Abstract
Background
G-protein β3 subunit (GNB3) gene C825T and endothelial nitric oxide (eNOS) gene G894T polymorphisms both influence arterial structure and function. However, information is scant regarding the interaction of these genes on arterial wall thickness.
Methods
This aspect was examined in 654 white and black subjects, aged 25–43 years (72.9% white, 39.3% male). Arterial wall thickness was assessed in terms of the average intima-media thickness (IMT) of common carotid, internal carotid, and carotid bulb segments by B-mode ultrasonography.
Results
Frequencies of T allele of the GNB3 C825T polymorphism (0.718 vs. 0.304, P < 0.0001) and G allele of the eNOS G894T polymorphism (0.868 vs. 0.661, P < 0.0001) were higher in blacks compared to whites. In a multivariate model including gender, age, mean arterial pressure, body mass index, triglycerides/HDL cholesterol ratio, insulin resistance index, smoking, and/or race, there was no significant genotypic effect on carotid IMT with respect to GNB3 C825T or eNOS G894T polymorphisms among whites, blacks, and total sample. However, the carriers of TT genotype of the GNB3 C825T and T allele of the eNOS G894T had a significantly lower carotid IMT among blacks (P = 0.003) and the total sample (P = 0.006).
Conclusion
These results indicate that the genetic variations of the eNOS gene in combination with the GNB3 gene jointly influence carotid artery wall thickening process in young adults, especially in blacks.
Alterations in arterial wall structure measured in terms of carotid intima-media thickness (IMT) has been increasingly recognized as a strong predictor of subsequent cardiovascular (CV) morbidity and mortality.1,2 Although carotid IMT is adversely associated with CV disease risk factors and their clustering,3–5 much of the variability is unexplained. Recent studies have suggested that variation in carotid IMT may be in part explained by genetic factors.6,7 Heritability of carotid IMT measures has been estimated to be 0.92 and 0.86 for common carotid and internal carotid segments, respectively.6 Information on genetic locus (or loci) contributing to the interindividual variability in carotid IMT is beginning to emerge.8–10
C825T polymorphism in exon 10 involving a thymidine for a cytosine of the G-protein β3 subunit (GNB3) gene has been identified.11 G proteins are expressed in all cells of the human body, and their pivotal role is to translate signals from the cell surface into a cellular response.12 Studies have shown that GNB3 C825T polymorphism adversely relates to CV risk factors, type 2 diabetes and carotid atherosclerosis.13–15 G894T polymorphism of the eNOS gene within exon 7 showed a transversion of G to T at nucleotide position 894 (G894T) resulting in a change of Glu298 to Asp298.16 Studies have found that eNOS gene G894T polymorphism was associated with carotid IMT.17–19 However, there is a paucity of information regarding the interaction effect of GNB3 C825T and eNOS G894T polymorphisms on carotid IMT, independent of blood pressure, in healthy young adults. As part of Bogalusa Heart Study, a community-based study of the early natural history of CV disease,20 this study investigates both the independent and joint effect of these two gene polymorphisms on carotid IMT, after controlling for CV risk factors including blood pressure.
METHODS
Study population
During the 2001–2002 survey of black and white young adults, aged 25–45 years (n = 1,203; 70% white), ultrasonography of carotid artery along with measurements of CV risk factor variables was performed. Of these, 654 participants (72.9% white, 39.3% male; mean age 36.7 years) who had genotype data of GNB3 C825T and eNOS G894T polymorphisms were included in this study. Tulane University Medical Center Institutional Review Board approved the Study. Informed consent was obtained from all participants.
General examination
Standardized techniques and protocols were used by trained field observers. Replicate measurements of height and weight were made twice and the mean values were used to calculate body mass index (BMI = weight in kilograms divided by the square of height in meters) as a measure of overall adiposity. Systolic and diastolic blood pressures were measured using a mercury sphygmomanometer three times by each of two randomly assigned observers on the right arm of participants in a relaxed, sitting position; the average value of the six blood pressure readings was used in the analyses. Mean arterial pressure was calculated as diastolic blood pressure plus one third of pulse pressure. Information on smoking status was obtained using a health habit questionnaires. Those who smoked at least one cigarette per week during the past 1 year were identified as current smokers. Participants were excluded if they were on antihypertensive medication.
Laboratory analysis
Serum total cholesterol and triglycerides were assayed on fasting samples using an enzymatic procedure on the Hitachi 902 Automatic Analyzer (Roche Diagnostics, Indianapolis, IN). Serum lipoprotein cholesterol levels were analyzed using a combination of heparin-calcium precipitation and agar-agarose gel electrophoresis procedures.21 The laboratory is being monitored for precision and accuracy of lipid measurements by the surveillance program of the Center for the Disease Control and Prevention (Atlanta, GA). A commercial radioimmunoassay kit was used for measuring plasma immunoreactive insulin levels (Pharmacia Diagnostics, Piscataway, NJ). Plasma glucose levels were measured by a glucose oxidase method as part of multiple chemistry profile (SMA20, Laboratory Corporation of America, Burlington, NC). Homeostasis model assessment of insulin resistance (HOMA-IR) was calculated based on the following formula: HOMA-IR = fasting insulin (µU/ml) × fasting glucose (mmol/l)/22.5. This model is considered useful to assess insulin resistance in epidemiologic studies.22
Genotyping for the G894T polymorphism
Genotyping of the eNOS G894T polymorphism was carried out using the Taqman assay. The sequences of the forward and reverse PCR primers were 5′-CCCCACAGCTCTGCATTCA-3′ and 5′-CACCCAGTCAATCCCTTTGG-3′, respectively. Allele-specific fluorogenic probes, labeled with different reporter dyes, were designed to hybridize to the target DNA in a sequence-specific manner. In this case, the two allele-specific probes were 6FAM-CCCCAGATGATCCCCCAGAACTC and VIC-CCCCAGATGAGCCCCCAGAAC. After PCR amplification, the increase in fluorescent intensity of the reporter dyes was detected by an end-point read using an ABI 7700 (Applied Biosystems, Foster City, CA). Analysis of the fluorescent signals lead to an automated genotype determination. Genotyping of the GNB3 C825T variant was performed using same method. An 80-bp product was amplified using 0.9 µmol/l each of the forward primer 5′-TCCCACGAGCATCATCTG-3′ and the reverse primer 5′-TCGTCGTAGCCAGCGAATAGT-3′, 0.2 µmol/leach of the sequence-specific probes 5′-6FAM-CATCACGTCCGTGGCCTTCTCC-TAMRA-3′ and 5′-VIC-CATCACGTCTGTGGCCTT CTCCCT-TAMRA-3′, 1 × TaqMan Universal PCR master mix containing AmpliTaq Gold DNA polymerase and 3 ng DNA in a 5.5 µl reaction volume.23 Based on the analysis of 67 pairs of blind duplicates, there was 100% concordance in genotyping.
Carotid ultrasonography
As previously described,4 images of the common carotid, bulb and internal carotid artery were recorded bilaterally by a Toshiba ultrasound instrument (Power Vision Toshiba SSH-380 ultrasound system, Toshiba America Medical System, Carrollton, TX) and a 7.5 MHz linear array transducer according to the protocol developed for the Atherosclerosis Risk in Communities Study.24 Images recorded on S-VHS tapes were read by certified readers at Wake Forest University School of Medicine using a semi-automatic ultrasound image processing program developed by the California Institute of Technology and Jet Propulsion Laboratory.24,25 The mean of the maximum common carotid, bulb and internal carotid IMT readings of three right and three left far walls was used. If bilateral images could not be obtained, one side was used in the calculation. The percentages of only one side vales available were 2.1, 9.3, and 15.2% for common carotid, carotid bulb and internal carotid, respectively. Carotid IMT was defined as the average of the three segmental carotid IMT measurements.
Statistical analyses
All analyses were conducted using SAS software, version 9.1 (SAS institute, Cary, NC). Differences in mean values of risk factor variables and composite carotid IMT between race–sex and genotype subgroups were tested using a general linear model. Hardy–Weinberg equilibrium and racial differences in genotype distributions and allele frequencies were tested using a χ2-test. Due to lower frequencies of C825 and T894 alleles among black individuals that generated an even smaller number of individuals when using the combination of the two genes, the genotypes of CC and CT for GNB3 and TT and GT for eNOS were combined for analyses. Multivariable regression models were applied to examine the genotype effect on carotid IMT, adjusting for covariates. Triglycerides/HDL cholesterol ratio and HOMA-IR levels were log-transformed in order to improve normality for regression analyses.
RESULTS
Mean levels of CV risk variables and composite carotid IMT in the study cohort are presented in Table 1 by race and gender. Blacks vs. whites had significantly higher mean arterial pressure and lower triglycerides/HDL cholesterol ratio; black females vs. white females higher BMI and HOMA-IR. Males vs. females were significantly older and displayed higher mean arterial pressure and triglycerides/HDL cholesterol ratio. Significant race (black > white) and sex (male > female) differences were noted.
Table 1.
Characteristics of study cohort by race and gender
| Variablesa | Male | Female | Comparisonb | |||
|---|---|---|---|---|---|---|
| White (n = 200) | Black (n = 57) | White (n = 277) | Black (n = 120) | Race | Gender | |
| Age (years) | 37.2 ± 4.2 | 37.3 ± 3.9 | 36.6 ± 4.2 | 35.7 ± 4.5 | ns | 0.02 |
| BMI (kg/m2) | 29.3 ± 5.6 | 30.7 ± 9.0 | 27.5 ± 6.4 | 32.3 ± 8.9 | <0.0001c | 0.02d |
| MAP (mm Hg) | 93 ± 8 | 100 ± 13 | 87 ± 9 | 95 ± 13 | <0.0001 | <0.0001 |
| TG /HDL cholesterol ratio | 4.6 ± 4.7 | 3.2 ± 3.9 | 2.7 ± 1.9 | 2.0 ± 1.1 | <0.0001 | <0.0001 |
| HOMA-IR | 3.3 ± 3.3 | 3.5 ± 3.6 | 2.4 ± 2.4 | 3.8 ± 5.7 | <0.0001c | 0.0003d |
| Smoking (%) | 48 | 50 | 44 | 42 | ns | ns |
| Carotid IMT (mm) | 0.83 ± 0.14 | 0.86 ± 0.14 | 0.76 ± 0.11 | 0.81 ± 0.13 | 0.0002 | <0.0001 |
BMI, body mass index; HDL, high-density lipoprotein; HOMA-IR, homeostasis model assessment of insulin resistance; IMT, intima-media thickness; MAP, mean arterial pressure; ns, not significant; TG, triglycerides.
Mean ± s.d. for continuous variables.
P values were adjusted for age.
Females only.
Whites only.
The genotype and allele frequencies of GNB3 C825T and eNOS G894T polymorphisms by race are displayed in Table 2. Both genotype distributions and allele frequencies were significantly different between races, with blacks showing higher frequencies of T allele of GNB3 C825T and lower frequency of the T allele of eNOS G894T than whites. The genotype distributions of both genes were in accordance with Hardy–Weinberg equilibrium expectations in both races. When both polymorphisms were examined jointly, as shown at the bottom of Table 2, the combined genotype distribution was also different between blacks and whites (P < 0.001). More blacks than whites had the combination of GNB3 TT genotype and eNOS GG genotype (42.4% vs. 5.7%), and less blacks than whites were carriers of both C825 and T894 alleles (10.2% vs. 52.2%). The genotypic effect on carotid IMT examined using multivariate regression models is presented by race in Table 3. In the total sample with blacks and whites combined, sex, age, mean arterial pressure and cigarette smoking were significantly associated with carotid IMT. The genotype effect on carotid IMT was not significant in both races and the total sample after adjusting for covariates.
Table 2.
Genotype and allele frequency of GNB3 C825T and eNOS G894T polymorphisms
| White (n = 477) | Black (n = 177) | |
|---|---|---|
| Count (%) | Count (%) | |
| GNB3 gene | ||
| Genotype* | ||
| C825/C825 | 235 (49.3) | 14 (7.9) |
| C825/t825 | 194 (40.7) | 72 (40.7) |
| T825/t825 | 48 (10.1) | 91 (51.4) |
| Allele* | ||
| C | 664 (69.6) | 100 (28.2) |
| T | 290 (30.4) | 254 (71.8) |
| eNOS gene | ||
| Genotype* | ||
| G894/g894 | 207 (43.4) | 143 (80.8) |
| G894/t894 | 217 (45.5) | 32 (18.1) |
| T894/t894 | 53 (11.1) | 2 (1.1) |
| Allele* | ||
| G | 631 (66.1) | 318 (89.8) |
| T | 323 (33.9) | 36 (10.2) |
| GNB3 + eNOS * | ||
| T825/T825 + G894/G894 | 27 (5.7) | 75 (42.4) |
| T825/T825 + T894 carriers | 21 (4.4) | 16 (9.0) |
| C825 carriers + G894/G894 | 180 (37.7) | 68 (38.4) |
| C825 carriers + T894 carriers | 249 (52.2) | 18 (10.2) |
C825 carriers = C825/C825 + C825/T825; T894 carriers = T894/T894 + G894/T894.
Race difference: P < 0.0001.
Table 3.
Multivariate regression of carotid IMT on genotypes and covariates by race
| White | Black | Total | ||||
|---|---|---|---|---|---|---|
| β | P | β | P | β | P | |
| Black race | — | — | — | — | 0.20 | 0.128 |
| Female sex | −0.052 | <0.0001 | −0.03 | 0.151 | −0.05 | <0.0001 |
| Age | 0.007 | <0.0001 | 0.008 | 0.0003 | 0.007 | <0.0001 |
| BMI | 0.0001 | 0.924 | −0.002 | 0.200 | −0.0009 | 0.324 |
| MAP | 0.002 | 0.002 | 0.003 | <0.0001 | 0.003 | <0.0001 |
| TG /HDLC | 0.02 | 0.087 | 0.015 | 0.366 | 0.01 | 0.069 |
| HOMA-IR | 0.02 | 0.140 | −0.003 | 0.857 | 0.01 | 0.099 |
| Smoking | 0.03 | 0.002 | −0.008 | 0.693 | 0.03 | 0.007 |
| GNB3 C825T | 0.0006 | 0.975 | −0.016 | 0.385 | −0.009 | 0.489 |
| eNOS G894T | −0.02 | 0.142 | −0.016 | 0.478 | −0.016 | 0.109 |
Smoking: 1 = smoker, 0 = nonsmoker.
GNB3 C825T: 1 = C825/C825 and C825/T825, 0 = T825/T825.
eNOS G894T: 1 = T894/T894 and G894/T894, 0 = G894/G894.
BMI, body mass index; eNOS, endothelial nitric oxide synthase; GNB3, G-protein β3 subunit; HDLC, high-density lipoprotein cholesterol ratio; HOMA-IR, homeostasis model assessment of insulin resistance; IMT, intima-media thickness; MAP, mean arterial pressure; TG, triglycerides.
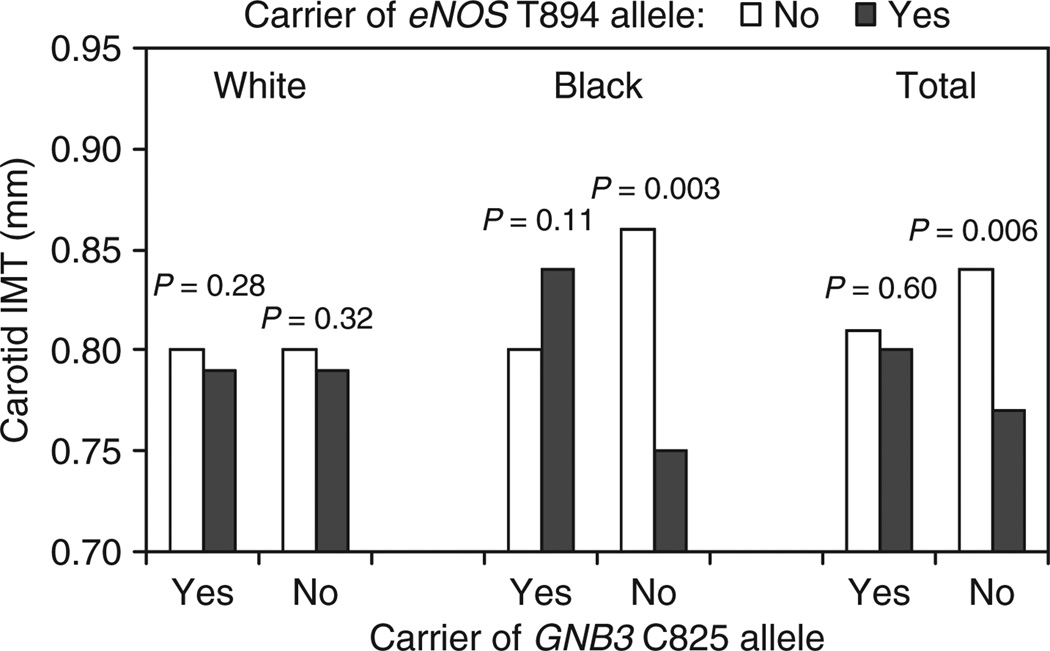
Figure 1 shows the interaction effect of eNOS T894 and GNB3 C825 alleles on carotid IMT by race. Among blacks, carriers of GNB3 T825/T825 genotype who also had at least one eNOS T894 allele (T894/T894 and G894/T894) showed lower carotid IMT than carriers of GNB3 T825/T825 genotype who had eNOS G894/G894 genotype (P = 0.003). Whites showed a similar, but nonsignificant trend (P = 0.32). A significant difference was noted in the total sample (P = 0.006) between subgroups with these combined genotypes after adjusting for above covariates. Carotid IMT did not differ between carriers and noncarriers of the eNOS T894 allele among subjects who had T825/T825 genotype.
Figure 1.
The interaction effect of endothelial nitric oxide (eNOS) T894 and G-protein β3 subunit (GNB3) C825 alleles on carotid intima-media thickness (IMT) by race: The Bogalusa Heart Study. P values were adjusted for gender, age, mean arterial pressure, body mass index, triglycerides/high-density lipoprotein cholesterol ratio, homeostasis model assessment of insulin resistance, smoking and/or race.
DISCUSSION
Studies on combined or the interaction effect of common, functionally relevant genetic variants on carotid IMT are beginning to emerge.14,23,26 The current study demonstrates the interaction effect of GNB3 C825T and eNOS G894T polymorphisms on carotid IMT in young adults. This observation on a community-based cohort is noteworthy in that the participants are free of selection bias of studies involving relatively older adults with clinical CV morbidity.
This study found a markedly higher frequency of T825 allele of GNB3 gene and lower frequency of the T894 allele of eNOS gene in black individuals which are similar to earlier studies in black and white populations.13,14,27,28 With respect to combined genotype distribution, the observed frequency of the combination of GNB3 T825/T825 and eNOS T allele carriers was similar in whites and blacks (4% vs. 9%). However, the observed frequency of combined GNB3 T825/T825 and eNOS G894/G894 was greater in blacks and the frequency of combined GNB3 C825 carriers and eNOS T894 carriers was greater in whites, reflecting the black–white difference on genotype distribution of each variant. No corresponding combined genotypes distribution data are available for comparison. More population-based studies are needed in this regard.
Although the eNOS G894T genotype was not significantly associated with carotid IMT in race groups, the T894 allele was associated with a lower common carotid IMT consistently in blacks and whites, with a P value of 0.109 in the total sample. The beneficial effect of this variant allele on blood pressure and arterial thickness and stiffness was noted earlier in this cohort.19,27,28 Site specific carotid IMT was also noted in previous studies although given the parodoxic findings,29 and as such was partly explained by hemodynamic shear stress-induced NO release that causes a lower carotid IMT in the common carotid IMT.30–32 On the other hand, nitric oxide production is genetically influenced, with 30% of phenotypic variance due to genes.33 Both exogenous and endogenous eNOS inhibitors, which decrease basal nitric oxide synthesis in endothelial cells, lead to an increase in carotid artery IMT and arterial stiffness.34,35 It has been suggested that basal nitric oxide synthesis is not only important for maintaining vascular tone, but for modulating arterial mechanical properties as well.36 In vitro studies have shown that nitric oxide/cyclic guanosine monophosphate (NO/cGMP) system inhibits vascular smooth cell proliferation, suggesting a potential role of nitric oxide in arterial wall thickness.37 But, very little information is available on the influence of G894T genotype on NO production and activity. The G894T genotype has been found in one study to have no appreciable effect on the eNOS enzyme activity,38 which does not preclude the increase in production rate. With respect to GNB3 gene, the TT genotype alone was not associated with carotid IMT in whites, blacks and total sample, although a study previously reported the association of T825 allele of GNB3 gene with carotid atherosclerosis in European white middle-aged individuals.15
Of interest, this study detected a beneficial effect of T allele of eNOS gene on carotid IMT among blacks who had TT genotype of the GNB3 gene. Previously we reported that the eNOS T894 allele of gene was associated with lower common carotid IMT in whites and combined sample of blacks and white.19 This study observed that the GNB3 genotype modulates the association of T allele of eNOS on carotid IMT. The mechanism(s) by which the eNOS T894 allele and GNB3 T825/T825 genotype interact to enhance the beneficial effect on carotid IMT is not clear. The observational study cannot address this issue. It is likely that the polymorphism of GNB3 gene or another linked polymorphism within the GNB3 gene may potentiate the expression of the eNOS variant and related IMT-lowering effect, as in a reverse direction previously demonstrated the epistatis interaction between angiotensin I converting enzyme and angiotensin II type 1 receptor gene on extent of carotid IMT.26
It is less likely that the observed gene–gene interaction effect of GNB3 and eNOS on carotid IMT were purely due to chance, because a similar trend was noted in whites, blacks and total samples, albeit not found to be significant in all cases. Moreover, as mentioned earlier the study cohort was an unselected community- based sample, which made the selection bias less likely. However, as mentioned earlier, the mechanism underlying the interaction effect cannot be addressed by this epidemiological study. Repeated studies are needed to confer the finding from this study and to overcome the weakness of genetic association studies.
In conclusion, the allelic variation (C825T) of GNB3 gene is not associated with arterial thickness in blacks, whites and total sample. But its combination with eNOS gene G894T was associated with lower carotid IMT in blacks and the total sample subjects. These findings from a community based, unselected cohort underscore the importance of gene–gene interactions in the assessment of genetic influences on complex traits such as atherosclerosis.
Acknowledgments
The Bogalusa Heart Study is a joint effort of many individuals whose cooperation is gratefully acknowledged. We are especially grateful to the study participants. This study was supported by grants 546145G1 from Tulane University, 0555168B from American Heart Association, AG -16592 from the National Institute on Aging and HL-38844 from the National Heart, Lung and Blood Institute.
Footnotes
Disclosure: The authors declared no conflict of interest.
References
- 1.Burke GL, Evans GW, Riley WA, Sharrett AR, Howard G, Barnes RW, Rosamond W, Crow RS, Rautaharju PM, Heiss G. Arterial wall thickness is associated with prevalent cardiovascular disease in middle-aged adults. The Atherosclerosis Risk in Communities (ARIC) Study. Stroke. 1995;26:386–391. doi: 10.1161/01.str.26.3.386. [DOI] [PubMed] [Google Scholar]
- 2.Hodis HN, Mack WJ, LaBree L, Selzer RH, Liu CR, Liu CH, Azen SP. The role of carotid arterial intima-media thickness in predicting clinical coronary events. Ann Intern Med. 1998;128:262–269. doi: 10.7326/0003-4819-128-4-199802150-00002. [DOI] [PubMed] [Google Scholar]
- 3.Heiss G, Sharrett AR, Barnes R, Chambless LE, Szklo M, Alzola C. Carotid atherosclerosis measured by B-mode ultrasound in populations: associations with cardiovascular risk factors in the ARIC study. Am J Epidemiol. 1991;134:250–256. doi: 10.1093/oxfordjournals.aje.a116078. [DOI] [PubMed] [Google Scholar]
- 4.Urbina EM, Srinivasan SR, Tang R, Bond MG, Kieltyka L, Berenson GS Bogalusa Heart Study. Impact of multiple coronary risk factors on the intima-media thickness of different segments of carotid artery in healthy young adults (The Bogalusa Heart Study) Am J Cardiol. 2002;90:953–958. doi: 10.1016/s0002-9149(02)02660-7. [DOI] [PubMed] [Google Scholar]
- 5.Bhuiyan AR, Srinivasan SR, Chen W, Paul TK, Berenson GS. Correlates of vascular structure and function measures in asymptomatic young adults: the Bogalusa Heart Study. Atherosclerosis. 2006;189:1–7. doi: 10.1016/j.atherosclerosis.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 6.Duggirala R, Gonzalez Villalpando C, O’Leary DH, Stern MP, Blangero L. Genetic basis of variation in carotid artery wall thickness. Stroke. 1996;27:833–837. doi: 10.1161/01.str.27.5.833. [DOI] [PubMed] [Google Scholar]
- 7.Zannad F, Visvikis S, Gueguen R, Sass C, Chapet O, Herbeth B, Siest G. Genetics strongly determines the wall thickness of the left and right carotid arteries. Hum Genet. 1998;103:183–188. doi: 10.1007/s004390050804. [DOI] [PubMed] [Google Scholar]
- 8.Arnett DK, Borecki IB, Ludwig EH, Pankow JS, Myers R, Evans G, Folsom AR, Heiss G, Higgins M. Angiotensinogen and angiotensin converting enzyme genotypes and carotid atherosclerosis: the atherosclerosis risk in communities and the NHLBI family heart studies. Atherosclerosis. 1998;138:111–116. doi: 10.1016/s0021-9150(98)00009-4. [DOI] [PubMed] [Google Scholar]
- 9.Bhuiyan AR, Chen W, Srinivasan SR, Rice J, Mock N, Tang R, Bond MG, Boerwinkle E, Berenson GS. G-6A Polymorphism of angiotensinogen gene modulates the effect of blood pressure on carotid intima-media thickness: The Bogalusa Heart Study. Am J Hypertens. 2007;20:1073–1078. doi: 10.1016/j.amjhyper.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 10.Bozec E, Fassot C, Tropeano AI, Boutouyrie P, Jeunemaitre X, Lacolley P, Dabire H, Laurent S. Angiotensinogen gene M235T polymorphism and reduction in wall thickness in response to antihypertensive treatment. Clin Sci. 2003;105:637–644. doi: 10.1042/CS20030156. [DOI] [PubMed] [Google Scholar]
- 11.Siffert W, Rosskopf D, Siffert G, Busch S, Moritz A, Erbel R, Sharma AM, Ritz E, Wichmann HE, Jakobs KH, Horsthemke B. Association of a human G-protein β3 subunit variant with hypertension. Nat Genet. 1998;18:45–48. doi: 10.1038/ng0198-45. [DOI] [PubMed] [Google Scholar]
- 12.Bourne HR. How receptors talk to trimeric G proteins. Curr Opin Cell Biol. 1997;9:134–142. doi: 10.1016/s0955-0674(97)80054-3. [DOI] [PubMed] [Google Scholar]
- 13.Siffert W, Forster P, Jockel KH, Mvere DA, Brinkmann B, Naber C, Crookes R, Du P Heyns A, Epplen JT, Fridey J, Freedman BI, Muller N, Stolke D, Sharma AM, Al Moutaery K, Grosse-Wilde H, Buerbaum B, Ehrlich T, Ahmad HR, Horsthemke B, Du Toit ED, Tiilikainen A, Ge J, Wang Y, Rosskopf D, et al. Worldwide ethnic distribution of the G protein β3 subunit 825T allele and its association with obesity in Caucasian, Chinese, and Black African individuals. J Am Soc Nephrol. 1999;10:1921–1930. doi: 10.1681/ASN.V1091921. [DOI] [PubMed] [Google Scholar]
- 14.Rosskopf D, Frey U, Eckhardt S, Schmidt S, Ritz E, Hofmann S, Jaksch M, Muller N, Husing J, Siffert W, Jocke KH. Interaction of the G protein β3 subunit T825 allele and the IRS-1 Arg972 variant in type 2 diabetes. Eur J Med Res. 2000;5:484–490. [PubMed] [Google Scholar]
- 15.Wascher TC, Paulweber B, Malaimare L, Stadlmayr A, Iglseder B, Schmoelzer I, Renner W. Associations of a human G protein β3 subunit dimorphism with insulin resistance and carotid atherosclerosis. Stroke. 2003;34:605–609. doi: 10.1161/01.STR.0000058159.63950.EA. [DOI] [PubMed] [Google Scholar]
- 16.Marsden PA, Heng HH, Scherer SW, Stewart RJ, Hall AV, Shi XM, Tsui LC, Schappert KT. Structure and chromosomal localization of the human constitutive endothelial nitric oxide synthase gene. J Biol Chem. 1993;268:17478–17488. [PubMed] [Google Scholar]
- 17.Lembo G, De Luca N, Battagli C, Iovino G, Aretini A, Musicco M, Frati G, Pompeo F, Vecchione C, Trimarco B. A common variant of endothelial nitric oxide synthase (Glu298Asp) is an independent risk factor for carotid atherosclerosis. Stroke. 2001;32:735–740. doi: 10.1161/01.str.32.3.735. [DOI] [PubMed] [Google Scholar]
- 18.Karvonen J, Kauma H, Kervinen K, Rantala M, Ikaheimo M, Paivansalo M, Savolainen MJ, Kesaniemi YA. Endothelial nitric oxide synthase gene Glu298Asp polymorphism and blood pressure, left ventricular mass and carotid artery atherosclerosis in a population-based cohort. J Intern Med. 2002;251:102–110. doi: 10.1046/j.1365-2796.2002.00933.x. [DOI] [PubMed] [Google Scholar]
- 19.Bhuiyan AR, Chen W, Srinivasan SR, Rice J, Mock N, Tang R, Bond MG, Boerwinkle E, Berenson GS. Influence of nitric oxide synthase gene polymorphism (G894T) on carotid intima-media thickness in adults: The Bogalusa Heart Study. J Am Soc Hypertens. 2007;1:362–368. doi: 10.1016/j.jash.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 20.The Bogalusa Heart Study 20th anniversary symposium. Am J Med Sci. 1995;310(Suppl 1):S1–S138. doi: 10.1097/00000441-199512000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Srinivasan SR, Berenson GS. Serum lipoproteins in children and methods for study. In: Lewis LA, editor. CRC Handbook of Electrophoresis. Volume III. Lipoprotein Methodology and Human Studies. Boca Raton, FL: CRC Press; 1983. pp. 185–204. [Google Scholar]
- 22.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 23.Grove ML, Morrison A, Folsom AR, Boerwinkle E, Hoelscher DM, Bray MS. Gene-environment interaction and the GNB3 gene in the Atherosclerosis Risk in Communities Study. Int J Obes (Lond) 2007;31:919–926. doi: 10.1038/sj.ijo.0803545. [DOI] [PubMed] [Google Scholar]
- 24.Bond MG, Barnes RW, Riley WA, Wilmoth SK, Chambless LE, Howard G, Owens B. ARIC Study Group High resolution B-mode ultrasound scanning methods in the Atherosclerosis Risk in Communities Study (ARIC) J Neuroimaging. 1991;1:68–73. [PubMed] [Google Scholar]
- 25.Tang R, Hennig M, Thomasson B, Scherz R, Ravinetto R, Catalini R, Rubba P, Zanchetti A, Bond MG. Baseline reproducibility of B-mode ultrasonic measurement of carotid artery intima-media thickness: the European Lacidipine Study on Atherosclerosis (ELSA) J Hypertens. 2000;18:197–201. doi: 10.1097/00004872-200018020-00010. [DOI] [PubMed] [Google Scholar]
- 26.Ye S, Dhillon S, Seear R, Dunleavey L, Day LB, Bannister W, Day IN, Simpson I. Epistatic interaction between variations in the angiotensin I converting enzyme and angiotensin II type 1 receptor genes in relation to extent of coronary atherosclerosis. Heart. 2003;89:1195–1199. doi: 10.1136/heart.89.10.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen W, Srinivasan SR, Li S, Boerwinkle E, Berenson GS. Gender specific influence of NO synthase gene on blood pressure since childhood. The Bogalusa Heart Study. Hypertension. 2004;44:668–673. doi: 10.1161/01.HYP.0000145474.23750.2b. [DOI] [PubMed] [Google Scholar]
- 28.Chen W, Srinivasan SR, Elkasabany A, Ellsworth DL, Boerwinkle E, Berenson GS. Combined effects of endothelial nitric oxide synthase gene polymorphism (G894T) and insulin resistance status on blood pressure and familial risk of hypertension in young adults: the Bogalusa Heart Study. Am J Hypertens. 2001;14:1046–1052. doi: 10.1016/s0895-7061(01)02192-6. [DOI] [PubMed] [Google Scholar]
- 29.Wolff B, Braun C, Schluter C, Grabe HJ, Popowski K, Volzke H, Ludemann J, John U, Cascorbi I. Endothelial nitric oxide synthase Glu(298)→Asp polymorphism, carotid atherosclerosis and intima-media thickness in a general population sample. Clin Sci (Lond) 2005;109:475–481. doi: 10.1042/CS20050090. [DOI] [PubMed] [Google Scholar]
- 30.Malek AM, Alper SL, Izumo S. Hemodynamic shear stress and its role in atherosclerosis. JAMA. 1999;282:2035–2042. doi: 10.1001/jama.282.21.2035. [DOI] [PubMed] [Google Scholar]
- 31.Corson MA, James NL, Latta SE, Nerem RM, Berk BC, Harrison DG. Phosphorylation of endothelial nitric oxide synthase in response to fluid shear stress. Circ Res. 1996;79:984–991. doi: 10.1161/01.res.79.5.984. [DOI] [PubMed] [Google Scholar]
- 32.Noris M, Morigi M, Donadelli R, Aiello S, Foppolo M, Todeschini M, Orisio S, Remuzzi G, Remuzzi A. Nitric oxide synthesis by cultured endothelial cells is modulated by flow conditions. Circ Res. 1995;76:536–543. doi: 10.1161/01.res.76.4.536. [DOI] [PubMed] [Google Scholar]
- 33.Wang XL, Mahaney MC, Sim AS, Wang J, Wang J, Blangero J, Almasy L, Badenhop RB, Wilcken DE. Genetic contribution of the endothelial constitutive nitric oxide synthase gene to plasma nitric oxide levels. Arterioscler Thromb Vasc Biol. 1997;17:3147–3153. doi: 10.1161/01.atv.17.11.3147. [DOI] [PubMed] [Google Scholar]
- 34.Wilkinson IB, MacCallum H, Cockcroft JR, Webb DJ. Inhibition of basal nitric oxide synthesis increases aortic augmentation index and pulse wave velocity in vivo. Br J Clin Pharmacol. 2002;53:189–192. doi: 10.1046/j.1365-2125.2002.1528adoc.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miyazaki H, Matsuoka H, Cooke JP, Usui M, Ueda S, Okuda S, Imaizumi T. Endogenous nitric oxide synthase inhibitor: a novel marker of atherosclerosis. Circulation. 1999;99:1141–1146. doi: 10.1161/01.cir.99.9.1141. [DOI] [PubMed] [Google Scholar]
- 36.Garg UC, Hassid A. Nitric oxide-generating vasodilators and 8-bromo-cyclic guanosine monophosphate inhibit mitogenesis and proliferation of cultured rat vascular smooth muscle cells. J Clin Invest. 1989;83:1774–1777. doi: 10.1172/JCI114081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jeremy JY, Rowe D, Emsley AM, Newby AC. Nitric oxide and the proliferation of vascular smooth muscle cells. Cardiovasc Res. 1999;43:580–594. doi: 10.1016/s0008-6363(99)00171-6. [DOI] [PubMed] [Google Scholar]
- 38.McDonald DM, Alp NJ, Channon KM. Functional comparison of the endothelial nitric Oxide synthase Glu298Asp polymorphic variants in human endothelial cells. Pharmacogenetics. 2004;14:831–839. doi: 10.1097/00008571-200412000-00006. [DOI] [PubMed] [Google Scholar]