Two sets of ABA-activated protein kinases and their interacting partners are required for plant growth under high external Mg2+ concentrations in Arabidopsis.
Abstract
Protein phosphorylation events play key roles in maintaining cellular ion homeostasis in higher plants, and the regulatory roles of these events in Na+ and K+ transport have been studied extensively. However, the regulatory mechanisms governing Mg2+ transport and homeostasis in higher plants remain poorly understood, despite the vital roles of Mg2+ in cellular function. A member of subclass III sucrose nonfermenting-1-related protein kinase2 (SnRK2), SRK2D/SnRK2.2, functions as a key positive regulator of abscisic acid (ABA)-mediated signaling in response to water deficit stresses in Arabidopsis (Arabidopsis thaliana). Here, we used immunoprecipitation coupled with liquid chromatography-tandem mass spectrometry analyses to identify Calcineurin B-like-interacting protein kinase26 (CIPK26) as a novel protein that physically interacts with SRK2D. In addition to CIPK26, three additional CIPKs (CIPK3, CIPK9, and CIPK23) can physically interact with SRK2D in planta. The srk2d/e/i triple mutant lacking all three members of subclass III SnRK2 and the cipk26/3/9/23 quadruple mutant lacking CIPK26, CIPK3, CIPK9, and CIPK23 showed reduced shoot growth under high external Mg2+ concentrations. Similarly, several ABA biosynthesis-deficient mutants, including aba2-1, were susceptible to high external Mg2+ concentrations. Taken together, our findings provided genetic evidence that SRK2D/E/I and CIPK26/3/9/23 are required for plant growth under high external Mg2+ concentrations in Arabidopsis. Furthermore, we showed that ABA, a key molecule in water deficit stress signaling, also serves as a signaling molecule in plant growth under high external Mg2+ concentrations. These results suggested that SRK2D/E/I- and CIPK26/3/9/23-mediated phosphorylation signaling pathways maintain cellular Mg2+ homeostasis.
As sessile organisms, plants have evolved multiple adaptive mechanisms to control growth and development under continuously changing environmental conditions. Plant hormones coordinate cellular and physiological responses to adjust growth and development. Abscisic acid (ABA), a key phytohormone involved in multiple biological processes, including plant development and responses to water deficit stresses caused by drought and high salinity, functions in a dose-dependent manner. At basal levels, endogenous ABA regulates the density of stomata during epidermal development (Tanaka et al., 2013). However, high levels of ABA, which are synthesized in response to water deficit stresses, induce the expression of stress-responsive genes and stomatal closure (Iuchi et al., 2001; Urano et al., 2009).
The Suc nonfermenting-1-related protein kinase2s (SnRK2s) form a unique family of plant-specific protein kinases involved in cellular signaling in response to water deficit stresses. In particular, subclass III SnRK2s play a pivotal role in coping with drought stress by regulating ABA-controlled biochemical and physiological responses in Arabidopsis (Arabidopsis thaliana; Fujii and Zhu, 2009; Fujita et al., 2009). The srk2d/e/i triple mutant, in which all three subclass III SnRK2s (SRK2D/SnRK2.2, SRK2E/SnRK2.6/OPEN STOMATA1 [OST1], and SRK2I/SnRK2.3) are mutated, displays near-perfect ABA insensitivity and exhibits greatly reduced tolerance to drought stress (Fujii and Zhu, 2009; Fujita et al., 2009). Under water deficit conditions, subclass III SnRK2s are activated in an ABA-dependent manner (Boudsocq et al., 2004; Yoshida et al., 2006). In recent years, substantial progress has been made in understanding how subclass III SnRK2s are activated in response to water deficit stresses. Cellular dehydration caused by water deficit stresses leads to increased levels of endogenous ABA; then, ABA-bound pyrabactin resistance1 (PYR1)/pyrabactin resistance1-like (PYL)/regulatory components of abscisic acid receptor (RCAR) proteins potentiate the formation of group A protein phosphatase2C (PP2C)-PYR/PYL/RCAR-ABA ternary complexes. These complexes inhibit PP2C activity, thereby enabling activation of subclass III SnRK2s (Ma et al., 2009; Park et al., 2009; Umezawa et al., 2009). The activated subclass III SnRK2s then positively regulate downstream transcription factors, such as abscisic acid-responsive elements binding proteins (AREBs)/abscisic acid-responsive elements binding factors (ABFs), by phosphorylating them, which results in the activation of ABA-responsive gene expression or other ABA-related responses (Kobayashi et al., 2005; Furihata et al., 2006; Fujii et al., 2009; Fujita et al., 2009; Yoshida et al., 2010). Other than their key roles in regulating gene expression, at least one of the subclass III SnRK2s, namely SRK2E/OST1, plays a key role in stomatal closure in response to water deficit stresses (Mustilli et al., 2002). SRK2E/OST1 activates the slow anion channel-associated1 (SLAC1) through its phosphorylation (Geiger et al., 2009; Lee et al., 2009). The activated SLAC1 mediates anion release, leading to depolarization of the guard cell plasma membrane. In turn, the depolarization-activated guard cell outward rectifying K+ channel mediates the release of K+ (Hosy et al., 2003). These changes lead to a decrease in turgor pressure in guard cells, resulting in stomatal closure. Despite the key roles of SRK2E/OST1 in regulating ion flux in guard cells, little is known about potential roles of subclass III SnRK2s (including SRK2D and SRK2I) in regulating ion flux or susceptibility to inorganic ions in tissues other than guard cells.
In this research, to explore a novel signaling pathway mediated by subclass III SnRK2s, we sought to identify proteins that physically interact with subclass III SnRK2s in planta. We focused mainly on SRK2D/SnRK2.2 because of its important role in ABA signaling during the vegetative growth stage (Fujii et al., 2007; Fujita et al., 2009). Using a combined immunoprecipitation (IP) and liquid chromatography (LC)-mass spectrometry (MS) approach, we identified Calcineurin B-like-interacting protein kinase26 (CIPK26) as a novel protein that physically interacts with SRK2D. We showed that, in addition to CIPK26, three other closely related protein kinases (CIPK3, CIPK9, and CIPK23) could also physically interact with SRK2D in planta. We generated multiple loss-of-function mutants, including a cipk26/3/9/23 quadruple mutant, and found that this quadruple mutant is hypersusceptible to high external Mg2+ concentrations. Our results showed that the srk2d/e/i triple mutant and some ABA-deficient mutants also show reduced shoot growth under high external Mg2+ concentrations. Based on our results, we discuss the novel roles of subclass III SnRK2s and CIPK26/3/9/23 in plant growth under high external Mg2+ concentrations and their potential roles in maintaining Mg2+ homeostasis in Arabidopsis.
RESULTS
Identification of SRK2D-Interacting Proteins by Coimmunoprecipitation Coupled with LC-MS/MS Analyses
To gain additional understanding of the subclass III SnRK2-mediated signaling pathway, we used IP coupled with LC-tandem mass spectrometry (MS/MS) to identify novel subclass III SnRK2-interacting proteins in Arabidopsis. Recently, studies integrating genetics with phosphoproteomics have identified undescribed downstream targets of protein kinases of interest, including targets of subclass III SnRK2s (Umezawa et al., 2013; Wang et al., 2013). However, the methods used in those studies cannot be used to identify associated proteins that are not phosphorylated by the protein kinases of interest (for example, proteins that function as upstream regulators of the protein kinases). Conversely, a recently developed method combining IP with LC-MS/MS has been used to identify proteins that interact with a protein of interest, including adaptor proteins and upstream regulators (Nishimura et al., 2010; Pauwels et al., 2010). Thus, the method combining IP with LC-MS/MS is complementary to the combined genetics and phosphoproteomics approach and can be used to further elucidate signaling pathways mediated by protein kinases. We focused mainly on SRK2D/SnRK2.2 because of its broad expression patterns in vegetative tissues and its important role in ABA responses (Fujii et al., 2007; Fujita et al., 2009), and we sought to identify SRK2D-interacting proteins in planta. We have generated transgenic Arabidopsis plants constitutively expressing the synthetic GFP (sGFP)-tagged SRK2D protein (SRK2D-sGFP) or sGFP alone under the control of the cauliflower mosaic virus (CaMV) 35S promoter in the wild-type background (Fujita et al., 2009). Fluorescence microscopy analyses showed that these two transgenic lines expressed SRK2D-sGFP and sGFP proteins, respectively, as previously reported (data not shown; Fujita et al., 2009). The growth of these transgenic plants was similar to that of wild-type plants on germination medium (GM) agar plates (Supplemental Fig. S1, A and B). We confirmed that the expressed SRK2D-sGFP proteins were activated in response to ABA treatment or hyperosmotic stress induced by mannitol treatment by in-gel kinase assay (Supplemental Fig. S1C). Constitutive expression of SRK2D-sGFP alleviated the impaired drought tolerance observed in the srk2d/e/i mutant (Supplemental Fig. S1D). These results indicate that the expressed SRK2D-sGFP proteins are functional in planta.
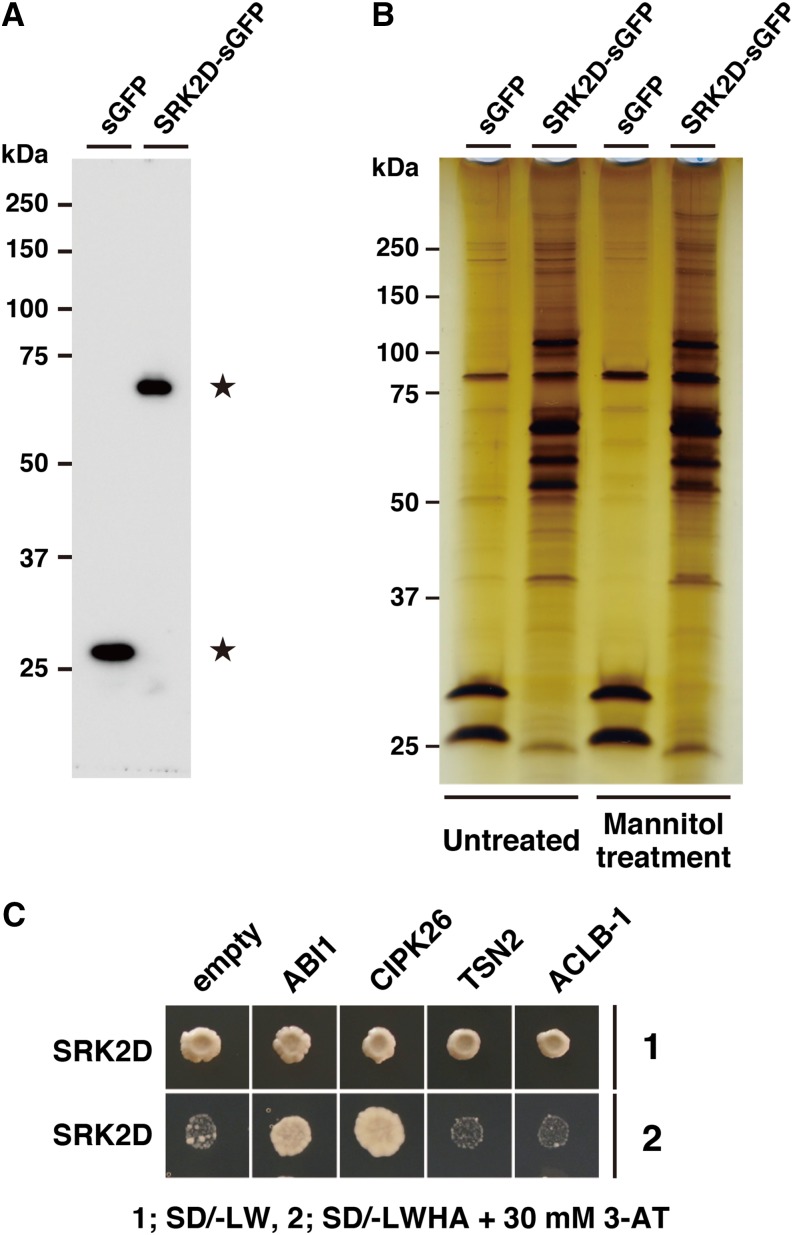
Next, we used the coimmunoprecipitation (co-IP) method to isolate SRK2D-sGFP protein complexes in planta by using an anti-GFP antibody. Detergent-solubilized fractions from the sGFP- or SRK2D-sGFP-expressing lines grown on GM plates for 3 weeks were subjected to co-IP. The immunoprecipitates were separated by SDS-PAGE followed by either the immunoblot analysis with the anti-GFP antibody or silver staining. A single band on the immunoblot confirmed the presence of intact sGFP or SRK2D-sGFP in each immunoprecipitate (Fig. 1A). Visualization by silver staining showed that the SRK2D-sGFP samples contained numerous bands that were absent from the sGFP samples (Fig. 1B), suggesting that SRK2D-interacting proteins may be included in these bands. Subsequently, the regions of the gels corresponding to these bands in each lane (including lanes containing sGFP and SRK2D-sGFP samples) were excised and subjected to in-gel trypsin digestion (Supplemental Fig. S1, E–G). The products of in-gel trypsin digestion were analyzed with an LTQ-Orbitrap LC-MS/MS instrument. MS and MS/MS spectra were assigned to specific peptide sequences by the MASCOT search engine.
Figure 1.
Identification of SRK2D-interacting proteins by co-IP coupled with LC-MS/MS analyses. A, Western-blot analysis of sGFP and SRK2D-sGFP after co-IP. SRK2D-sGFP and associated proteins were purified from transgenic Arabidopsis plants expressing SRK2D-sGFP by co-IP using anti-GFP antibody. In control experiments, transgenic Arabidopsis plants expressing only sGFP were subjected to co-IP. Immunoprecipitates from the sGFP-expressing line and the SRK2D-sGFP-expressing line (line 1; Supplemental Fig. S1, A and B) were subjected to immunoblot analysis with the anti-GFP antibody. Stars indicate predicted sGFP and SRK2D-sGFP bands. B, Immunoprecipitates from plants of the sGFP-expressing line or the SRK2D-sGFP-expressing line (line 1) treated with or without 0.8 m mannitol for 1 h were analyzed and visualized by silver staining. C, Validation of physical interactions between SRK2D and candidate interactors by yeast two-hybrid assay. Representative growth status of yeast cells is shown on synthetic dextrose medium agar plates without Leu, Trp, His, and adenine (SD/−LWHA) with 30 mm 3-amino-1,2,4-triazole (3-AT) from duplicate independent trials. Photographs were taken 7 d after inoculation. ACLB-1, ATP-citrate lyase B-1; TSN2, TUDOR-SN protein2.
Our three independent LC-MS/MS analyses (of two independent untreated samples and an independent mannitol-treated sample) allowed identification of numerous candidate proteins as interactors of SRK2D-sGFP. We further screened the proteins to narrow down the candidate SRK2D-interacting proteins using the following criteria: (1) the protein should contain more than two unique peptides (with confidence > 95%), (2) the peptides should be specifically detected in the SRK2D-sGFP samples but not the sGFP samples (in at least two of three independent analyses), and (3) the protein should be predicted to localize in the cytoplasm, nucleus, or plasma membrane by the Subcellular Localization Database for Arabidopsis Proteins, version 3 program (Tanz et al., 2013) based on the fact that SRK2D-sGFP localizes in both the cytoplasm and the nucleus (Fujita et al., 2009; Supplemental Fig. S2B). In total, 25 proteins met these criteria (Supplemental Table S1). Importantly, ABSCISIC ACID INSENSITIVE1 (ABI1), which is known to be a negative regulator of subclass III SnRK2s (Fujii et al., 2009; Umezawa et al., 2009), met all of these criteria. This indicated that the SRK2D-ABI1 protein complex remained at least partially intact during the purification step in co-IP and implied that this method would be useful to identify known or previously undescribed interactors of SRK2D. Next, we tested whether SRK2D was able to physically interact with the candidates using a yeast (Saccharomyces cerevisiae) two-hybrid assay. As far as we tested, in addition to ABI1, CIPK26 could physically interact with SRK2D in yeast, but TUDOR-SN protein2 and ATP-citrate lyase B-1 could not (Fig. 1C). These results suggest that the plant-specific protein kinase CIPK26 is a potent and novel interactor of SRK2D.
Physical Interactions between CIPK26 and SnRK2s
We evaluated the tissue-specific expression profiles of CIPK26 by generating transgenic Arabidopsis plants carrying the CIPK26 promoter fused to the GUS gene. GUS activity was widely observed in both aerial parts and roots of the CIPK26pro:GUS plant (Supplemental Fig. S2A), suggesting that the CIPK26 gene is broadly expressed in vegetative tissues. The subcellular localization of CIPK26 was then analyzed in Nicotiana benthamiana leaves transiently coexpressing both sGFP-tagged CIPK26 (sGFP-CIPK26) and mCherry-tagged SRK2D (SRK2D-mCherry). Confocal microscopic analyses revealed that sGFP-CIPK26 accumulated mainly in the cytoplasm, whereas SRK2D-mCherry accumulated in both the cytoplasm and the nucleus (Supplemental Fig. S2B). Also, GFP fluorescence was observed mainly in the cytoplasm in the transgenic Arabidopsis plants expressing sGFP-CIPK26 under the control of its own promoter (Supplemental Fig. S2C). These observations suggest that CIPK26 is mainly localized in the cytoplasm, whereas SRK2D is localized in both the cytoplasm and the nucleus.
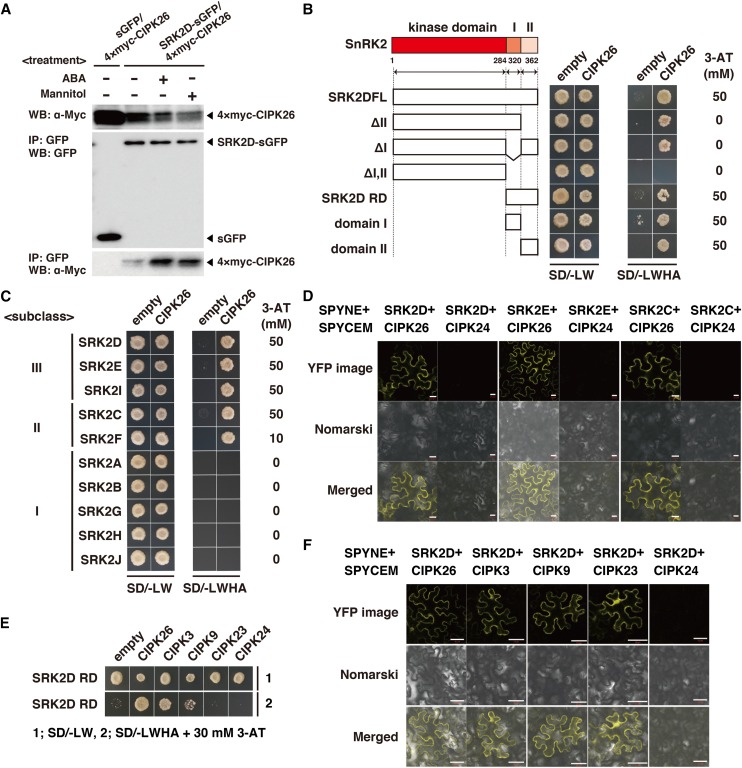
To further investigate the physical interaction between SRK2D and CIPK26, we conducted a co-IP assay using untreated, ABA-treated, or mannitol-treated plants expressing both 4×myc epitope-tagged CIPK26 (4×myc-CIPK26) and sGFP or SRK2D-sGFP under the control of the CaMV 35S promoter. We observed that the 4×myc-CIPK26 proteins were coimmunoprecipitated with the SRK2D-sGFP proteins but not the sGFP proteins in extracts from untreated plants (Fig. 2A). The 4×myc-CIPK26 proteins were also coimmunoprecipitated with SRK2D-sGFP proteins with an apparent enhancement upon ABA or mannitol treatment (Fig. 2A). This result suggests that CIPK26 can physically interact with SRK2D in both the presence and absence of stress conditions. To narrow down the regions of SRK2D responsible for the physical interaction with CIPK26, we generated SRK2D derivatives and tested the physical interaction between each of the SRK2D derivatives and CIPK26 using a yeast two-hybrid assay. The yeast two-hybrid assay showed that the regulatory domain (RD) of SRK2D (SRK2D RD) is necessary and sufficient for the physical interaction with CIPK26 (Fig. 2B). In more detail, both domains I and II in the RD are sufficient for the physical interaction with CIPK26 (Fig. 2B). The Arabidopsis SnRK2 family consists of 10 members, which are classified into subclass I, II, or III (Boudsocq et al., 2004). Considering that subclass III SnRK2s (SRK2D, SRK2E/OST1, and SRK2I) have distinct but overlapping functions in regulating ABA-mediated physiological responses (Fujii and Zhu, 2009; Fujita et al., 2009; Nakashima et al., 2009), we wondered whether CIPK26 could physically interact with SnRK2s other than SRK2D. The yeast two-hybrid assay showed that, in addition to SRK2D, CIPK26 could physically interact with members of subclass II SnRK2s (SRK2C/SnRK2.8 and SRK2F/SnRK2.7) and subclass III SnRK2s (SRK2E and SRK2I) but not members of subclass I SnRK2s (SRK2A/SnRK2.4, SRK2B/SnRK2.10, SRK2G/SnRK2.1, SRK2H/SnRK2.5, and SRK2J/SnRK2.9) in yeast (Fig. 2C). The bimolecular fluorescence complementation (BiFC) analyses showed that, in addition to SRK2D, SRK2E and SRK2C could physically interact with CIPK26 but not CIPK24/Salt Overly Sensitive2 (SOS2), an outer group of CIPK, in N. benthamiana leaves (Fig. 2D; Supplemental Fig. S3B). BiFC visualization showed that the physical interactions occurred mainly in the cytoplasm. These results indicate that CIPK26 can physically interact with subclasses II and III SnRK2s, mainly in the cytoplasm.
Figure 2.
Physical interactions between SnRK2s and CIPKs. A, Co-IP of CIPK26 with SRK2D in Arabidopsis plants. Transgenic lines harboring both pGH-35Spro:4×myc-CIPK26 and pGK-35Spro:SRK2D-sGFP or pGK-35Spro:sGFP were subjected to co-IP using anti-GFP antibody. Immunoprecipitates were analyzed by immunoblotting with anti-GFP or anti-myc antibody. Similar results were obtained in independent experiments; representative data are shown. B, Physical interactions between CIPK26 and SRK2D derivatives analyzed by yeast two-hybrid assay. Representative growth status of yeast cells is shown on synthetic dextrose medium agar plates without Leu, Trp, His, and adenine (SD/−LWHA) with or without 3-amino-1,2,4-triazole (3-AT) from duplicate independent trials. Photographs were taken 7 d after inoculation. C, Yeast two-hybrid assay to validate interaction between CIPK26 and members of SnRK2s. Photographs were taken 7 d after inoculation. D, BiFC analyses of physical interactions between SRK2D, SRK2E, or SRK2C and CIPK26 or CIPK24 in N. benthamiana leaves. Transiently transformed N. benthamiana epidermal cells harboring indicated plasmid combinations were analyzed by confocal microscopy. Yellow fluorescent protein (YFP) fluorescence and Nomarski images are shown. Bars = 20 µm. E, Physical interactions between the RD of SRK2D (SRK2D RD) and CIPK26, CIPK3, CIPK9, CIPK23, or CIPK24 analyzed by yeast two-hybrid assay. Representative growth status of yeast cells is shown on SD/−LWHA agar medium with 30 mm 3-AT from duplicate independent trials. F, BiFC visualization of interaction between SRK2D and CIPK26, CIPK3, CIPK9, and CIPK23 but not CIPK24 in N. benthamiana leaves. The experimental procedure was as described in D. Bars = 50 µm. WB, Western blot.
CIPK26/3/9/23 Can Physically Interact with SRK2D
CIPK26 belongs to the CIPK family (Weinl and Kudla, 2009). Four Arabidopsis CIPKs (CIPK26, CIPK3, CIPK9, and CIPK23 [CIPK26/3/9/23]) were grouped in the same clade in a phylogenetic tree of CIPKs from land plants (Supplemental Fig. S3), implying that CIPK26/3/9/23 has some overlapping functions in planta. To investigate whether, in addition to CIPK26, CIPK3, CIPK9, and CIPK23 can physically interact with SRK2D, we performed yeast two-hybrid assays and BiFC assays. The yeast two-hybrid assays showed that SRK2D RD, which is necessary and sufficient for the interaction with CIPK26 (Fig. 2B), could interact with CIPK3 and CIPK9 but not CIPK23 and CIPK24 (Fig. 2E), suggesting that CIPK3 and CIPK9 can physically interact with SRK2D in yeast. Also, BiFC assays in N. benthamiana leaves showed that, in addition to CIPK26, CIPK3, CIPK9, and CIPK23 could physically interact with SRK2D, whereas there was no evidence of an interaction between SRK2D and CIPK24 in the BiFC assay (Fig. 2F). Taken together, these data suggest that, in addition to CIPK26, CIPK3, CIPK9, and CIPK23 are potent and novel interactors of SRK2D in planta.
CIPK26 Can Phosphorylate SRK2D in Vitro
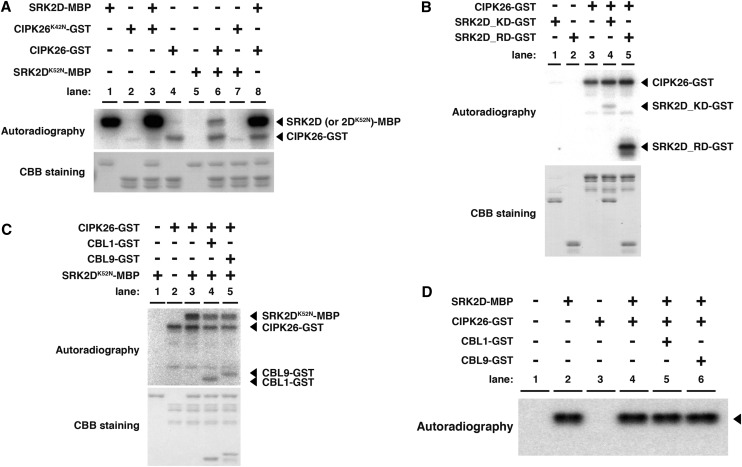
To explore the functional relevance of the physical interaction between SRK2D and CIPK26 at the molecular level, we next investigated whether recombinant SRK2D proteins can phosphorylate CIPK26 proteins or conversely, whether recombinant CIPK26 proteins can phosphorylate SRK2D proteins in vitro. The recombinant maltose-binding protein (MBP) -tagged SRK2D (SRK2D-MBP) proteins and glutathione S-transferase (GST)-tagged CIPK26 (CIPK26-GST) proteins showed autophosphorylation activity and protein kinase activity toward myelin basic protein (Supplemental Fig. S4A, lanes 1, 3, 6, and 8). This result indicates that both SRK2D-MBP and CIPK26-GST are functional protein kinases. To clarify whether SRK2D and CIPK26 phosphorylate each other, we generated kinase-inactive forms of SRK2D and CIPK26, because their autophosphorylation activities make it difficult to assess the possibility of transphosphorylation events between these protein kinases. Lys-42 of CIPK26 and Lys-52 of SRK2D correspond to a highly conserved residue that is required for activity in most protein kinases (Hanks et al., 1988). The Lys-42 to Asn mutation of CIPK26 and the Lys-52 to Asn mutation of SRK2D abolished their autophosphorylation activities and their kinase activities toward myelin basic protein (Supplemental Fig. S4A, lanes 2, 4, 7, and 9). This result confirmed that the mutated GST-tagged CIPK26 (CIPK26K42N-GST) proteins and the mutated MBP-tagged SRK2D (SRK2DK52N-MBP) proteins were dysfunctional protein kinases. We conducted in vitro phosphorylation reactions using CIPK26K42N-GST or SRK2DK52N-MBP as substrate. The SRK2D-MBP proteins could not phosphorylate the CIPK26K42N-GST proteins (Fig. 3A, lane 3), whereas CIPK26-GST proteins phosphorylated SRK2DK52N-MBP proteins (Fig. 3A, lane 6). These results suggest that CIPK26 cannot be a phosphorylation substrate for SRK2D but rather, that SRK2D is a potential substrate for CIPK26 in vitro.
Figure 3.
CIPK26 can phosphorylate SRK2D in vitro. A, Phosphorylation of SRK2DK52N-MBP by CIPK26-GST in vitro. In vitro phosphorylation assays were conducted with 200 ng of SRK2D (or SRK2DK52N)-MBP and 800 ng of CIPK26 (or CIPK26K42N)-GST. Proteins were separated on 10% (w/v) SDS-polyacrylamide gel after incubation in protein kinase assay buffer containing [γ-32P]ATP. Each lane represents an independent reaction, in which the indicated combinations of recombinant proteins were tested. Radioactively labeled proteins were visualized by autoradiography. Protein abundance was visualized by Coomassie Brilliant Blue staining. Similar results were obtained in independent experiments; representative data are shown. B, Phosphorylation of the RD of SRK2D by CIPK26-GST in vitro. In vitro phosphorylation assays were conducted with 200 ng of SRK2D KD-GST or SRK2D RD-GST and 800 ng of CIPK26-GST. C, Effects of coincubation of CBL1-GST or CBL9-GST with CIPK26-GST on the phosphorylation level of SRK2DK52N-MBP. In vitro phosphorylation assays were conducted with 200 ng of SRK2DK52N-MBP, 800 ng of CIPK26-GST, and 200 ng of CBL1-GST or CBL9-GST. D, Effects of coincubation of CIPK26-GST and CBL1-GST or CBL9-GST on the SRK2D-MBP activity. The kinase activity of SRK2D-MBP toward myelin basic protein was analyzed by in vitro preincubation of SRK2D-MBP with CIPK26-GST and CBL1/CBL9-GST followed by an in-gel kinase assay. In vitro preincubation was performed as described in C, except for the absence of [γ-32P]ATP and the addition of 0.1 mm Na3VO4 in the protein kinase assay buffer. After preincubation, samples were subjected to an in-gel kinase assay. The phosphorylation reaction was performed in the protein kinase assay buffer containing [γ-32P]ATP as described in “Materials and Methods.” Myelin basic protein was embedded in the gel as the substrate. Arrowheads indicate the bands corresponding to the kinase activity of SRK2D-MBP toward myelin basic protein. CBB, Coomassie Brilliant Blue.
To narrow down the region of SRK2D subjected to phosphorylation by CIPK26, we tested whether the kinase domain (KD; SRK2D KD) and RD (SRK2D RD) of SRK2D could be phosphorylated by CIPK26 in vitro. CIPK26-GST could efficiently phosphorylate the SRK2D RD, whereas only a very weak phosphorylation signal from the SRK2D KD was detected when coincubated with CIPK26-GST (Fig. 3B). This result indicated that the SRK2D RD could be phosphorylated by CIPK26. Based on a previous report that CIPK26 can activate the activity of the NADPH oxidase RESPIRATORY BURST OXIDASE PROTEIN F (RBOHF) only when together with the calcineurin B-like (CBL) calcium sensors CBL1 or CBL9 (Drerup et al., 2013), it is possible that these CBLs affect the phosphorylation of SRK2D by CIPK26. We tested the effects of coincubation of CBL1-GST or CBL9-GST with CIPK26-GST on the phosphorylation level of SRK2DK52N-MBP. The addition of CBL1-GST or CBL9-GST did not enhance but rather, slightly reduced the phosphorylation level of SRK2DK52N-MBP by CIPK26-GST (Fig. 3C). These results suggest that, at least under in vitro conditions, CIPK26 is capable of recognizing SRK2D as a substrate and phosphorylating it independent of CBL1/CBL9. Competitive binding of CBL1/CBL9-GST to CIPK26-GST with SRK2DK52N-MBP might explain the slightly reduced phosphorylation of SRK2DK52N-MBP.
We then tested whether coincubation of CIPK26 and CBL1/CBL9 with SRK2D could enhance SRK2D activity in vitro. No obvious synergistic effect on the phosphorylation level of myelin basic protein was observed when CIPK26-GST and CBL1/CBL9 were coincubated with SRK2D-MBP in vitro (Supplemental Fig. S4B, lanes 6–8). To dissect the phosphorylation of myelin basic protein by SRK2D-MBP from that by CIPK26-GST, we further tested the SRK2D activity using an in-gel kinase assay (Fig. 3D). After coincubation of SRK2D-MBP with CIPK26-GST and CBL1/CBL9-GST in the presence of ATP but not [γ-32P]ATP, the kinase activity of SRK2D-MBP toward myelin basic protein was tested by an in-gel kinase assay in the presence of [γ-32P]ATP. Despite preincubation of SRK2D-MBP with CIPK26-GST and CBL1/CBL9-GST, the phosphorylation level of myelin basic protein by SRK2D-MBP remained unchanged (Fig. 3D). We could not detect the kinase activity of CIPK26-GST in our experimental conditions, probably because of misfolding of CIPK26-GST proteins during the renaturation step after SDS-PAGE (Fig. 3D). Taken together, these results suggest that CIPK26 can phosphorylate SRK2D in vitro; however, despite the presence of CBL1/CBL9, CIPK26 is unlikely to substantially enhance the kinase activity of SRK2D in vitro. Although we did not observe that CIPK26 activated SRK2D in vitro, it is still possible that CIPK26 is involved in modulating the activity of SRK2D in vivo.
cipk26/3/9 Triple and cipk26/3/9/23 Quadruple Mutants Show Severely Impaired Growth Phenotypes
To gain insight into the functional relationship between CIPK26/3/9/23 and subclass III SnRK2s in planta, we first examined the effect of disruptions in these CIPKs on the growth of Arabidopsis plants. We obtained transfer DNA (T-DNA) insertion lines of CIPK26, CIPK3, CIPK9, and CIPK23 in the Columbia-0 (Col-0) accession from the Arabidopsis Biological Resource Center (ABRC) at The Ohio State University. We isolated homozygous mutants for each line (Supplemental Fig. S5A) and confirmed that the expression of the relevant gene was completely interrupted by the T-DNA insertion in each homozygous mutant by reverse transcription (RT)-PCR (Supplemental Fig. S5B). Considering that CIPK26/3/9/23 formed a monophyletic group (Supplemental Fig. S3B) and could physically interact with SRK2D in planta (Fig. 2F), we considered that CIPK26, CIPK3, CIPK9, and CIPK23 could be functionally redundant to some extent. Hence, we generated and analyzed multiple mutants of cipk26, cipk3, cipk9, and cipk23 (Fig. 4, A–F; Supplemental Fig. S5, B–H). We confirmed that the expression of CIPK26/3/9 was disrupted in the corresponding multiple mutants (Supplemental Fig. S5B). However, in contrast to the complete disruption of CIPK23 expression in the cipk23 single mutant, we detected weak but significant expression of CIPK23 in the cipk23/9 double mutant, the cipk26/3/23, cipk26/9/23, and cipk3/9/23 triple mutants, and the cipk26/3/9/23 quadruple mutant (Supplemental Fig. S5B), despite the homozygous T-DNA insertions in CIPK23. A similar phenomenon has been reported in other multiple loss-of-function mutants (Tokunaga et al., 2012). Quantitative RT-PCR analyses showed that the expression level of CIPK23 in each mutant was reduced to 5% to 21% of that in the wild type (Supplemental Fig. S5C), indicating a significant reduction in CIPK23 expression in these mutants. Compared with the wild type, all single and double mutants showed similar growth phenotypes with respect to the growth of rosette leaves on GM agar plates (Supplemental Fig. S5, D and E) and the growth of rosette leaves and inflorescence height when grown in soil in pots (Fig. 4, A–E; Supplemental Fig. S5, F and G). By contrast, when grown in soil, the cipk26/3/9 triple mutant and the cipk26/3/9/23 quadruple mutant displayed severely impaired growth phenotypes represented by small rosettes and necrotic symptoms on the leaf tips at the vegetative growth stage (Fig. 4, A–C), reduced inflorescence height and necrotic symptoms on the shoot apex at the reproductive growth stage (Fig. 4, D–F), and reduced seed yield (Supplemental Fig. S5H), whereas they grew normally on GM agar plates (Supplemental Fig. S5, D and E). The cipk26/9/23 triple mutant showed a moderately impaired growth phenotype when grown in soil, whereas the other triple mutants (cipk3/9/23 and cipk26/3/23) grew normally both on GM agar plates and in soil (Fig. 4, A–E; Supplemental Fig. S5, D and E).
Figure 4.
Growth retardation of the cipk26/3/9 triple mutant and the cipk26/3/9/23 quadruple mutant is rescued under low external Mg2+ concentrations. A, Growth phenotypes of plants grown on GM agar plates for 2 weeks and then in soil for an additional 10 d. Bars = 1 cm. B, Maximum rosette radius of each plant grown as described in A. Experiment was performed two times; a representative result is shown. Bars indicate sd (n = 6). Asterisks indicate statistically significant difference compared with the wild type. **, P < 0.01, one-way ANOVA followed by a post hoc Dunnett’s multiple comparison test. C, Representative images of rosette leaves of the wild type, the cipk26/3/9 triple mutant, and the cipk26/3/9/23 quadruple mutant. D, Growth phenotypes of plants grown on GM agar plates for 2 weeks and then in soil for another 14 d. Bars = 1 cm. E, Inflorescence height of each plant grown as described in D. Bars indicate sd (n = 6). Experiment was performed two times; a representative result is shown. Asterisks indicate statistically significant difference compared with the wild type as described in B. F, Representative images of shoot apexes of the wild type, the cipk26/3/9 triple mutant, and the cipk26/3/9/23 quadruple mutant. G, Representative images of plants of the wild type, the cipk26/3/9 triple mutant, and the cipk26/3/9/23 quadruple mutant grown in a hydroponic culture system for 24 d. Left, Plants grown hydroponically in modified low-calcium solution (“Materials and Methods”) supplemented with indicated concentrations of CaCl2. Right, Plants grown hydroponically in low-magnesium solution (“Materials and Methods”) supplemented with indicated concentrations of MgCl2. Bars = 1 cm. H, Fresh weight of aerial parts of each plant grown as described in G. Columns marked with different lowercase letters represent significantly different means (P < 0.01) according to two-way ANOVA followed by a post hoc Tukey-Kramer multiple comparison test. Experiment was performed two times; a representative result is shown. Bars indicate sd (n = 6). WT, Wild type.
Subclass III SnRK2s play a pivotal role in ABA signaling (Fujii and Zhu, 2009; Fujita et al., 2009; Nakashima et al., 2009), and it has been reported that the srk2d/e/i triple mutant showed a near-perfect ABA-insensitive phenotype during the germination and vegetative growth stages (Fujita et al., 2009; Nakashima et al., 2009). Thus, it is possible that CIPK26/3/9/23 participates in the ABA signaling pathway. Accordingly, we tested the ABA sensitivity of seedlings of the cipk26/3/9 triple and cipk26/3/9/23 quadruple mutants. Unlike that of the srk2d/e/i mutant, the ABA sensitivity of the cipk26/3/9 triple and cipk26/3/9/23 quadruple mutants was similar to that of the wild type (Supplemental Fig. S6, A and B). This result suggested that CIPK26/3/9/23 is unlikely to play a key role in ABA signaling during the vegetative growth stage. Consistent with this observation, the activation patterns of subclass III SnRK2s in response to ABA or mannitol treatment in the cipk26/3/9/23 quadruple mutants were comparable with those in the wild-type plants (Supplemental Fig. S6C).
cipk26/3/9 Triple and cipk26/3/9/23 Quadruple Mutants Are Hypersusceptible to High External Mg2+ Concentrations
To gain additional insight into the physiological functions of CIPK26, CIPK3, CIPK9, and CIPK23 in planta, we next focused our attention on the impaired growth phenotypes of the cipk26/3/9 triple mutant and the cipk26/3/9/23 quadruple mutant (Fig. 4, A–F). Hitherto, similar phenotypes (necrotic symptoms on the leaf tips and shoot apex) have been reported for a cation exchanger1 (cax1)/cax3 double mutant, in which CAX1 and CAX3, which encode tonoplast-localized Ca2+/H+ antiporters, are disrupted (Cheng et al., 2005). The cax1/cax3 double mutant is impaired in vacuolar H+/Ca2+ antiport and H+-ATPase activity and hypersensitive to high external Ca2+ concentrations but tolerant to high external Mg2+ concentrations (Cheng et al., 2005). Considering the apparently similar phenotypes of the cipk26/3/9 triple mutant, the cipk26/3/9/23 quadruple mutant, and the cax1/cax3 double mutant, it is possible that the impaired growth phenotypes in these cipk mutants resulted from the external Ca2+-Mg2+ conditions.
Accordingly, we used a hydroponic culture system to evaluate the growth of seedlings of the wild type, the cipk26/3/9 triple mutant, and the cipk26/3/9/23 quadruple mutant in media containing various concentrations of Ca2+ and Mg2+ (Fig. 4, G and H). Under normal growth conditions (media supplemented with 2 mm CaCl2 or 2 mm MgCl2; i.e. 2 mm Ca2+ or Mg2+), the cipk26/3/9 triple mutant and the cipk26/3/9/23 quadruple mutant showed growth retardation similar to that observed when they were grown in soil. Contrary to our expectations, the growth retardation of the cipk26/3/9 triple mutant and cipk26/3/9/23 quadruple mutant became more severe at a higher external Mg2+ concentration (4 mm MgCl2; Fig. 4, G and H, right) and lower external Ca2+ concentrations (0.1 or 0.2 mm CaCl2; Fig. 4, G and H, left), whereas the growth retardation was rescued under low external Mg2+ concentrations (0.1 or 0.2 mm MgCl2; Fig. 4, G and H, right). These results indicate that the cipk26/3/9 triple mutant and the cipk26/3/9/23 quadruple mutant are hypersusceptible to external Ca2+ and Mg2+ concentrations. Furthermore, considering that the growth retardation of these cipk mutants was not rescued under a high external Ca2+ concentration (4 mm CaCl2; Fig. 4, G and H, left), these results suggest that the growth retardation in the cipk26/3/9 triple mutant and the cipk26/3/9/23 quadruple mutant is not simply because of Ca2+ deficiency but rather, because of Mg2+ toxicity. Consistent with these observations, the impaired growth phenotype (reduced inflorescence height) observed in the cipk26/3/9 triple mutant and the cipk26/3/9/23 quadruple mutant grown in soil was partially rescued by decreasing the concentration of MgCl2 in the liquid medium from 2 to 0.1 mm (Supplemental Fig. S7).
To investigate whether ion homeostasis was affected in the cipk26/3/9 triple and the cipk26/3/9/23 quadruple mutants grown in the hydroponic culture system, we measured contents of magnesium, calcium, potassium, and sodium in aerial parts of these mutants by inductively coupled plasma (ICP)-MS. ICP-MS analyses revealed that the growth retardation in the cipk26/3/9 triple and the cipk26/3/9/23 quadruple mutants was accompanied by a significant reduction in either calcium or magnesium content and an increase in sodium content compared with those of the wild type (Supplemental Fig. S8). The potassium content in these mutants was comparable with that of the wild type, with a few exceptions (Supplemental Fig. S8). Under a low external Mg2+ concentration (0.1 mm MgCl2), in which the growth retardation of the cipk26/3/9 triple and the cipk26/3/9/23 quadruple mutants was rescued, the contents of calcium, magnesium, and sodium were comparable with those of the wild type (Supplemental Fig. S8). These results suggest that the growth retardation of the cipk26/3/9 triple and the cipk26/3/9/23 quadruple mutants can be at least partly attributed to a disruption in Mg2+ and Ca2+ homeostasis. The impaired growth phenotypes (small rosettes and reduced inflorescence height) of the cipk26/3/9/23 quadruple mutant grown in soil were rescued by expression of CIPK26 under the control of its own promoter (Supplemental Fig. S9, A–E). In addition, the reduced shoot growth of the cipk26/3/9/23 quadruple mutant under relatively higher external Mg2+ concentrations (2 or 4 mm MgCl2) in hydroponic culture was also rescued by expressing CIPK26 under the control of its own promoter (Supplemental Fig. S9, F and G).
To assess whether overexpression of CIPK26 affects susceptibility of shoot growth to high external Mg2+ concentrations or low external Ca2+ concentrations, we generated transgenic Arabidopsis plants overexpressing CIPK26 under the control of the CaMV 35S promoter. We evaluated the growth of the transgenic plants under high external Mg2+ concentrations or low external Ca2+ concentrations. The expression of CIPK26 in two independent overexpressors was confirmed to be higher than that in wild-type or vector-control plants by quantitative RT-PCR (Supplemental Fig. S10A). Then, we tested the plants’ susceptibility to high external Mg2+ concentrations on agar plates. The CIPK26-overexpressing plants were significantly more tolerant than vector-control plants to a high external Mg2+ concentration (25 mm MgCl2) on agar plates (Supplemental Fig. S10, B and C). These CIPK26-overexpressing plants also grew better under a low external Ca2+ concentration (0.1 mm CaCl2) than did vector-control plants in the hydroponic culture system (Supplemental Fig. S10, D and E). Taken together, these results support the view that CIPK26 plays an important role in plant growth under both high external Mg2+ and low external Ca2+ conditions in a dose-dependent manner.
Hypersusceptibility of srk2d/e/i Triple and srk2d/e/i/cipk26/3/9/23 Septuple Mutants to a High External Mg2+ Concentration
Considering that CIPK26 was identified as a novel interactor of subclass III SnRK2s (Fig. 2, A–D), we considered whether subclass III SnRK2s are also required for plant growth under high external Mg2+ concentrations. We investigated whether SRK2D can still physically interact with CIPK26 under a high external Mg2+ concentration by co-IP. We observed that the 4×myc-CIPK26 proteins were coimmunoprecipitated with the SRK2D-sGFP proteins in extracts from plants treated with or without 20 mm MgCl2 (Supplemental Fig. S11). This result indicates that SRK2D forms a protein complex with CIPK26 under high external Mg2+ concentrations.
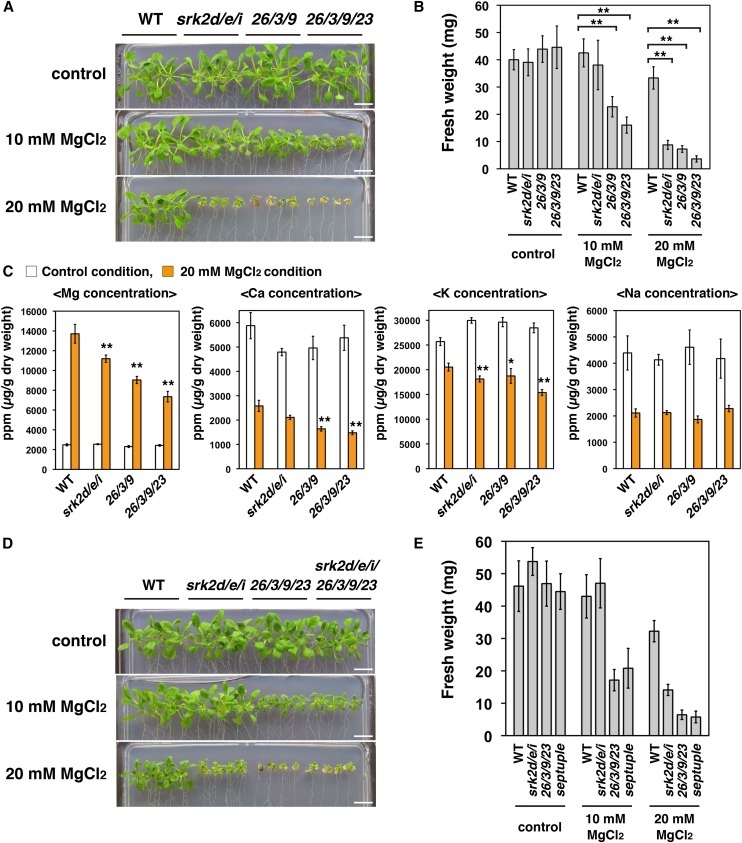
To test the susceptibility of the srk2d/e/i mutant to high external Mg2+ concentrations, we used an assay system on agar plates, because it was difficult to grow the srk2d/e/i mutant hydroponically in view of its extremely drought-sensitive phenotype (Fujii and Zhu, 2009; Fujita et al., 2009). Consistent with the patterns of plant growth in the hydroponic culture system (Fig. 4G), the cipk26/3/9 triple and the cipk26/3/9/23 quadruple mutants showed increased susceptibility (defined as increased susceptibility to inhibition of shoot growth) to high external Mg2+ concentrations on agar plates. Using this experimental system, we found that, as well as the cipk26/3/9 triple and the cipk26/3/9/23 quadruple mutants, the srk2d/e/i mutant also showed increased susceptibility to 20 mm MgCl2 (Fig. 5, A and B). This observation indicated that, other than CIPK26/3/9/23, subclass III SnRK2s play an important role in plant growth under high external Mg2+ concentrations. In addition, ICP-MS analyses showed that the magnesium and potassium contents in the aerial parts of the srk2d/e/i mutant grown with 20 mm MgCl2 were significantly lower than those of the wild type, which was the case in the cipk26/3/9 triple and the cipk26/3/9/23 quadruple mutants (Fig. 5C, orange bars). In contrast, the sodium content in the aerial parts of the srk2d/e/i mutant grown with 20 mm MgCl2 was similar to that of the wild type (Fig. 5C, orange bars).
Figure 5.
The srk2d/e/i triple mutant shows increased susceptibility to a high external Mg2+ concentration. A, Growth phenotypes of the wild type, srk2d/e/i and cipk26/3/9 triple mutants, and the cipk26/3/9/23 quadruple mutant grown under a high external Mg2+ concentration. Photographs show representative phenotypes of 18-d-old seedlings grown vertically for 4 d on GM plates and then for 14 d on one-half-strength Murashige and Skoog medium agar plates containing indicated concentrations of MgCl2. Bars = 1 cm. B, Fresh weight of seedlings treated as described in A. Similar results were obtained in independent experiments; representative data are shown. Bars indicate sd (n = 8). Asterisks indicate statistically significant difference. **, P < 0.01, two-way ANOVA followed by a post hoc Tukey-Kramer multiple comparison test. C, ICP-MS analyses of magnesium, calcium, potassium, and sodium concentrations in aerial parts of plants grown vertically for 4 d on GM plates and then for 12 d on one-half-strength Murashige and Skoog agar plates with or without the addition of 20 mm MgCl2. For each biological replicate, material from four plants was pooled to make one sample for ICP-MS analysis. Data represent means and sds (n = 6). Orange bars, 20 mm MgCl2; white bars, control. Asterisks indicate that the corresponding mean is significantly different from the mean value of the wild type within each condition. *, P < 0.05; **, P < 0.01, two-way ANOVA followed by a post hoc Tukey-Kramer multiple comparison test. D, Susceptibility of the srk2d/e/i/cipk26/3/9/23 septuple mutant to high external Mg2+ concentrations. Plants were grown as described in A. Bars = 1 cm. E, Fresh weight of seedlings treated as described in D. Similar results were obtained in independent experiments; representative data are shown. Bars indicate sd (n = 8). WT, Wild type.
To analyze the functional redundancy among CIPK26/3/9/23 and subclass III SnRK2s in modulating Mg2+ susceptibility (Mg2+ susceptibility is defined as susceptibility to shoot growth inhibition in response to increased external Mg2+ concentrations), we tested the susceptibility of the various mutants to a high external Mg2+ concentration. We analyzed the cipk26, cipk3, cipk9, and cipk23 single mutants and multiple cipk mutants and srk2d, srk2e, and srk2i single mutants and multiple snrk2 mutants. All of the tested single and double cipk mutants, except for the cipk26/3 double mutant, showed a similar susceptibility to a high external Mg2+ concentration as that of the wild type (Supplemental Fig. S12A). In contrast, the cipk26/3 double mutant and the cipk26/3/9, cipk26/3/23, and cipk26/9/23 triple mutants showed greater Mg2+ susceptibility than that of the wild type, whereas the cipk3/9/23 triple mutant did not (Supplemental Fig. S12A). All of the single and double snrk2 mutants showed similar susceptibility to a high external Mg2+ concentration as that of the wild type, whereas the srk2d/e/i triple mutant was significantly hypersusceptible to a high external Mg2+ concentration (Supplemental Fig. S12B).
We also tested whether the srk2d/e/i and cipk26/3/9 triple mutants and the cipk26/3/9/23 quadruple mutant were hypersusceptible to high external K+, Na+, or Ca2+ concentrations on agar plates (Supplemental Fig. S13). The srk2d/e/i triple mutant was specifically hypersusceptible to a high external Mg2+ concentration. As well as showing hypersusceptibility to a high external Mg2+ concentration, the cipk26/3/9 triple and the cipk26/3/9/23 quadruple mutants were slightly susceptible to a high external Ca2+ concentration.
To reveal the genetic interactions between CIPK26/3/9/23 and SRK2D/E/I in modulating Mg2+ susceptibility, we generated an srk2d/e/i/cipk26/3/9/23 septuple mutant. In this mutant, both the SRK2D/E/I and CIPK26/3/9/23 gene groups were disrupted. We tested the susceptibility of the wild type, the srk2d/e/i triple mutant, the cipk26/3/9/23 quadruple mutant, and the srk2d/e/i/cipk26/3/9/23 septuple mutant to high external Mg2+ concentrations (Fig. 5, D and E). The srk2d/e/i/cipk26/3/9/23 septuple mutant and the cipk26/3/9/23 quadruple mutant showed similar growth inhibition under high external Mg2+ concentrations (10 or 20 mm MgCl2; Fig. 5, D and E). This result suggests that SRK2D/E/I and CIPK26/3/9/23 modulate Mg2+ susceptibility through a common pathway.
We also evaluated the activation patterns of SRK2D-sGFP proteins in response to high external Mg2+ concentrations using myelin basic protein as substrate. In contrast to the significant activation of SRK2D-sGFP proteins in response to ABA or mannitol treatment, the activation status of SRK2D-sGFP proteins in response to a high external Mg2+ concentration remained comparable with that of nontreated plants (Supplemental Fig. S14).
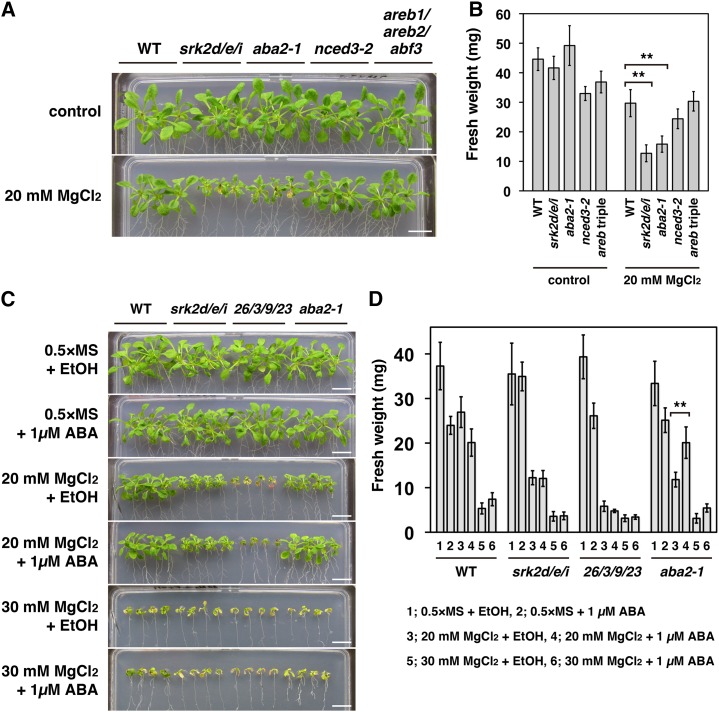
ABA2-Mediated ABA Biosynthesis Is Important for Modulating Susceptibility to a High External Mg2+ Concentration
Considering that the activities of subclass III SnRK2s are modulated by ABA (Boudsocq et al., 2004; Kobayashi et al., 2005; Furihata et al., 2006), we wondered whether ABA biosynthesis plays a role in plant growth under high external Mg2+ concentrations. We tested the susceptibility of the ABA biosynthesis-deficient mutants aba2-1 (Léon-Kloosterziel et al., 1996) and nine-cis-epoxycarotenoid dioxygenase3-2 (nced3-2) (Urano et al., 2009) to a high external Mg2+ concentration. In the aba2-1 mutant, accumulation of ABA both to basal levels under well-watered conditions and in response to drought stress is impaired (Léon-Kloosterziel et al., 1996). The nced3-2 mutant is impaired in drought-responsive ABA accumulation, but it can accumulate basal levels of ABA (Urano et al., 2009). The aba2-1 mutant showed increased susceptibility to a high external Mg2+ concentration (20 mm MgCl2), whereas the nced3-2 mutant did not (Fig. 6, A and B). We also tested the susceptibility of the areb1/areb2/abf3 triple mutant to a high external Mg2+ concentration. This mutant lacks three AREB/ABF transcription factors (AREB1, AREB2, and ABF3) and shows impaired expression of many genes downstream of subclass III SnRK2 in ABA signaling in response to osmotic stress (Yoshida et al., 2010). The susceptibility of the areb1/areb2/abf3 triple mutant to a high external Mg2+ concentration (20 mm MgCl2) was similar to that of the wild type (Fig. 6, A and B).
Figure 6.
ABA-mediated rescue of hypersusceptibility of the aba2-1 mutant to a high external Mg2+ concentration. A, Susceptibility to a high external Mg2+ concentration (20 mm MgCl2) in the aba2-1, the nced3-2, and the areb1/areb2/abf3 triple (areb triple) mutants. Photographs show representative phenotypes of 18-d-old seedlings grown as described in Fig. 5A. Bars = 1 cm. B, Fresh weight of seedlings treated as described in A. Similar results were obtained in independent experiments. Representative data are shown. Bars indicate sd (n = 9). Asterisks indicate statistically significant difference. **, P < 0.01, two-way ANOVA followed by a post hoc Tukey-Kramer multiple comparison test. C, Growth phenotypes of the wild-type, the srk2d/e/i triple, the cipk26/3/9/23 quadruple, and the aba2-1 mutant plants grown vertically for 4 d on GM plates and then for 14 d on one-half-strength Murashige and Skoog agar plates with or without the addition of 20 or 30 mm MgCl2 and 1 µm ABA. In the control experiment, an equivalent amount of ethanol (the solvent for ABA) was added to the media. Bars = 1 cm. D, Fresh weight of seedlings treated as described in C. Similar results were obtained in independent experiments; representative data are shown. Bars indicate sd (n = 8). Asterisks indicate statistically significant difference as described in B. WT, Wild type.
To further test the hypothesis that ABA biosynthesis plays an important role in modulating Mg2+ susceptibility, we tested whether two other ABA biosynthesis-deficient mutants, abscisic aldehyde oxidase3-4 (aao3-4; Seo et al., 2004) and aba3-1 (Léon-Kloosterziel et al., 1996), showed increased susceptibility to a high external Mg2+ concentration (Supplemental Fig. S15). Like aba2-1, the aao3-4 and aba3-1 mutants showed increased susceptibility to a high external Mg2+ concentration (20 mm MgCl2; Supplemental Fig. S15). We further investigated whether the hypersusceptibility of the aba2-1 mutant to a high external Mg2+ concentration results from an ABA deficiency. Addition of 1 µm ABA to the medium rescued the hypersusceptibility of the aba2-1 mutant to a high external Mg2+ concentration (20 mm MgCl2) but did not rescue the hypersensitivity of the srk2d/e/i triple and the cipk26/3/9/23 quadruple mutants (Fig. 6, C and D). Taken together, these results support the idea that ABA synthesized through ABA2 plays a key role in plant growth under high external Mg2+ concentrations.
DISCUSSION
Recent advances have furthered our understanding of the roles of protein phosphorylation in regulating Na+ and K+ transport (Qiu et al., 2002; Li et al., 2006; Xu et al., 2006); however, the regulatory mechanisms by which plants modulate cellular Mg2+ transport and maintain Mg2+ homeostasis in response to changes in external ion concentrations remain poorly understood, despite the pivotal functions of Mg2+ in plant cells. Here, we reveal that two distinct families of plant-specific protein kinases, subclass III SnRK2s (SRK2D/E/I) and CIPK26/3/9/23, modulate the susceptibility to shoot growth inhibition in response to increased external Mg2+ concentrations (Mg2+ susceptibility) in Arabidopsis. To date, many studies on subclass III SnRK2s have focused on their functions as positive regulators of ABA signaling in response to water deficit stress (Mustilli et al., 2002; Kobayashi et al., 2005; Fujii and Zhu, 2009; Fujita et al., 2009). Conversely, a recent phosphoproteomic analysis identified proteins that are involved in flowering time regulation, such as MODIFIER OF SNC1, 3 (MOS3) and 5′-3′ exoribonuclease3 (XRN3), as possible substrates for subclass III SnRK2s (Wang et al., 2013), which was consistent with the early flowering phenotype of the srk2d/e/i triple mutant (Wang et al., 2013). This suggested that subclass III SnRK2s play diverse roles in modulating plant growth under not only water deficit stress conditions but also, normal growth conditions. In this study, we revealed a novel role of subclass III SnRK2s in plant growth under high external Mg2+ concentrations. Previous studies on CIPK26/3/9/23 have characterized the diverse and distinct functions of each of these CIPK genes (Kim et al., 2003; Li et al., 2006; Xu et al., 2006; Cheong et al., 2007; Pandey et al., 2007; Drerup et al., 2013; Kimura et al., 2013; Lyzenga et al., 2013). Other than the individual functions of CIPK26, CIPK3, CIPK9, and CIPK23, it seems that these CIPK genes also have some overlapping functions in planta, because CIPK26/3/9/23 formed a monophyletic group in the phylogenetic analysis (Supplemental Fig. S3B), and all of them could physically interact with SRK2D in planta (Fig. 2F).
In this research, we found that the cipk26/3/9 triple and the cipk26/3/9/23 quadruple mutants displayed impaired growth phenotypes represented by small rosettes and necrotic symptoms on the leaf tips and shoot apex when grown in soil (Fig. 4, A–F). When grown in a hydroponic culture system, the growth retardation observed in the cipk26/3/9 triple and the cipk26/3/9/23 quadruple mutants was rescued by decreasing the concentration of external Mg2+ (Fig. 4, G and H). Consistent with this observation, the impaired growth phenotype (reduced inflorescence height) of these mutants when grown in soil was partially rescued by decreasing the concentration of MgCl2 (Supplemental Fig. S7). These results indicate that CIPK26/3/9/23 plays a fundamental role in plant growth, even under normal external Mg2+ concentrations. The cipk26/3/9 triple and the cipk26/3/9/23 quadruple mutants showed increased susceptibility to high external Mg2+ concentrations on agar plates (Fig. 5, A and B). Other than these cipk mutants, the srk2d/e/i triple mutant was also hypersusceptible to a high external Mg2+ concentration (Fig. 5, A and B). By contrast, Arabidopsis plants overexpressing CIPK26 showed significantly higher tolerance to a high external Mg2+ concentration (Supplemental Fig. S10, A–C). In addition, the CIPK26-overexpressing lines grew well under a low external Ca2+ concentration (Supplemental Fig. S10, D and E). Given the fact that genes closely related to CIPK26 are conserved in monocots and eudicots (Supplemental Fig. S3B), CIPK26 may be a strong candidate as a gene to improve crop growth under Mg2+-toxic and/or Ca2+-deficient conditions (e.g. in serpentine soils).
We considered that CIPK26, CIPK3, CIPK9, and CIPK23 could have a degree of functional redundancy in modulating Mg2+ susceptibility (Supplemental Fig. S12A). All single and double cipk mutants, except for the cipk26/3 double mutant, showed similar susceptibility to a high external Mg2+ concentration as that of the wild type (Supplemental Fig. S12A). Conversely, the cipk26/3/9, cipk26/3/23, and cipk26/9/23 triple mutants showed increased Mg2+ susceptibility, whereas the cipk3/9/23 triple mutant did not (Supplemental Fig. S12A). This result suggests that the functions of CIPK26, CIPK3, CIPK9, and CIPK23 in modulating Mg2+ susceptibility overlap to some extent and that CIPK26 plays a particularly important role in modulating Mg2+ susceptibility. We also considered that SRK2D, SRK2E, and SRK2I could have overlapping functions in modulating Mg2+ susceptibility (Supplemental Fig. S12B). The srk2d/e/i triple mutant was hypersusceptible to a high external Mg2+ concentration, whereas none of the single or double T-DNA mutants were hypersusceptible (Supplemental Fig. S12B). This result suggests that SRK2D, SRK2E, and SRK2I modulate Mg2+ susceptibility in a redundant manner.
Under a high external Mg2+ concentration (20 mm MgCl2), the srk2d/e/i and cipk26/3/9 triple mutants and the cipk26/3/9/23 quadruple mutant accumulated much lower levels of magnesium in their aerial parts than did the wild type (Fig. 5C), although these mutants showed greater susceptibility to a high external Mg2+ concentration (Fig. 5, A and B). These data suggested that intracellular Mg2+ homeostasis is disrupted in these mutants; however, it remains unclear how and where Mg2+ homeostasis is affected. As an abundant intracellular divalent cation, Mg2+ stabilizes phosphate compounds (e.g. DNA, RNA, and ATP) and is essential for the function of many enzymes. It also coordinates with the porphyrin ring of chlorophyll molecules. Therefore, it is essential for photosynthesis. Recent studies have revealed that several mitochondrial RNA splicing2 (MRS2)/magnesium transport (MGT) proteins, which are structurally homologous to bacterial Co2+ resistance A (CorA) proteins and ALUMINUM RESISTANCE1 (ALR1), ALR2, and MRS2 in yeast, play an important role in Mg2+ transport and plant growth under low external Mg2+ conditions (Gebert et al., 2009; Lenz et al., 2013; Mao et al., 2014). However, it remains largely unknown how plants maintain cellular Mg2+ homeostasis under high external Mg2+ conditions. Taken together with a previous report that mesophyll vacuoles can accumulate up to 80 mm Mg2+ in leaves of Arabidopsis fed with high-magnesium artificial sap solutions (Conn et al., 2011a), impaired Mg2+ transport into the vacuole with a resulting increase in Mg2+ concentration in the cytoplasm might, at least partly, explain the increased susceptibility of the srk2d/e/i and cipk26/3/9 triple mutants and the cipk26/3/9/23 quadruple mutant to high external Mg2+ concentrations and the decreased accumulation of Mg2+ in aerial parts. The report also showed that the disruption of MRS2-1/MGT2 and MRS2-5/MGT3, which are tonoplast-localized MRS family Mg2+ transporters, partially impaired compartmentalization of Mg2+ into the vacuole under a high external Mg2+ concentration (Conn et al., 2011a). In addition, the SOS2/CIPK24 protein kinase can activate the tonoplast-localized Ca2+/H+ antiporter CAX1 (Cheng et al., 2004). Based on these reports, subclass III SnRK2s and CIPK26/3/9/23 protein kinases might target certain tonoplast-localized Mg2+ transporters and/or channels and modulate their activities under high external Mg2+ concentrations to maintain the cytoplasmic Mg2+ concentration (Fig. 7). More precisely, these protein kinases might activate certain tonoplast-localized proteins involved in active Mg2+ transport into the vacuole and in parallel, might inactivate certain tonoplast-localized proteins involving in Mg2+ passive transport between the cytoplasm and the vacuole. In future research, it will be important to measure the magnesium contents in the cytoplasm and various organelles, such as the vacuole, to unravel how Mg2+ homeostasis is affected in the srk2d/e/i and cipk26/3/9 triple mutants and the cipk26/3/9/23 quadruple mutant.
Figure 7.
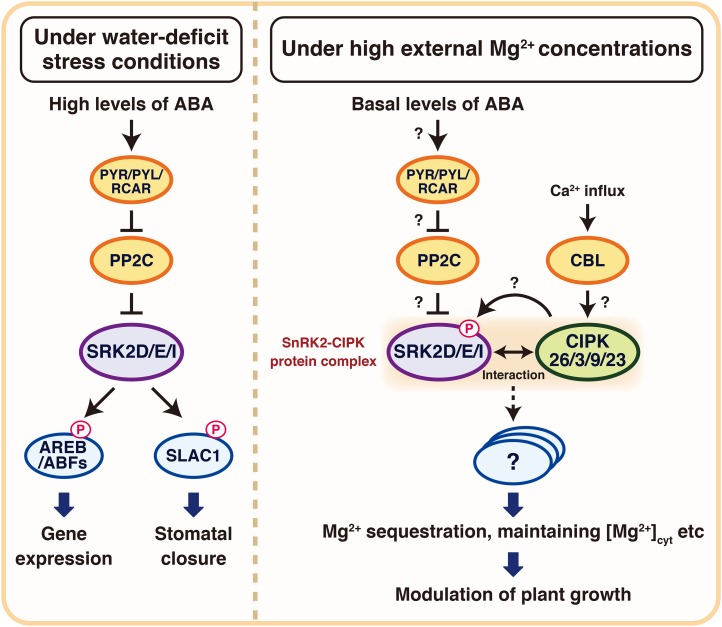
A hypothesized schematic model of the novel roles of SRK2D/E/I and CIPK26/3/9/23 in plant growth under high external Mg2+ concentrations in Arabidopsis. Under water deficit stress conditions, subclass III SnRK2s (SRK2D, SRK2I, and SRK2E/OST1) are activated by high levels of ABA, and they then modulate the activities of downstream targets, including transcription factors, such as AREB/ABFs and channels (e.g. SLAC1). Under high external Mg2+ concentrations, subclass III SnRK2s, in response to basal levels of ABA, play a key role in plant growth while interacting with CIPK26/3/9/23. Subclass III SnRK2s and CIPK26/3/9/23 function as key modulators of the susceptibility to shoot growth inhibition in response to increased external Mg2+ concentrations (Mg2+ susceptibility) probably through phosphorylating and, thus, modulating downstream targets, which are assumed to modulate intracellular Mg2+ transport and maintain Mg2+ homeostasis. Dashed lines indicate possible but not confirmed routes.
CIPK26 physically interacts with SRK2D in planta (Fig. 2, A and D; Supplemental Fig. S11). CIPK26 and SRK2D are Ser/Thr protein kinases; therefore, it is possible that these two proteins could phosphorylate each other. SRK2D-MBP could not phosphorylate CIPK26K42N-GST in vitro (Fig. 3A), suggesting that CIPK26 is not a phosphorylation substrate for SRK2D. Conversely, CIPK26-GST was able to phosphorylate SRK2DK52N-MBP in vitro (Fig. 3A), suggesting that SRK2D is a potential substrate for CIPK26. The signal from transphosphorylation of SRK2DK52N-MBP by CIPK26-GST was weaker than that from autophosphorylation of SRK2D-MBP (Fig. 3A). This may be because of the multiple phosphorylations of several Ser/Thr residues in the autophosphorylation of SRK2D, which is the case in the autophosphorylation of SRK2E/OST1 (Belin et al., 2006). In addition, an in vitro phosphorylation assay showed that the RD of SRK2D could be phosphorylated by CIPK26 (Fig. 3B). These results raised the possibility that CIPK26 affects the activity of SRK2D. We then considered whether CIPK26-GST could enhance the kinase activity of SRK2D-MBP in vitro (Fig. 3D; Supplemental Fig. S4B). Based on a previous report that CBL1/CBL9 work together with CIPK26 in the activation of RBOHF (Drerup et al., 2013), we also considered whether the addition of CBL1/CBL9-GST to CIPK26-GST would affect the kinase activity of SRK2D-MBP (Fig. 3D). Despite the presence of CBL1/CBL9-GST, coincubation of CIPK26-GST with SRK2D-MBP could not activate the kinase activity of SRK2D-MBP in vitro (Fig. 3D). Although we did not observe that CIPK26 activated SRK2D in vitro, these results could not rule out the possibility that unknown factors other than CBL1/CBL9 together with CIPK26 modulate the activity of SRK2D in vivo. In future research, additional analyses are required to clarify whether CIPK26 is involved in modulating the activity of SRK2D in vivo, especially under high external Mg2+ concentrations. It will be important to explore novel player(s) involved in CIPK26- and SRK2D-mediated modulation of Mg2+ susceptibility by using co-IP coupled with LC-MS/MS method.
Previous reports showed that the phosphorylation of the activation loop plays a crucial role in the activation of subclass III SnRK2s (Belin et al., 2006; Umezawa et al., 2009) and that a Glycogen synthase kinase3-like kinase, brassinosteroid insensitive2 (BIN2), phosphorylates the activation loop in the KD of SRK2D and activates its autophosphorylation activity and the kinase activity toward ABF2/AREB1 (Cai et al., 2014). Thus, an alternative possibility is that the phosphorylation of SRK2D by CIPK26 has functions other than activation. It has been reported that the phosphorylation of SOS3-LIKE CALCIUM BINDING PROTEIN8 (SCaBP8)/CBL10 by SOS2/CIPK24 stabilized the SCaBP8-SOS2 interaction (Lin et al., 2009). Taken together with our finding that the RD of SRK2D is necessary and sufficient for the physical interaction with CIPK26 (Fig. 2B), the phosphorylation of the RD of SRK2D by CIPK26 might play a role in modulating the physical interactions between these protein kinases. In additional research, it will be also important to identify the phosphorylation site(s) of SRK2D by CIPK26 and then analyze mutated forms of SRK2D, in which the residues phosphorylated by CIPK26 are substituted with Ala or Asp/Glu, to consider how the phosphorylation of SRK2D by CIPK26 affects the function of SRK2D in planta.
Considering that the srk2d/e/i/cipk26/3/9/23 septuple mutant and the cipk26/3/9/23 quadruple mutant show similar hypersusceptibility to high external Mg2+ concentrations (Fig. 5, D and E), subclass III SnRK2s and CIPK26/3/9/23 may modulate Mg2+ susceptibility through a common pathway. Moreover, CIPK26 physically interacts with SRK2D under a high external Mg2+ concentration (Supplemental Fig. S11). This result provides evidence that SRK2D forms a protein complex with CIPK26 under a high external Mg2+ concentration, where subclass III SnRK2s and CIPK26/3/9/23 play an important role in modulating Mg2+ susceptibility. Recently, it was reported that both SRK2E/OST1 and CIPK26 are able to phosphorylate the NADPH oxidase RBOHF (Sirichandra et al., 2009; Drerup et al., 2013; Kimura et al., 2013). The effect of the phosphorylation of RBOHF by SRK2E/OST1 is still unknown (Sirichandra et al., 2009), but it was reported that CIPK26 enhances the activity of RBOHF through its phosphorylation together with CBL1 and CBL9 in human embryonic kidney 293T cells (Drerup et al., 2013). These findings together with our finding that CIPK26 interacts with SRK2D in planta to form a protein complex under a high external Mg2+ concentration (Supplemental Fig. S11) support the view that subclass III SnRK2s and CIPK26 cooperatively regulate activities of some downstream targets that play important roles in modulating Mg2+ susceptibility and/or Mg2+ transport (Fig. 7). To validate this hypothesis, it will be important to comprehensively identify downstream targets of SRK2D/E/I and CIPK26/3/9/23. This can be investigated using a phosphoproteomics approach to analyze the srk2d/e/i and cipk26/3/9 triple mutants and the cipk26/3/9/23 quadruple mutant.
Several ABA biosynthesis-deficient mutants showed increased susceptibility to a high external Mg2+ concentration (Supplemental Fig. S15), and the hypersusceptibility of the aba2-1 mutant to a high external Mg2+ concentration (20 mm MgCl2) was rescued by adding ABA (Fig. 6, C and D). These findings indicate that ABA, a key molecule in water deficit stress signaling, also serves as a signaling molecule in plant growth under high external Mg2+ conditions in Arabidopsis. Several studies have reported that ABA modulates the activities of subclass III SnRK2s (Mustilli et al., 2002; Boudsocq et al., 2004; Furihata et al., 2006; Yoshida et al., 2006) and that subclass III SnRK2s are key positive regulators of ABA signaling (Fujii and Zhu, 2009; Fujita et al., 2009; Nakashima et al., 2009; Umezawa et al., 2009). In addition, the srk2d/e/i triple mutant showed increased susceptibility to a high external Mg2+ concentration along with aba2-1 (Fig. 6, A and B). Therefore, it is likely that ABA modulates Mg2+ susceptibility through a subclass III SnRK2s-mediated pathway. Additional research is needed to examine the involvement of PYR/PYLs/RCARs and PP2Cs in ABA-mediated modulation of Mg2+ susceptibility (Fig. 7). In contrast to the aba2-1, aao3-4, and aba3-1 mutants, the nced3-2 mutant showed a similar susceptibility to a high external Mg2+ concentration as that of the wild type (Supplemental Fig. S15), probably because of the redundant role(s) of other NCED gene(s). Considering that the nced3-2 mutant was impaired only in drought-responsive ABA accumulation and not in accumulation of basal levels of ABA (Urano et al., 2009), basal levels of ABA synthesized by other NCED(s), ABA2, and AAO3 may play a key role in modulating Mg2+ susceptibility. This hypothesis seemed to be consistent with our observation that the activation status of SRK2D-sGFP proteins under a high external Mg2+ concentration remained at a basal level (Supplemental Fig. S14). Basal subclass III SnRK2s activity, in response to basal levels of ABA, may be necessary to modulate Mg2+ susceptibility (Fig. 7). We observed that the susceptibility of the areb1/areb2/abf3 triple mutant to a high Mg2+ concentration was similar to that of the wild type (Fig. 6, A and B). This result indicated that ABA-mediated modulation of susceptibility to a high external Mg2+ concentration is independent of osmotic stress-responsive gene expression mediated by the SnRK2-AREB/ABF pathways (Yoshida et al., 2014b). Therefore, it is likely that subclass III SnRK2s modulate Mg2+ susceptibility through other downstream targets (Fig. 7).
Considering that CIPK26/3/9/23 physically interacted with SRK2D (Fig. 2F), it is possible that CIPK26/3/9/23 plays a role in the ABA signaling pathway. However, our data indicated that neither the cipk26/3/9 triple mutant nor the cipk26/3/9/23 quadruple mutant exhibited any visible changes in ABA responses compared with those of the wild type during the vegetative growth stage (Fig. 6, C and D; Supplemental Fig. S6, A and B). Consistently, the activation patterns of subclass III SnRK2s in response to ABA or mannitol treatment in the cipk26/3/9/23 quadruple mutants were similar to those in wild-type plants (Supplemental Fig. S6C). These observations suggested that CIPK26/3/9/23 is unlikely to play a key role in ABA signaling during the vegetative growth stage and unlikely to be involved in modulating subclass III SnRK2s activity under water deficit stress conditions. Conversely, CIPK26 overexpressors showed a slightly ABA-hypersensitive phenotype during the germination stage, and it was suggested that CIPK26 could function as a positive regulator of plant responses to ABA (Lyzenga et al., 2013). The discrepancy between our results and the previous report could, at least partially, be explained by a difference in growth stages. Given that CIPK26 mainly localizes in the cytoplasm (Supplemental Fig. S2, B and C), whereas the localization of SRK2D is cytoplasmic and nuclear (Supplemental Fig. S2B), where it physically interacts with AREB/ABFs (Fujita et al., 2009; Yoshida et al., 2014a), it is likely that SRK2D regulates AREB/ABFs in the nucleus to induce osmotic stress-responsive gene expression and other ABA-related responses independent of CIPK26. Conversely, a previous study reported that CIPK26 localizes to both the cytoplasm and the nucleus and physically interacts with ABI5 mainly in the nucleus (Lyzenga et al., 2013). Another report showed that CIPK26 predominantly localizes in the cytoplasm, partially localizes in the nucleus, and physically interacts with plasma membrane-localized RBOHF (Drerup et al., 2013). Additional analyses are required to clarify the overall functions and the downstream targets of CIPK26 and other CIPKs during ABA signaling throughout the plant’s lifecycle.
Importantly, administration of ABA could not rescue the increased Mg2+ susceptibility of the cipk26/3/9/23 quadruple mutant (Fig. 6, C and D). This finding together with our observation that the cipk26/3/9/23 quadruple mutant displayed similar ABA sensitivity to that of the wild type (Supplemental Fig. S6, A and B) indicate that the increased Mg2+ susceptibility of the cipk26/3/9/23 quadruple mutant was not because of ABA deficiency or disruption of ABA signaling. Taken together, our data support the view that subclass III SnRK2s, in response to basal levels of ABA synthesized by ABA2 and AAO3 together with CIPK26/3/9/23, play a key role in plant growth under high external Mg2+ concentrations (Fig. 7). In addition, our findings suggest that the role of subclass III SnRK2s in modulating Mg2+ susceptibility is different from their pivotal roles in ABA signaling in response to water deficit stress conditions, which require a considerable level of ABA accumulation synthesized by NCED3 (Fig. 7).
Collectively, our findings provide genetic and physiological evidence that SRK2D/E/I and CIPK26/3/9/23 are required for plant growth under high external Mg2+ concentrations in Arabidopsis. Our results also show that ABA serves as an important signaling molecule in modulating Mg2+ susceptibility in Arabidopsis. Our research suggests that plants modulate susceptibility to Mg2+ through phosphorylation signaling mediated by SRK2D/E/I and CIPK26/3/9/23. It will be challenging to understand the physiological importance of the physical interactions between CIPK26/3/9/23 and SRK2D and reveal the molecular mechanisms by which these two distinct protein kinases modulate susceptibility to high external Mg2+ concentrations. In future research, it will be important to identify the downstream targets of these protein kinases that are responsible for modulating Mg2+ susceptibility and/or Mg2+ transport and maintaining cellular Mg2+ homeostasis. This will increase our understanding of how plants control cellular Mg2+ transport and maintain cellular Mg2+ homeostasis in response to changes in external Mg2+concentrations.
MATERIALS AND METHODS
Plant Materials, Growth Conditions, and Generation of Transgenic Plants
Arabidopsis thaliana (Arabidopsis) ‘Heynh’ ecotype Col-0 was used in this study. Seeds were surface sterilized and sown on GM agar plates. After stratification at 4°C for 3 d in the dark, seeds were grown on GM agar plates in a growth chamber under a 16-h-light/8-h-dark photoperiod (40 ± 10 µE m−2 s−1) at 22°C as described previously (Kim et al., 2012). For growing plants in soil, 2-week-old plants grown on GM agar plates were transferred into a plastic pot (approximately 7 cm with a height of 6 cm or approximately 8 cm with a height of 6.5 cm) filled with vermiculite supplemented with a modified basal nutrient solution [2 mm NH4NO3, 3 mm KNO3, 0.1 mm CaCl2, 2 mm KCl, 2 mm Ca(NO3)2, 2 mm MgSO4, 0.6 mm KH2PO4, 2 mm NaCl with micronutrients, 50 µm NaFe(III)EDTA, 50 µm H3BO3, 5 µm MnCl2, 10 µm ZnSO4, 0.5 µm CuSO4, 0.1 µm Na2MoO4, pH 5.6, and NaOH; Conn et al., 2011b] and grown under a 16-h-light/8-h-dark photoperiod (60 ± 10 µE m−2 s−1) at 22°C. The T-DNA insertion lines cipk26 (SALK_005859C), cipk3 (SALK_137779C), cipk9 (SALK_058629), cipk23 (SALK_032341), and aao3-4 (N572361) were obtained from the ABRC or the European Arabidopsis Stock Centre. A series of multiple cipk26, cipk3, cipk9, and cipk23 mutants in the Col-0 ecotype was constructed by genetic crosses and screened by genomic PCR using primers recommended by the ABRC (listed in Supplemental Table S2). The detailed procedure for construction of pGreenII-based plasmids for plant transformation is described in Supplemental Data. Plants were transformed using Agrobacterium tumefaciens strain GV3101 as described previously (Kim et al., 2012).
Co-IP, Silver Staining, and Immunoblot Analysis
Proteins were extracted from 3-week-old seedlings (4–6 g of fresh weight) grown on GM agar plates. Samples were ground to a powder in liquid nitrogen and homogenized on ice in 3× extraction buffer (50 mm Tris-HCl, pH 8.0, 0.2% [w/v] Triton X-100, and one tablet of complete protease inhibitor cocktail tablet per 25 mL). Crude extracts were then centrifuged at 5,000g for 10 min at 4°C to remove cellular debris. The supernatants were passed through one layer of Miracloth (Calbiochem) and centrifuged at 20,000g for 20 min at 4°C. The supernatant was mixed with 100 µL of µMACS Anti-GFP MicroBeads (130-091-125; Miltenyi Biotec) and then incubated for 30 min at 4°C. The mixtures were applied to M columns (130-042-801; Miltenyi Biotec) placed in the magnetic field of a MiniMACS Separator to retain magnetically labeled proteins. After extensive washing of the columns with extraction buffer, immunoprecipitated protein complexes were eluted with 100 µL of elution buffer (50 mm Tris-HCl, pH 6.8, 50 mm dithiothreitol, 1% [w/v] SDS, 1 mm EDTA, 0.005% [w/v] bromophenol blue, and 10% [v/v] glycerol). The immunoprecipitates were separated by SDS-PAGE followed by either silver staining or immunoblot analysis. Silver staining was performed using the SilverQuest Silver Staining Kit (Life Technologies Corporation). Immunoblot analyses were performed using the anti-GFP antibody (11814460001; 1:2,500; Roche) or the anti-Myc antibody (562; 1:1,000; MBL). Signals were detected with the ECL Prime Western Blotting Detection Reagent (GE Healthcare Life Sciences).
Hydroponic Culture
Hydroponic cultivation was performed using the Araponics Growing System (Araponics; http://www.araponics.com/) under a 16-h-light/8-h-dark photoperiod (40 ± 10 µE m−2 s−1) at 22°C. Plants were grown hydroponically in either of the following nutrient solutions: (1) a modified low-calcium solution (Conn et al., 2011b) [2 mm NH4NO3, 5 mm KNO3, 2 mm MgSO4, 0.6 mm KH2PO4, 2 mm NaNO3 with micronutrients, 50 µm NaFe(III)EDTA, 50 µm H3BO3, 5 µm MnCl2, 10 µm ZnSO4, 0.5 µm CuSO4, 0.1 µm Na2MoO4, pH 5.6, and NaOH] supplemented with various concentrations of CaCl2 as indicated; or (2) a low-magnesium solution (2 mm NH4NO3, 5 mm KNO3, 0.1 mm CaCl2, 2 mm CaSO4, 0.6 mm KH2PO4, 2 mm NaNO3 with micronutrients as described above, pH 5.6, and NaOH) supplemented with various concentrations of MgCl2 as indicated. Nutrient solutions were constantly aerated and replaced every week.
Physiological Assays
For Mg2+ susceptibility assays at the seedling stage, seeds were surface sterilized, sown on GM agar plates solidified with 1.2% (w/v) bactoagar (BD Biosciences), stratified at 4°C for 3 d in the dark, and then grown vertically in a growth room under a 16-h-light/8-h-dark photoperiod (60 ± 10 µE m−2 s−1) at 22°C. Four-day-old seedlings were transferred to fresh agar plates containing one-half-strength Murashige and Skoog medium, 1% (w/v) Suc, and 0.5 g L−1 MES-KOH (pH 5.7) supplemented with various concentrations of MgCl2, solidified with 1.2% (w/v) bactoagar, and then grown vertically for an additional 14 d. Photographs were then taken, and the fresh weight of each plant was determined. For K+, Na+, and Ca2+ susceptibility assays, KCl, NaCl, and CaCl2 were added to the growth medium at indicated concentrations instead of MgCl2.
In Vitro Phosphorylation Assay
The bacterial expression plasmids used for expression of recombinant proteins were constructed using pGEX-4T-2 (GE Healthcare Life Sciences) and pMALc2X (New England Biolabs) as described in Supplemental Data. The pGEX-4T-2- and pMALc2X-based plasmids were transformed into Escherichia coli strain BL21-Gold (DE3; Agilent Technologies). The GST- and MBP-fused proteins were bacterially expressed and affinity purified with glutathione sepharose 4B (GE Healthcare Life Sciences) and amylose resin (New England Biolabs), respectively, according to the manufacturer’s instructions. For in vitro phosphorylation assays, purified proteins and/or myelin basic protein (Sigma-Aldrich Japan) were incubated in a total volume of 15 µL of protein kinase assay buffer (50 mm Tris-HCl, pH 7.5, 100 mm NaCl, 5 mm MnSO4, 0.5 mm CaCl2, 2 mm dithiothreitol, 10 µm ATP, and 10 µCi [γ-32P]ATP) for 60 min at 30°C. The reaction was terminated by adding 5 µL of 4× SDS-PAGE sample buffer and heating samples at 95°C for 3 min. The protein samples were then separated by SDS-PAGE, and the gel was subsequently dried and exposed to a Fujifilm imaging plate (BAS-MS; GE Healthcare Life Sciences) for 1 d. Incorporation of a radioactive phosphate group was visualized by autoradiography using an FLA-5000 Phosphor Imager (Fujifilm). The protein level was analyzed by Coomassie Brilliant Blue staining.
Sequence data from this article can be found in the Arabidopsis Genome Initiative database under the following accession numbers: CIPK26 (At5g21326), CIPK3 (At2g26980), CIPK9 (At1g01140), CIPK23 (At1g30270), CIPK24/SOS2 (At5g35410), SRK2D (At3g50500), SRK2E/OST1 (At4g33950), SRK2I (At5g66880), SRK2C (At1g78290), SRK2F (At4g40010), SRK2A (At1g10940), SRK2B (At1g60940), SRK2G (At5g08590), SRK2H (At5g63650), SRK2J/SnRK2.9 (At2g23030), ABI1 (At4g26080), TSN2 (At5g61780), ACLB-1 (At3g06650), CBL1 (At4g17615), CBL9 (At5g47100), ABA2 (At1g52340), NCED3 (At3g14440), AAO3 (At2g27150), ABA3 (At1g16540), AREB1 (At1g45249), AREB2 (At3g19290), and ABF3 (At4g34000).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Functionality of constitutively expressed SRK2D-sGFP and visualization of immunoprecipitated proteins for LC-MS/MS analyses by silver staining.
Supplemental Figure S2. Expression patterns of CIPK26 and subcellular localization of sGFP-CIPK26 in leaf epidermal cells.
Supplemental Figure S3. Phylogenetic analysis of CIPK family members from Arabidopsis, rice (Oryza sativa), Selaginella moellendorffii, and Physcomitrella patens.
Supplemental Figure S4. Both SRK2D-MBP and CIPK26-GST are functional protein kinases.
Supplemental Figure S5. Growth phenotypes of cipk26, cipk3, cipk9, and cipk23 single mutants and multiple mutants.
Supplemental Figure S6. The cipk26/3/9 triple and the cipk26/3/9/23 quadruple mutants show similar ABA sensitivity to that of the wild type.
Supplemental Figure S7. Improved growth of the cipk26/3/9 and cipk26/3/9/23 mutants under a low external Mg2+ concentration in soil.
Supplemental Figure S8. ICP-MS analyses of magnesium, calcium, potassium, and sodium concentrations in aerial parts of hydroponically grown plants.
Supplemental Figure S9. Complementation tests of the cipk26/3/9/23 quadruple mutant by CIPK26.
Supplemental Figure S10. CIPK26-overexpressing lines grow well under both high external Mg2+ and low external Ca2+ concentrations.
Supplemental Figure S11. SRK2D physically interacts with CIPK26 under a high external Mg2+ concentration.
Supplemental Figure S12. Mg2+ susceptibility of cipk single mutants, multiple cipk mutants, snrk2 single mutants, and snrk2 multiple mutants.
Supplemental Figure S13. Susceptibility of srk2d/e/i and cipk26/3/9 triple mutants and the cipk26/3/9/23 quadruple mutant to high external K+, Na+, Mg2+, and Ca2+ concentrations.
Supplemental Figure S14. The activation status of SRK2D-sGFP under high external Mg2+ concentrations as validated by an in-gel kinase assay.
Supplemental Figure S15. Susceptibility of ABA biosynthesis-deficient mutants to a high external Mg2+ concentration.
Supplemental Table S1. Candidates of SRK2D-interacting proteins identified by LC-MS/MS analyses.
Supplemental Table S2. Primer pairs used in this study.
Supplementary Material
Acknowledgments
We thank Kaori Amano, Kyoko Murai, Emiko Kishi, and Kyouko Yoshiwara (Japan International Research Center for Agricultural Sciences) and Saho Mizukado (RIKEN Center for Sustainable Resource Science) for excellent technical assistance; Etsuko Toma (University of Tokyo) for skillful editorial assistance; Dr. Kohji Yamada (Max Planck Institute for Plant Breeding Research, Cologne, Germany) for figure illustration; Dr. Tomoaki Horie (Shinshu University, Nagano, Japan) for helpful suggestions; Dr. Tsuyoshi Nakagawa (Shimane University, Izumo, Japan) for providing the pGWB17 vector; Dr. Hirofumi Yoshioka (Nagoya University, Nagoya, Japan) for teaching us how to perform the agroinfiltration assay in Nicotiana benthamiana; Dr. David Baulcombe (University of Cambridge, Cambridge, United Kingdom) for providing A. tumefaciens carrying pBin-P19; and the ABRC and European Arabidopsis Stock Centre for supplying T-DNA insertion mutants.
Glossary
- ABRC
Arabidopsis Biological Resource Center
- BiFC
bimolecular fluorescence complementation
- co-IP
coimmunoprecipitation
- Col-0
Columbia-0
- GM
germination medium
- ICP
inductively coupled plasma
- IP
immunoprecipitation
- KD
kinase domain
- LC
liquid chromatography
- MS
mass spectrometry
- RD
regulatory domain
- RT
reverse transcription
- ABA
abscisic acid
Footnotes
This work was supported by the Ministry of Education, Culture, Sports, Science and Technology of Japan (Grant-in-Aid for Scientific Research C no. 24510312 to Y.F., Grant-in-Aid for Young Scientists A no. 24688007 to H.N., Grant-in-Aid for Challenging Exploratory Research no. 26650106 to H.N., Grant-in-Aid for Scientific Research S no. 25221202 to T.F., and Grants-in-Aid for Scientific Research on Innovative Areas nos. 22119002 to T.F. and 22119004 to K.Y.-S.), the Program for the Promotion of Basic Research Activities for Innovative Biosciences (BRAIN) of Japan, and the Science and Technology Research Partnership for Sustainable Development (SATREPS) of the Japan Science and Technology Agency/Japan International Cooperation Agency (to K.Y.-S.).
References
- Belin C, de Franco PO, Bourbousse C, Chaignepain S, Schmitter JM, Vavasseur A, Giraudat J, Barbier-Brygoo H, Thomine S (2006) Identification of features regulating OST1 kinase activity and OST1 function in guard cells. Plant Physiol 141: 1316–1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudsocq M, Barbier-Brygoo H, Laurière C (2004) Identification of nine sucrose nonfermenting 1-related protein kinases 2 activated by hyperosmotic and saline stresses in Arabidopsis thaliana. J Biol Chem 279: 41758–41766 [DOI] [PubMed] [Google Scholar]
- Cai Z, Liu J, Wang H, Yang C, Chen Y, Li Y, Pan S, Dong R, Tang G, Barajas-Lopez JD, et al. (2014) GSK3-like kinases positively modulate abscisic acid signaling through phosphorylating subgroup III SnRK2s in Arabidopsis. Proc Natl Acad Sci USA 111: 9651–9656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng NH, Pittman JK, Shigaki T, Lachmansingh J, LeClere S, Lahner B, Salt DE, Hirschi KD (2005) Functional association of Arabidopsis CAX1 and CAX3 is required for normal growth and ion homeostasis. Plant Physiol 138: 2048–2060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng NH, Pittman JK, Zhu JK, Hirschi KD (2004) The protein kinase SOS2 activates the Arabidopsis H+/Ca2+ antiporter CAX1 to integrate calcium transport and salt tolerance. J Biol Chem 279: 2922–2926 [DOI] [PubMed] [Google Scholar]
- Cheong YH, Pandey GK, Grant JJ, Batistic O, Li L, Kim BG, Lee SC, Kudla J, Luan S (2007) Two calcineurin B-like calcium sensors, interacting with protein kinase CIPK23, regulate leaf transpiration and root potassium uptake in Arabidopsis. Plant J 52: 223–239 [DOI] [PubMed] [Google Scholar]
- Conn SJ, Conn V, Tyerman SD, Kaiser BN, Leigh RA, Gilliham M (2011a) Magnesium transporters, MGT2/MRS2-1 and MGT3/MRS2-5, are important for magnesium partitioning within Arabidopsis thaliana mesophyll vacuoles. New Phytol 190: 583–594 [DOI] [PubMed] [Google Scholar]
- Conn SJ, Gilliham M, Athman A, Schreiber AW, Baumann U, Moller I, Cheng NH, Stancombe MA, Hirschi KD, Webb AA, et al. (2011b) Cell-specific vacuolar calcium storage mediated by CAX1 regulates apoplastic calcium concentration, gas exchange, and plant productivity in Arabidopsis. Plant Cell 23: 240–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drerup MM, Schlücking K, Hashimoto K, Manishankar P, Steinhorst L, Kuchitsu K, Kudla J (2013) The Calcineurin B-like calcium sensors CBL1 and CBL9 together with their interacting protein kinase CIPK26 regulate the Arabidopsis NADPH oxidase RBOHF. Mol Plant 6: 559–569 [DOI] [PubMed] [Google Scholar]
- Fujii H, Chinnusamy V, Rodrigues A, Rubio S, Antoni R, Park SY, Cutler SR, Sheen J, Rodriguez PL, Zhu JK (2009) In vitro reconstitution of an abscisic acid signalling pathway. Nature 462: 660–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii H, Verslues PE, Zhu JK (2007) Identification of two protein kinases required for abscisic acid regulation of seed germination, root growth, and gene expression in Arabidopsis. Plant Cell 19: 485–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii H, Zhu JK (2009) Arabidopsis mutant deficient in 3 abscisic acid-activated protein kinases reveals critical roles in growth, reproduction, and stress. Proc Natl Acad Sci USA 106: 8380–8385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita Y, Nakashima K, Yoshida T, Katagiri T, Kidokoro S, Kanamori N, Umezawa T, Fujita M, Maruyama K, Ishiyama K, et al. (2009) Three SnRK2 protein kinases are the main positive regulators of abscisic acid signaling in response to water stress in Arabidopsis. Plant Cell Physiol 50: 2123–2132 [DOI] [PubMed] [Google Scholar]
- Furihata T, Maruyama K, Fujita Y, Umezawa T, Yoshida R, Shinozaki K, Yamaguchi-Shinozaki K (2006) Abscisic acid-dependent multisite phosphorylation regulates the activity of a transcription activator AREB1. Proc Natl Acad Sci USA 103: 1988–1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebert M, Meschenmoser K, Svidová S, Weghuber J, Schweyen R, Eifler K, Lenz H, Weyand K, Knoop V (2009) A root-expressed magnesium transporter of the MRS2/MGT gene family in Arabidopsis thaliana allows for growth in low-Mg2+ environments. Plant Cell 21: 4018–4030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger D, Scherzer S, Mumm P, Stange A, Marten I, Bauer H, Ache P, Matschi S, Liese A, Al-Rasheid KA, et al. (2009) Activity of guard cell anion channel SLAC1 is controlled by drought-stress signaling kinase-phosphatase pair. Proc Natl Acad Sci USA 106: 21425–21430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks SK, Quinn AM, Hunter T (1988) The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science 241: 42–52 [DOI] [PubMed] [Google Scholar]
- Hosy E, Vavasseur A, Mouline K, Dreyer I, Gaymard F, Porée F, Boucherez J, Lebaudy A, Bouchez D, Very AA, et al. (2003) The Arabidopsis outward K+ channel GORK is involved in regulation of stomatal movements and plant transpiration. Proc Natl Acad Sci USA 100: 5549–5554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iuchi S, Kobayashi M, Taji T, Naramoto M, Seki M, Kato T, Tabata S, Kakubari Y, Yamaguchi-Shinozaki K, Shinozaki K (2001) Regulation of drought tolerance by gene manipulation of 9-cis-epoxycarotenoid dioxygenase, a key enzyme in abscisic acid biosynthesis in Arabidopsis. Plant J 27: 325–333 [DOI] [PubMed] [Google Scholar]
- Kim JS, Mizoi J, Kidokoro S, Maruyama K, Nakajima J, Nakashima K, Mitsuda N, Takiguchi Y, Ohme-Takagi M, Kondou Y, et al. (2012) Arabidopsis GROWTH-REGULATING FACTOR7 functions as a transcriptional repressor of abscisic acid- and osmotic stress-responsive genes, including DREB2A. Plant Cell 24: 3393–3405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KN, Cheong YH, Grant JJ, Pandey GK, Luan S (2003) CIPK3, a calcium sensor-associated protein kinase that regulates abscisic acid and cold signal transduction in Arabidopsis. Plant Cell 15: 411–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura S, Kawarazaki T, Nibori H, Michikawa M, Imai A, Kaya H, Kuchitsu K (2013) The CBL-interacting protein kinase CIPK26 is a novel interactor of Arabidopsis NADPH oxidase AtRbohF that negatively modulates its ROS-producing activity in a heterologous expression system. J Biochem 153: 191–195 [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Murata M, Minami H, Yamamoto S, Kagaya Y, Hobo T, Yamamoto A, Hattori T (2005) Abscisic acid-activated SNRK2 protein kinases function in the gene-regulation pathway of ABA signal transduction by phosphorylating ABA response element-binding factors. Plant J 44: 939–949 [DOI] [PubMed] [Google Scholar]
- Lee SC, Lan W, Buchanan BB, Luan S (2009) A protein kinase-phosphatase pair interacts with an ion channel to regulate ABA signaling in plant guard cells. Proc Natl Acad Sci USA 106: 21419–21424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz H, Dombinov V, Dreistein J, Reinhard MR, Gebert M, Knoop V (2013) Magnesium deficiency phenotypes upon multiple knockout of Arabidopsis thaliana MRS2 clade B genes can be ameliorated by concomitantly reduced calcium supply. Plant Cell Physiol 54: 1118–1131 [DOI] [PubMed] [Google Scholar]
- Léon-Kloosterziel KM, Gil MA, Ruijs GJ, Jacobsen SE, Olszewski NE, Schwartz SH, Zeevaart JA, Koornneef M (1996) Isolation and characterization of abscisic acid-deficient Arabidopsis mutants at two new loci. Plant J 10: 655–661 [DOI] [PubMed] [Google Scholar]
- Li L, Kim BG, Cheong YH, Pandey GK, Luan S (2006) A Ca2+ signaling pathway regulates a K+ channel for low-K response in Arabidopsis. Proc Natl Acad Sci USA 103: 12625–12630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H, Yang Y, Quan R, Mendoza I, Wu Y, Du W, Zhao S, Schumaker KS, Pardo JM, Guo Y (2009) Phosphorylation of SOS3-LIKE CALCIUM BINDING PROTEIN8 by SOS2 protein kinase stabilizes their protein complex and regulates salt tolerance in Arabidopsis. Plant Cell 21: 1607–1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyzenga WJ, Liu H, Schofield A, Muise-Hennessey A, Stone SL (2013) Arabidopsis CIPK26 interacts with KEG, components of the ABA signalling network and is degraded by the ubiquitin-proteasome system. J Exp Bot 64: 2779–2791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Szostkiewicz I, Korte A, Moes D, Yang Y, Christmann A, Grill E (2009) Regulators of PP2C phosphatase activity function as abscisic acid sensors. Science 324: 1064–1068 [DOI] [PubMed] [Google Scholar]
- Mao D, Chen J, Tian L, Liu Z, Yang L, Tang R, Li J, Lu C, Yang Y, Shi J, et al. (2014) Arabidopsis transporter MGT6 mediates magnesium uptake and is required for growth under magnesium limitation. Plant Cell 26: 2234–2248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustilli AC, Merlot S, Vavasseur A, Fenzi F, Giraudat J (2002) Arabidopsis OST1 protein kinase mediates the regulation of stomatal aperture by abscisic acid and acts upstream of reactive oxygen species production. Plant Cell 14: 3089–3099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima K, Fujita Y, Kanamori N, Katagiri T, Umezawa T, Kidokoro S, Maruyama K, Yoshida T, Ishiyama K, Kobayashi M, et al. (2009) Three Arabidopsis SnRK2 protein kinases, SRK2D/SnRK2.2, SRK2E/SnRK2.6/OST1 and SRK2I/SnRK2.3, involved in ABA signaling are essential for the control of seed development and dormancy. Plant Cell Physiol 50: 1345–1363 [DOI] [PubMed] [Google Scholar]
- Nishimura N, Sarkeshik A, Nito K, Park SY, Wang A, Carvalho PC, Lee S, Caddell DF, Cutler SR, Chory J, et al. (2010) PYR/PYL/RCAR family members are major in-vivo ABI1 protein phosphatase 2C-interacting proteins in Arabidopsis. Plant J 61: 290–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey GK, Cheong YH, Kim BG, Grant JJ, Li L, Luan S (2007) CIPK9: a calcium sensor-interacting protein kinase required for low-potassium tolerance in Arabidopsis. Cell Res 17: 411–421 [DOI] [PubMed] [Google Scholar]
- Park SY, Fung P, Nishimura N, Jensen DR, Fujii H, Zhao Y, Lumba S, Santiago J, Rodrigues A, Chow TF, et al. (2009) Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. Science 324: 1068–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauwels L, Barbero GF, Geerinck J, Tilleman S, Grunewald W, Pérez AC, Chico JM, Bossche RV, Sewell J, Gil E, et al. (2010) NINJA connects the co-repressor TOPLESS to jasmonate signalling. Nature 464: 788–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu QS, Guo Y, Dietrich MA, Schumaker KS, Zhu JK (2002) Regulation of SOS1, a plasma membrane Na+/H+ exchanger in Arabidopsis thaliana, by SOS2 and SOS3. Proc Natl Acad Sci USA 99: 8436–8441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo M, Aoki H, Koiwai H, Kamiya Y, Nambara E, Koshiba T (2004) Comparative studies on the Arabidopsis aldehyde oxidase (AAO) gene family revealed a major role of AAO3 in ABA biosynthesis in seeds. Plant Cell Physiol 45: 1694–1703 [DOI] [PubMed] [Google Scholar]
- Sirichandra C, Gu D, Hu HC, Davanture M, Lee S, Djaoui M, Valot B, Zivy M, Leung J, Merlot S, et al. (2009) Phosphorylation of the Arabidopsis AtrbohF NADPH oxidase by OST1 protein kinase. FEBS Lett 583: 2982–2986 [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Nose T, Jikumaru Y, Kamiya Y (2013) ABA inhibits entry into stomatal-lineage development in Arabidopsis leaves. Plant J 74: 448–457 [DOI] [PubMed] [Google Scholar]
- Tanz SK, Castleden I, Hooper CM, Vacher M, Small I, Millar HA (2013) SUBA3: a database for integrating experimentation and prediction to define the SUBcellular location of proteins in Arabidopsis. Nucleic Acids Res 41: D1185–D1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokunaga H, Kojima M, Kuroha T, Ishida T, Sugimoto K, Kiba T, Sakakibara H (2012) Arabidopsis lonely guy (LOG) multiple mutants reveal a central role of the LOG-dependent pathway in cytokinin activation. Plant J 69: 355–365 [DOI] [PubMed] [Google Scholar]
- Umezawa T, Sugiyama N, Mizoguchi M, Hayashi S, Myouga F, Yamaguchi-Shinozaki K, Ishihama Y, Hirayama T, Shinozaki K (2009) Type 2C protein phosphatases directly regulate abscisic acid-activated protein kinases in Arabidopsis. Proc Natl Acad Sci USA 106: 17588–17593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umezawa T, Sugiyama N, Takahashi F, Anderson JC, Ishihama Y, Peck SC, Shinozaki K (2013) Genetics and phosphoproteomics reveal a protein phosphorylation network in the abscisic acid signaling pathway in Arabidopsis thaliana. Sci Signal 6: rs8. [DOI] [PubMed] [Google Scholar]
- Urano K, Maruyama K, Ogata Y, Morishita Y, Takeda M, Sakurai N, Suzuki H, Saito K, Shibata D, Kobayashi M, et al. (2009) Characterization of the ABA-regulated global responses to dehydration in Arabidopsis by metabolomics. Plant J 57: 1065–1078 [DOI] [PubMed] [Google Scholar]
- Wang P, Xue L, Batelli G, Lee S, Hou YJ, Van Oosten MJ, Zhang H, Tao WA, Zhu JK (2013) Quantitative phosphoproteomics identifies SnRK2 protein kinase substrates and reveals the effectors of abscisic acid action. Proc Natl Acad Sci USA 110: 11205–11210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinl S, Kudla J (2009) The CBL-CIPK Ca2+-decoding signaling network: function and perspectives. New Phytol 184: 517–528 [DOI] [PubMed] [Google Scholar]
- Xu J, Li HD, Chen LQ, Wang Y, Liu LL, He L, Wu WH (2006) A protein kinase, interacting with two calcineurin B-like proteins, regulates K+ transporter AKT1 in Arabidopsis. Cell 125: 1347–1360 [DOI] [PubMed] [Google Scholar]
- Yoshida R, Umezawa T, Mizoguchi T, Takahashi S, Takahashi F, Shinozaki K (2006) The regulatory domain of SRK2E/OST1/SnRK2.6 interacts with ABI1 and integrates abscisic acid (ABA) and osmotic stress signals controlling stomatal closure in Arabidopsis. J Biol Chem 281: 5310–5318 [DOI] [PubMed] [Google Scholar]
- Yoshida T, Fujita Y, Maruyama K, Mogami J, Todaka D, Shinozaki K, Yamaguchi-Shinozaki K (2014a) Four Arabidopsis AREB/ABF transcription factors function predominantly in gene expression downstream of SnRK2 kinases in abscisic acid signalling in response to osmotic stress. Plant Cell Environ 38: 35–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Fujita Y, Sayama H, Kidokoro S, Maruyama K, Mizoi J, Shinozaki K, Yamaguchi-Shinozaki K (2010) AREB1, AREB2, and ABF3 are master transcription factors that cooperatively regulate ABRE-dependent ABA signaling involved in drought stress tolerance and require ABA for full activation. Plant J 61: 672–685 [DOI] [PubMed] [Google Scholar]
- Yoshida T, Mogami J, Yamaguchi-Shinozaki K (2014b) ABA-dependent and ABA-independent signaling in response to osmotic stress in plants. Curr Opin Plant Biol 21C: 133–139 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.