82 patients and tacrolimus in 96 patients) associated with mycophenolate mofetil and prednisolone. Target tacrolimus and cyclosporin levels were set according to standard recommendations. By the end of the 12th week after transplantation, the maintenance dose of prednisolone was reduced to 5 mg/d. Five patients developed post-transplant diabetes during the 6-month follow-up period, and all patients were on prednisolone treatment during the same period (dose range=5–60 mg/d).
As a control group, we enrolled 96 healthy controls recruited by in-hospital advertisement, and the vast majority of these volunteers was hospital personnel. These healthy volunteers were specifically selected to match the age and sex distributions of patients in this study. Routine analyses documented that healthy volunteers had normal renal function (GFR>90 ml/min), no hypertension, diabetes, or disorders of lipid metabolism, and no other relevant disease.
Study Protocol
In living transplant recipients, baseline biochemical and IMT assessments were performed within 4 weeks before transplantation. In cadaveric transplant recipients, the same measurements were performed during the routine clinical evaluation performed at the time of renal transplantation. Both in living and cadaveric transplant recipients, biochemical and IMT assessments were repeated 6 months after transplantation. eGFR was calculated according to the simplified version of the Modification of Diet in Renal Disease equation. Homeostatic model assessment (HOMA), an index of insulin sensitivity, was calculated with the formula HOMA=fasting plasma glucose (milligrams per deciliter)×immunoreactive insulin (microinternational units per milliliter)/405. The methods of measurement of 25 hydroxy vitamin D (25OHVD), PTH, FGF23 (second generation, two-site mAb ELISA; Kainos Laboratories, Tokyo, Japan), high-sensitivity C-reactive protein (hsCRP), and serum insulin were described in detail in a previous publication (24).
Common Carotid B-Mode Doppler Ultrasound Measurement of IMT
Scanning was performed using the recommendations by the American Society of Echocardiography (12) using an instrument generating a wide-band ultrasonic pulse with a middle frequency of 12 MHz (ATL 5000; Advanced Technology Laboratories Inc., Bothell, WA). After an initial overview of vessel orientation, wall thickness, plaques, and surrounding structures, in all participants, a high-resolution B-mode ultrasound of the common carotid arteries with scanning of the longitudinal axis until the bifurcation and the transversal axis was performed. Measurements were made in triplicate on each side by tracing far-wall blood intima and media adventitia interfaces using the leading edge to leading edge method. All patients and controls were blindly examined by one experienced operator (a licensed radiology technologist). IMT was measured, always in plaque-free areas, at 1 cm proximal to the bifurcation on each side, and the average value was taken as an estimate of the IMT. The intraoperator variability for IMT at our laboratory was 4%. The upper limit of the normal range of IMT in our laboratory (established in 93 healthy controls of the study) is 0.71 mm.
Statistical Analyses
Non-normally distributed variables were expressed as medians (interquartile ranges), and normally distributed variables were expressed as means±SDs. A P value <0.05 was considered to be statistically significant. Variables with a non-normal distribution were appropriately log transformed before analysis. Comparisons between two groups were assessed by the paired t and Wilcoxon rank sums tests as appropriate. Multiple regression analysis was applied to identify the independent correlates of IMT. Tested risk factors included all Framingham risk factors except for diabetes and smoking (because smokers and patients with diabetes were excluded from the study), biomarkers of bone mineral disorders (P, Ca, 25OHVD, PTH, and FGF23), CRP, albumin and insulin resistance as measured by the HOMA index, and the type of renal transplantation (living/cadaveric). Multiple regression models were built by including into the models all significant bivariate correlates of the main outcome measure (IMT). The models had sufficient power to test the independent association of this outcome measure with relevant correlates (i.e., at least 10 observations per covariate in the same models) (25). All statistical analyses were performed by using the SPSS 15.0 (SPSS Inc., Chicago, IL) statistical package.
Results
The flowchart of patients enrolled into this study is presented in Figure 1. Living related and cadaveric kidney transplants (Table 1) had similar ages and causes of CKD. Antihypertensive and immune-suppressive agents were also similar along with the dialysis treatment modality before transplantation. Therefore, additional analyses were performed in the combined population of patients with living and cadaveric transplants. The mean age in the control group of healthy subjects (33±10 years old) was very close to that of patients (32±9 years old), and the sex distribution was also similar (control group: 73% men and 27% women; patient group: 71% men and 29% women).
Table 1.
Demographic and clinical characteristics of the study population according to the type of transplant received
| Patient Characteristics | Living (n=124) | Cadaveric (n=54) | P Value |
|---|---|---|---|
| Sex (men/women) | 93/31 | 33/21 | 0.06 |
| Age (yr) | 31±10 | 33±8 | |
| Etiology of chronic renal failure, n (%) | 0.44 | ||
| GN | 29 (23) | 10 (18) | |
| Hypertension | 23 (18) | 6 (11) | |
| ADPKD | 8 (6) | 5 (9) | |
| Chronic pyelonephritis | 5 (4) | — | |
| Reflux nephropathy | 8 (6) | 5 (9) | |
| Amyloidosis | 4 (3) | 3 (5) | |
| Unknown | 47 (38) | 25 (46) | |
| Antihypertensive drugs, n (%) | 0.52 | ||
| α-Blockers | 5 (4) | 1 (2) | |
| Calcium-channel blockers | 19 (15) | 5(9) | |
| β-Blockers | 6 (5) | 1 (2) | |
| αβ-Blockers | 6 (5) | 4 (7) | |
| Immunosuppressant agents, n (%) | <0.001 | ||
| Cyclosporin | 66 (53) | 16 (30) | |
| Tacrolimus | 58 (47) | 38 (70) | |
| Dialysis modality, n (%) | 0.63 | ||
| Hemodialysis | 111 (89) | 47 (87) | |
| Peritoneal dialysis | 13 (11) | 7 (13) |
ADPKD, autosomal dominant polycystic kidney disease.
As shown in Table 2, average eGFR after transplantation was 86±14 ml/min per 1.73 m2, and no patient had a GFR<60 ml/min per 1.73 m2. After transplantation, there was a mild fall in systolic and diastolic BP (−4 and −2 mmHg, respectively), a modest (+1.1 kg/m2) increase in body mass index, and a rise in serum total and LDL cholesterol and triglycerides. HOMA index reduced after transplantation. Likewise, biomarkers of nutrition and inflammation underwent favorable changes as indicated by an 11% increase in serum albumin and a substantial reduction in hsCRP. Among CKD-MBD biomarkers, FGF23 fell dramatically after transplantation (from 6443 to 30 pg/ml). Serum calcium and phosphate reverted to the normal range, and PTH fell to 60 pg/ml. Before transplantation, all patients had either vitamin D insufficiency (i.e., plasma 25OHVD levels between 50 and 72 nmol/L [n=61; 34.3%]) or frank deficiency (i.e., 25OHVD levels≤50 nmol/L [n=117; 65.7%]). After transplantation, 25OHVD increased by about 22%, and 62 patients (38%) attained vitamin D sufficiency.
Table 2.
Clinical and hemodynamic data before and after transplantation
| Parameters | Before Transplantation | After Transplantation | P Value |
|---|---|---|---|
| Systolic BP (mmHg) | 136 (110–195) | 132 (90–155) | <0.001 |
| Diastolic BP (mmHg) | 84 (65–110) | 82 (50–97) | <0.001 |
| Body mass index (kg/m2) | 25.4±2.6 | 26.5±2.3 | <0.001 |
| Total cholesterol (mg/dl) | 188.5±23.1 | 207.5±26.2 | <0.001 |
| Triglycerides (mg/dl) | 132.9±22.3 | 140.2±19.1 | <0.001 |
| LDL cholesterol (mg/dl) | 117.4±17.3 | 119.8±17.3 | 0.18 |
| HDL cholesterol (mg/dl) | 41.1±5.9 | 43.8±6.1 | <0.001 |
| Serum glucose (mg/dl) | 86.7±10.1 | 84.4±10.7 | 0.04 |
| Insulin (IU/L) | 7.1±1.9 | 5.9±1.4 | <0.001 |
| Homeostasis model assessment IR | 1.5±0.5 | 1.2±0.3 | <0.001 |
| eGFR (ml/min per 1.73 m2) | 6.3±4.0 | 85.8±13.7 | <0.001 |
| Serum albumin (g/dl) | 3.7±0.3 | 4.1±0.3 | <0.001 |
| High-sensitivity C-reactive protein (mg/L) | 25 (4–53) | 3.0 (1.0–9.2) | <0.001 |
| Serum calcium (mg/dl) | 8.2±0.6 | 8.8±0.5 | <0.001 |
| Serum phosphate (mg/dl) | 6.5±1.3 | 4.1±0.9 | <0.001 |
| Intact parathyroid hormone (pg/ml) | 234 (89–776) | 60 (21–132) | <0.001 |
| 25 Hydroxy vitamin D (nmol/L) | 44.8±9.9 | 54.4±11.1 | <0.001 |
| Fibroblast growth factor 23 (pg/ml) | 6443 (378–77,812) | 30.4 (10.1–59.5) | <0.001 |
| Logn fibroblast growth factor 23 (pg/ml) | 9.0±0.9 | 3.3±0.3 | <0.001 |
| Intima media thickness (mm) | 0.9±0.2 | 0.7±0.1 | <0.001 |
Data are means±SDs or medians (ranges) as appropriate. IR, insulin resistance; logn, natural logarithm.
IMT: Effect of Renal Transplantation
Before transplantation, IMT in patients on dialysis (0.9±0.2 mm) was higher than in age- and sex-matched healthy controls (0.6±0.1 mm) and exceeded the upper limit of the normal range in 83.1% of patients. IMT decreased by 22% after transplantation to 0.68±0.13 mm (P=0.001).
Cross-Sectional Analyses of the Relationship between Risk Factors and IMT before and after Transplantation
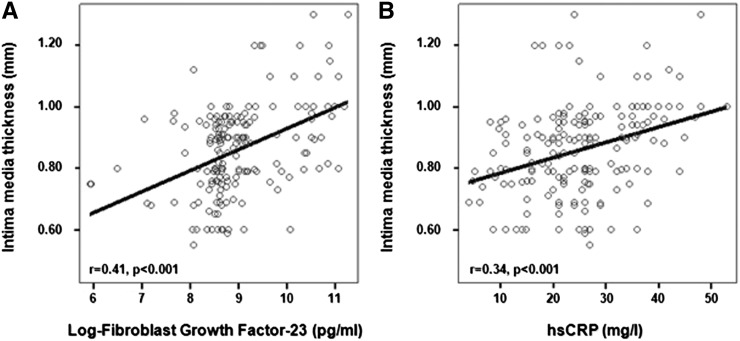
Before transplantation, FGF23, hsCRP, systolic BP, and HOMA index (P<0.001) as well as eGFR and to a weaker extent, 25OHVD and serum phosphate were all related to IMT (Table 3, pretransplantation IMT bivariate analyses). The GFR, FGF23, and CRP were the strongest correlates of IMT before transplantation (Figure 2). Similarly, post-transplantation (Table 3, post-transplantation IMT bivariate analyses) FGF23, eGFR, PTH, and phosphate associated strongly with IMT, whereas diastolic BP, hsCRP, HOMA, 25OHVD, and serum calcium showed weaker correlations with the same parameter. Of note, hsCRP and FGF23 before transplantation were directly related each other (r=0.30), and this relationship was highly significant (P<0.001).
Table 3.
Bivariate and multivariate standardized correlation coefficients (β) and P values between intima media thickness and pertinent variables before and after transplantation
| Parameters | Pretransplantation IMT β (P Value) | Post-Transplantation IMT β (P Value) | ||
|---|---|---|---|---|
| Bivariate | Multiple regression | Bivariate | Multiple regression | |
| Age (yr) | 0.06 (0.43) | 0.06 (0.45) | −0.04 (0.63) | −0.13 (0.10) |
| Sex | −0.08 (0.28) | 0.01 (0.94) | 0.01 (0.96) | 0.05 (0.55) |
| Body mass index (kg/m2) | −0.02 (0.82) | −0.09 (0.22) | 0.09 (0.26) | 0.10 (0.19) |
| LDL cholesterol (mg/dl) | 0.08 (0.32) | 0.10 (0.20) | 0.04 (0.62) | 0.12 (0.13) |
| Systolic BP (mmHg) | 0.27 (<0.001)a | 0.17 (0.01)a | 0.02 (0.79) | 0.01 (0.90) |
| Diastolic BP (mmHg) | 0.07 (0.36) | −0.05 (0.50) | 0.15 (0.04)a | 0.05 (0.49) |
| High-sensitivity C-reactive protein (mg/L) | 0.34 (<0.001)a | 0.15 (0.03)a | 0.16 (0.04)a | 0.10 (0.18) |
| Serum albumin (g/dl) | −0.06 (0.42) | −0.07 (0.33) | 0.07 (0.39) | 0.11 (0.15) |
| Homeostasis model assessment index | 0.26 (<0.001)a | 0.04 (0.59) | 0.15 (0.05)a | 0.01 (0.88) |
| eGFR (ml/min per 1.73 m2) | −0.40 (<0.001)a | −0.23 (<0.001)a | −0.29 (<0.001)a | −0.16 (0.03)a |
| Fibroblast growth factor 23 (pg/ml) | 0.41 (<0.001)a | 0.28 (<0.001)a | 0.36 (<0.001)a | 0.31 (<0.001)a |
| 25 Hydroxy vitamin D (nmol/L) | −0.22 (0.003)a | −0.04 (0.64) | −0.19 (0.01)a | −0.09 (0.25) |
| Serum calcium (mg/dl) | −0.09 (0.24) | −0.07 (0.39) | −0.22 (0.03)a | −0.15 (0.03)a |
| Serum phosphate (mg/dl) | 0.20 (<0.01)a | 0.11 (0.17) | 0.29 (<0.001)a | 0.14 (0.04)a |
| Intact parathyroid hormone (pg/ml) | 0.13 (0.09) | 0.08 (0.29) | 0.31 (<0.001)a | 0.15 (0.03)a |
| Living/cadaveric transplantation | −0.02 (0.81) | 0.02 (0.77) | 0.03 (0.69) | −0.04 (0.56) |
IMT, Intima media thickness.
Significant association.
Figure 2.
Relationship of the intimate media thickness before transplantation to the levels of fibroblast growth factor 23 (A) and high-sensitivity C-reactive protein (hsCRP) (B).
Multiple regression models were built and included variables associated with IMT at bivariate analyses (see above) as well as other established risk factors for IMT, including age, sex, body mass index, LDL cholesterol, and serum albumin. Before transplantation (Table 3, pretransplantation IMT multiple regression analyses), FGF23 (β=0.28; P<0.001), eGFR (β=0.23; P=0.002), systolic BP (β=0.17; P=0.01), and hsCRP (β=0.15; P=0.03) were all independent correlates of IMT. After transplantation (Table 3, post-transplantation IMT multiple regression analyses), FGF23 and to a very slight degree, eGFR, serum calcium and phosphate, and PTH maintained an independent association with IMT. The type of renal transplantation (living/cadaveric) had no independent relationship with IMT both pre- and post-transplantation (Table 3).
Longitudinal Analyses of Changes in Risk Factors after Transplantation and Changes in IMT
In these analyses (Table 4), we tested the association between changes (i.e., the difference between values of each variable after transplantation minus the value of the same variable before transplantation) in individual risk factors with simultaneous changes in IMT brought about by renal transplantation.
Table 4.
Bivariate and multivariate associations between changes (Δ) in intima media thickness after renal transplantation and relevant parameters
| Parameters | ΔIMT β (P Value) | |
|---|---|---|
| Bivariate | Multiple regression | |
| ΔSystolic BP (mmHg) | 0.11 (0.16) | 0.03 (0.74) |
| ΔDiastolic BP (mmHg) | 0.04 (0.64) | −0.06 (0.45) |
| ΔLDL cholesterol (mg/dl) | 0.02 (0.79) | 0.04 (0.61) |
| ΔAlbumin (g/L) | 0.06 (0.44) | 0.01 (0.91) |
| ΔhsCRP (mg/L) | 0.29 (<0.001)a | 0.25 (0.001)a |
| ΔHOMA | 0.23 (<0.001)a | 0.13 (0.10) |
| ΔeGFR (ml/min/1.73 m2) | −0.21 (<0.01)a | −0.16 (0.02)a |
| ΔLog FGF23 (pg/ml) | 0.33 (<0.001)a | 0.26 (<0.001)a |
| Δ25OHVD (nmol/L) | −0.16 (0.04)a | −0.08 (0.28) |
| ΔSerum calcium (mg/dl) | −0.23 (<0.001)a | −0.14 (0.06) |
| ΔSerum phosphate (mg/dl) | −0.27 (0.001)a | 0.15 (0.05)a |
| ΔiPTH (pg/ml) | 0.16 (0.04)a | −0.17 (0.34) |
| Living/cadaveric renal transplantation | 0.02 (0.82) | −0.06 (0.46) |
Data are standardized correlation coefficients (β) and P values. IMT, intima media thickness; hsCRP, high-sensitivity C-reactive protein; HOMA, homeostasis model assessment index; FGF23, fibroblast growth factor 23; 25OHVD, 25 hydroxy vitamin D; iPTH, intact parathyroid hormone.
Statistically significant relationship.
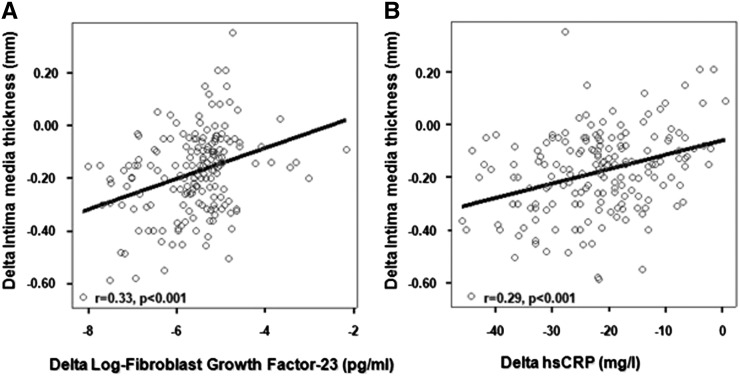
Changes in FGF23 and CRP were the strongest bivariate correlates of IMT (Figure 3, Table 4). In these unadjusted analyses, IMT was also associated with eGFR, serum phosphate and calcium, and to a weak extent, 25OHVD and PTH.
Figure 3.
Relationship of the alteration of the intimate media thickness 2 weeks after the transplantation with the alteration of fibroblast growth factor 23 (A) or high-sensitivity C-reactive protein (hsCRP) levels (B).
On multiple regression analysis, changes in FGF23 (β=0.26; P<0.001) and hsCRP (β=0.25; P=0.001) emerged as the strongest independent correlates of IMT changes. Changes in eGFR and serum phosphate maintained an independent association with changes in IMT, but these relationships were fairly weak (Table 4). Similar to cross-sectional analyses, the type of renal transplantation (living/cadaveric) had no independent relationship with changes in IMT after renal transplantation (Table 4).
Discussion
This study shows that IMT of the carotid arteries, an intermediate phenotype and a valid surrogate biomarker of atherosclerosis denoting intimal fibroplasia and muscle cell hypertrophy in the vascular wall, reduces after renal transplantation. Reduction in IMT after renal transplantation parallels the decline in CRP levels and the marked fall in FGF23 associated with restored renal function. Classic risk factors and other risk factors apparently do not contribute to explain the improvement in IMT after transplantation.
Successful renal transplantation substantially reduces the risk for cardiovascular complications in patients with transplants compared with well matched patients on dialysis on the waiting list (1). In a study by Kasiske et al. (26) in the 1990s, no relationship was observed between Framingham risk factors, including smoking, hypertension, and hyperlipidemia, and the risk of myocardial infarction, cardiac death, and coronary interventions in recipients of renal transplants (26). Among nonclassic risk factors, CRP (9) and biomarkers of CKD-MBD (10) undergo dramatic changes after transplantation, and FGF23 and 1,25(OH)2 vitamin levels approach the normal range in most patients, whereas serum PTH often remains mildly to moderately elevated (27). Numerous low-powered cross-sectional ultrasound studies of carotid arteries have been performed in patients with renal transplants (28–32). The vast majority of these studies considered isolated biomarkers (mainly biomarkers of inflammation) (29,31,32) or just a limited set of purported risk factors. Longitudinal studies have several advantages over cross-sectional studies for exploring causation. Indeed, these studies provide information about individual changes in the variables of interest, exclude between-subjects variation from error, and allow investigation of the relationship between predictor variables with relevant study end points (33).
With 178 patients, our longitudinal study had sufficient power for testing the independent contribution of a large set of potential risk factors to the variance of IMT, including classic (Framingham) risk factors and risk factors related with CKD, such as inflammation, insulin resistance, low albumin, and biomarkers of CKD-MBD. Even brief periods of systemic inflammation by infectious diseases determine a relevant increase in IMT (+17%) within 3 months of the infectious process, and such an increase is attenuated by appropriated treatment with antibiotics (34). Furthermore, this study is the first testing the evolution of the same parameters and FGF23 along with simultaneous IMT measurements before and after renal transplantation. FGF23 is strongly associated with whole-body atherosclerosis burden as measured by nuclear magnetic resonance imaging in a large study in the general population in Sweden (35). It is, therefore, of relevance to investigate whether similar links also exist in patients on dialysis before and after renal transplantation. In this study, we tried to minimize the confounding effects of preexisting cardiovascular damage (myocardial infarction, peripheral vascular disease, or cerebrovascular disease), diabetes, and smoking on the evolution of arterial damage by excluding patients with these risk factors. Although this approach may be difficult to apply in the aging dialysis population with a high cardiovascular burden in most centers in northern Europe and the United States, in Turkey, 65% of transplants are made in patients on dialysis in the age range of 20–44 years old (36) (i.e., an age range substantially lower than in northern Europe and the United States). Likewise, we tried to limit the confounding effect of drug treatment by excluding patients on renin angiotensin blockers, statins, erythropoietin, active forms of vitamin D, and inhibitors of mammalian target of rapamycin, all drugs that per se may influence vascular disease. Our analyses in this selected population show that FGF23 and serum CRP are the strongest independent correlates of IMT in both baseline analyses before transplantation and longitudinal analyses incorporating measurements made before and after transplantation. Our observations suggest that attenuation of inflammation and almost complete correction of high FGF23 levels may play a relevant role in the amelioration of arterial disease after successful renal transplantation. CRP and FGF23 are associated with each other in patients with CKD (37), and these risk factors were directly related in baseline analyses (before transplantation) in this study. However, both of these biomarkers resulted in independent correlates of the evolution of IMT after transplantation, suggesting that amelioration of inflammation and correction of high FGF23 may attenuate carotid atherosclerosis by separate biologic pathways. In line with observational studies in patients with transplants showing no relationship between classic risk factors and all-cause and cardiovascular risk factors (26), in this study, hypertension, other Framingham risk factors, and insulin resistance were not associated with longitudinal changes in IMT after transplantation. Overall, our data are in keeping with the hypothesis that the reduction of CRP levels and abatement of FGF23 levels brought about by restored renal function contribute to reverse arterial damage in patients with transplants.
Our study has limitations. First, although useful to explore causal hypotheses, longitudinal studies do not prove causality. Second, as briefly alluded to before, patients in our cohort were all Turkish and younger than patients with transplants in American and European registries of dialysis and transplantation, which limits the generalizability of our findings. Third, although we tried to limit the effect of drug treatment on data interpretation, calcineurin inhibitors per se have an adverse effect on arterial disease in patients with transplants. Patients in our cohort were all treated with these drugs. Therefore, being evenly administered, it is unlikely that these immunosuppressant agents could have disturbed (to an important extent) the appreciation of the relationships emerged in this study.
In conclusion, IMT improves after renal transplantation, and such an improvement parallels the reduction in CRP levels and the dramatic fall in FGF23 associated with restored renal function. These findings are in keeping with the hypothesis that nonclassic risk factors may play a role in vascular disease in patients with coeval stage 5 CKD on dialysis and that the reduction in cardiovascular risk after transplantation is, at least in part, driven by an amelioration of these risk factors, like CRP and CKD-MBD.
Disclosures
None.
Acknowledgments
The authors thank the Familial Mediterranean Fever Arthritis Vasculitis and Orphan Diseases Research (FAVOR; www.favor.org.tr) web registries at Gulhane Medical Academy, Institute of Health Sciences for statistical analysis and contribution to the preparation of this manuscript.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, Held PJ, Port FK: Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med 341: 1725–1730, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Hunsicker LG: A survival advantage for renal transplantation. N Engl J Med 341: 1762–1763, 1999 [DOI] [PubMed] [Google Scholar]
- 3.van Dijk PC, Jager KJ, de Charro F, Collart F, Cornet R, Dekker FW, Grönhagen-Riska C, Kramar R, Leivestad T, Simpson K, Briggs JD, ERA-EDTA registry : Renal replacement therapy in Europe: The results of a collaborative effort by the ERA-EDTA registry and six national or regional registries. Nephrol Dial Transplant 16: 1120–1129, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Kasiske BL, Chakkera HA, Roel J: Explained and unexplained ischemic heart disease risk after renal transplantation. J Am Soc Nephrol 11: 1735–1743, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Stenvinkel P, Wanner C, Metzger T, Heimbürger O, Mallamaci F, Tripepi G, Malatino L, Zoccali C: Inflammation and outcome in end-stage renal failure: Does female gender constitute a survival advantage? Kidney Int 62: 1791–1798, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Benedetto FA, Tripepi G, Mallamaci F, Zoccali C: Rate of atherosclerotic plaque formation predicts cardiovascular events in ESRD. J Am Soc Nephrol 19: 757–763, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hruska KA, Mathew S, Lund RJ, Memon I, Saab G: The pathogenesis of vascular calcification in the chronic kidney disease mineral bone disorder: The links between bone and the vasculature. Semin Nephrol 29: 156–165, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.London GM, Guérin AP, Verbeke FH, Pannier B, Boutouyrie P, Marchais SJ, Mëtivier F: Mineral metabolism and arterial functions in end-stage renal disease: Potential role of 25-hydroxyvitamin D deficiency. J Am Soc Nephrol 18: 613–620, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Simmons EM, Langone A, Sezer MT, Vella JP, Recupero P, Morrow JD, Ikizler TA, Himmelfarb J: Effect of renal transplantation on biomarkers of inflammation and oxidative stress in end-stage renal disease patients. Transplantation 79: 914–919, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Molnar MZ, Naser MS, Rhee CM, Kalantar-Zadeh K, Bunnapradist S: Bone and mineral disorders after kidney transplantation: Therapeutic strategies. Transplant Rev (Orlando) 28: 56–62, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group : KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl 113: S1–S130, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Stein JH, Korcarz CE, Hurst RT, Lonn E, Kendall CB, Mohler ER, Najjar SS, Rembold CM, Post WS, American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine : Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: A consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. J Am Soc Echocardiogr 21: 93–111, quiz 189–190, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Tardif JC, Heinonen T, Orloff D, Libby P: Vascular biomarkers and surrogates in cardiovascular disease. Circulation 113: 2936–2942, 2006 [DOI] [PubMed] [Google Scholar]
- 14.European Medicines Agency: Pre-Authorisation Evaluation of Medicines for Human Use. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003242.pdf. Accessed January 10, 2010
- 15.Szeto CC, Chow KM, Woo KS, Chook P, Ching-Ha Kwan B, Leung CB, Kam-Tao Li P: Carotid intima media thickness predicts cardiovascular diseases in Chinese predialysis patients with chronic kidney disease. J Am Soc Nephrol 18: 1966–1972, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Benedetto FA, Mallamaci F, Tripepi G, Zoccali C: Prognostic value of ultrasonographic measurement of carotid intima media thickness in dialysis patients. J Am Soc Nephrol 12: 2458–2464, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Suwelack B, Gerhardt U, Witta J, Hillebrandt U, Hohage H: Effect of parathyroid hormone levels on carotid intima-media thickness after renal transplantation. Am J Hypertens 14: 1012–1018, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Wolf M: Update on fibroblast growth factor 23 in chronic kidney disease. Kidney Int 82: 737–747, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meier-Kriesche HU, Schold JD, Srinivas TR, Reed A, Kaplan B: Kidney transplantation halts cardiovascular disease progression in patients with end-stage renal disease. Am J Transplant 4: 1662–1668, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Yilmaz MI, Sonmez A, Saglam M, Yaman H, Kilic S, Turker T, Unal HU, Gok M, Cetinkaya H, Eyileten T, Oguz Y, Caglar K, Vural A, Mallamaci F, Zoccali C: Longitudinal analysis of vascular function and biomarkers of metabolic bone disorders before and after renal transplantation. Am J Nephrol 37: 126–134, 2013 [DOI] [PubMed] [Google Scholar]
- 21.Joannides R, Etienne I, Iacob M, Hurault de Ligny B, Barbier S, Bellien J, Lebranchu Y, Thuillez C, Godin M: Comparative effects of sirolimus and cyclosporin on conduit arteries endothelial function in kidney recipients. Transpl Int 23: 1135–1143, 2010 [DOI] [PubMed] [Google Scholar]
- 22.Seckinger J, Sommerer C, Hinkel UP, Hoffmann O, Zeier M, Schwenger V: Switch of immunosuppression from cyclosporine A to everolimus: Impact on pulse wave velocity in stable de-novo renal allograft recipients. J Hypertens 26: 2213–2219, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Beiras-Fernandez A, Walther S, Thein E, Muenzing S, Hammer C: Influence of polyclonal ATGs on expression of adhesion molecules: An experimental study. Transplant Proc 37: 1944–1946, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Yilmaz MI, Sonmez A, Saglam M, Yaman H, Kilic S, Demirkaya E, Eyileten T, Caglar K, Oguz Y, Vural A, Yenicesu M, Zoccali C: FGF-23 and vascular dysfunction in patients with stage 3 and 4 chronic kidney disease. Kidney Int 78: 679–685, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Kleinbaum DG, Kupper LL, Muller KE,Nizam A: Applied Regression Analysis and Other Multivariable Methods, Boston, Duxbury Press, 1998, pp 389–390 [Google Scholar]
- 26.Kasiske BL, Guijarro C, Massy ZA, Wiederkehr MR, Ma JZ: Cardiovascular disease after renal transplantation. J Am Soc Nephrol 7: 158–165, 1996 [DOI] [PubMed] [Google Scholar]
- 27.Kalantar-Zadeh K, Molnar MZ, Kovesdy CP, Mucsi I, Bunnapradist S: Management of mineral and bone disorder after kidney transplantation. Curr Opin Nephrol Hypertens 21: 389–403, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cañas L, Bayés B, Granada ML, Ibernon M, Porrini E, Benítez R, Díaz JM, Lauzurica R, Moreso F, Torres A, Lampreabe I, Serra A, Romero R: Is adiponectin a marker of preclinical atherosclerosis in kidney transplantation? Clin Transplant 26: 259–266, 2012 [DOI] [PubMed] [Google Scholar]
- 29.Turkmen K, Tonbul HZ, Erdur FM, Toker A, Biyik Z, Ozbiner H, Gaipov A, Gul EE, Kayrak M, Solak Y, Ozbek O, Turk S, Covic A: Soluble TWEAK independently predicts atherosclerosis in renal transplant patients. BMC Nephrol 14: 144, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uchida J, Machida Y, Iwai T, Kuwabara N, Kabei K, Naganuma T, Kumada N, Nakatani T: Glucose intolerance is associated with increased intimal-medial thickness of the carotid artery and increased pulse-wave velocity in renal transplant recipients. Transplant Proc 45: 1535–1539, 2013 [DOI] [PubMed] [Google Scholar]
- 31.Cobanoglu AK, Gungor O, Kircelli F, Altunel E, Asci G, Ozbek SS, Toz H, Ok E: Role of asymmetric dimethylarginine in the progression of carotid atherosclerosis in renal transplant patients. Int Urol Nephrol 45: 1463–1469, 2013 [DOI] [PubMed] [Google Scholar]
- 32.Gungor O, Kismali E, Sisman AR, Kircelli F, Carrero JJ, Tatar E, Asci G, Toz H: The relationships between serum sTWEAK, FGF-23 levels, and carotid atherosclerosis in renal transplant patients. Ren Fail 35: 77–81, 2013 [DOI] [PubMed] [Google Scholar]
- 33.Grootendorst DC, Jager KJ, Zoccali C, Dekker FW: Observational studies are complementary to randomized controlled trials. Nephron Clin Pract 114: c173–c177, 2010 [DOI] [PubMed] [Google Scholar]
- 34.Liuba P, Persson J, Luoma J, Ylä-Herttuala S, Pesonen E: Acute infections in children are accompanied by oxidative modification of LDL and decrease of HDL cholesterol, and are followed by thickening of carotid intima-media. Eur Heart J 24: 515–521, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Mirza MA, Hansen T, Johansson L, Ahlström H, Larsson A, Lind L, Larsson TE: Relationship between circulating FGF23 and total body atherosclerosis in the community. Nephrol Dial Transplant 24: 3125–3131, 2009 [DOI] [PubMed] [Google Scholar]
- 36.Serdengecti K, Suleymanlar G, Altiparmak MR, Seyahi N: Turkish Registry of Dialysis and Transplantation. Available at: http://tsn.org.tr/folders/file/registry%20kitap.pdf. Accessed March 25, 2010
- 37.Munoz Mendoza J, Isakova T, Ricardo AC, Xie H, Navaneethan SD, Anderson AH, Bazzano LA, Xie D, Kretzler M, Nessel L, Hamm LL, Negrea L, Leonard MB, Raj D, Wolf M, Chronic Renal Insufficiency Cohort : Fibroblast growth factor 23 and Inflammation in CKD. Clin J Am Soc Nephrol 7: 1155–1162, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]