A transcription factor represses abscisic acid biosynthesis and fruit ripening.
Abstract
Abscisic acid (ABA) regulates plant development and adaptation to environmental conditions. Although the ABA biosynthesis pathway in plants has been thoroughly elucidated, how ABA biosynthetic genes are regulated at the molecular level during plant development is less well understood. Here, we show that the tomato (Solanum lycopersicum) zinc finger transcription factor SlZFP2 is involved in the regulation of ABA biosynthesis during fruit development. Overexpression of SlZFP2 resulted in multiple phenotypic changes, including more branches, early flowering, delayed fruit ripening, lighter seeds, and faster seed germination, whereas down-regulation of its expression caused problematic fruit set, accelerated ripening, and inhibited seed germination. SlZFP2 represses ABA biosynthesis during fruit development through direct suppression of the ABA biosynthetic genes NOTABILIS, SITIENS, and FLACCA and the aldehyde oxidase SlAO1. We also show that SlZFP2 regulates fruit ripening through transcriptional suppression of the ripening regulator COLORLESS NON-RIPENING. Using bacterial one-hybrid screening and a selected amplification and binding assay, we identified the (A/T)(G/C)TT motif as the core binding sequence of SlZFP2. Furthermore, by RNA sequencing profiling, we found that 193 genes containing the SlZFP2-binding motifs in their promoters were differentially expressed in 2 d post anthesis fruits between the SlZFP2 RNA interference line and its nontransgenic sibling. We propose that SlZFP2 functions as a repressor to fine-tune ABA biosynthesis during fruit development and provides a potentially valuable tool for dissecting the role of ABA in fruit ripening.
Abscisic acid (ABA) plays important roles in seed maturation and germination as well as in responses to stresses, such as cold, drought, and salinity (Wasilewska et al., 2008). ABA is derived from apocarotenoids, and the synthetic pathway involves several enzymatic reactions (Nambara and Marion-Poll, 2005; Wasilewska et al., 2008; Hauser et al., 2011). Zeaxanthin derived from β-carotene is first converted to violaxanthin by zeaxanthin epoxidase (ZEP) and then to xanthoxin by 9-cis-epoxycarotenoid dioxygenase (NCED) in plastid. Then, xanthoxin is subsequently converted to ABA in the cytoplasm via the intermediate ABA aldehyde by xanthoxin oxidase/short-chain dehydrogenase/reductase and aldehyde oxidase (AO) together with its cofactor molybdenum-cofactor sulfurase (MoCOS). In tomato (Solanum lycopersicum), high pigment-3 (hp-3), notabilis (not), sitiens (sit), and flacca (flc) contain mutations in ZEP, LeNCED1, an AO, and a MoCOS gene, respectively (Burbidge et al., 1999; Sagi et al., 2002; Galpaz et al., 2008; Harrison et al., 2011). It is well known that the expression of many ABA biosynthetic genes is induced by stresses to increase ABA production (Seo and Koshiba, 2002). For example, ABA1/ZEP, NCEDs, AOs, and ABA3/MoCOS are up-regulated by water stress, salt, and high temperature in Arabidopsis (Arabidopsis thaliana) (Xiong et al., 2001b, 2002; Barrero et al., 2006; Toh et al., 2008; Frey et al., 2012). Likewise, NOT/LeNCED1 and HP-3/ZEP are induced by drought in tomato leaves and roots (Thompson et al., 2000). ABA biosynthesis is also developmentally regulated, especially during seed maturation and germination (Xiong and Zhu, 2003). In tomato, pollination triggers down-regulated NOT/LeNCED1 expression in ovary during fruit set, which is likely responsible for the rapid decline in ABA levels after anthesis (Vriezen et al., 2008; Nitsch et al., 2009). Later during fruit development, ABA production is gradually elevated to its maximal level when ripening occurs and then decreases afterward (Gillaspy et al., 1993; Buta and Spaulding, 1994; McAtee et al., 2013).
Despite such insights, the molecular mechanisms whereby the transcription of ABA biosynthetic genes is regulated, especially during plant development, are not well understood. Under stress conditions, the Arabidopsis gene SOMNUS promotes ABA biosynthesis by enhancing ABA1, NCED6, and NCED9 expression (Kim et al., 2008). SUPERSENSITIVE TO ABSCISIC ACID AND DROUGHT1 also is required for ABA production through modulating ABA3 and Arabidopsis ALDEHYDE OXIDASE3 (AAO3) expression (Xiong et al., 2001a). The exosome subunit Ribonuclease-PH domain subunit RRP41L controls mRNA decay of the ABA biosynthetic genes NCED3, NCED5, NCED6, and NCED9 (Yang et al., 2013). Overexpression of the salt-inducible RING-H2 zinc finger gene XERICO increases NCED3 expression and produces more cellular ABA in Arabidopsis (Ko et al., 2006). The C2H2 zinc finger gene INDETERMINATE DOMAIN1/ENHYDROUS negatively regulates ABA biosynthesis in Arabidopsis during seed development, likely through a DELLA-mediated pathway (Feurtado et al., 2011). Nevertheless, there are only several transcription factors identified to directly target ABA biosynthetic genes. The rice (Oryza sativa) APETALA2 (AP2)-like gene OsAP2-39 targets OsNECD1, and the Arabidopsis NECD3 is the direct target of the WRKY transcription factor ACQUIRED DROUGHT TOLERANCE and the NAC (for no apical meristem [NAM], Arabidopsis transcription activation factor [ATAF], and cup-shaped cotyledon [CUC]) family member ATAF1 (Yaish et al., 2010; Jiang et al., 2012; Jensen et al., 2013). Recently, two more transcription factors, the AP2/ETHYLENE RESPONSE FACTOR (ERF) family member dehydration-responsive element-binding protein 2C and the NAC-like gene ACTIVATED BY AP3/PI, have been shown to directly activate NCED9 and AAO3 expression in Arabidopsis during seed germination and leaf senescence (Je et al., 2014; Yang et al., 2014).
Ripening of climacteric fruits, such as tomato, is regulated mainly by the ethylene pathway but also by several transcription factors acting upstream (Klee and Giovannoni, 2011; Seymour et al., 2013). The MADS box gene RIPENING INHIBITOR (RIN) in the AP1/FRUITFULL (FUL) subfamily controls the early phase of ripening via both ethylene-dependent and independent mechanisms (Vrebalov et al., 2002). RIN induces ethylene production through transcriptional regulation on two developmentally controlled 1-amino-1-carboxylic acid (ACC) synthases, LeACS1A and LeACS4; it also regulates the expression of LeACS2 and two ACC oxidases, LeACO1 and LeACO3, that are mainly responsible for ethylene production during ripening (Barry et al., 2000). The known ripening regulators acting upstream of the ethylene pathway also include the NAC transcription factor NONRIPENING (NOR), the SQUAMOSA PROMOTER BINDING box gene COLORLESS NON-RIPENING (CNR), and an AP2/ERF member, AP2a, in addition to several other MADS box genes, such as TDR4/SlFUL1, SlFUL2, TOMATO AGAMOUS-LIKE1 (TAGL1), and TOMATO AGAMOUS1 (TAG1; Vrebalov et al., 2002, 2009; Manning et al., 2006; Itkin et al., 2009; Chung et al., 2010; Karlova et al., 2011; Bemer et al., 2012). In addition, LeMADS1 (also named SlMADS1) has been shown to weaken RIN action during fruit ripening; down-regulation of its expression in tomato elevated ethylene production (Dong et al., 2013). An epimutation in the CNR promoter inhibits fruit ripening, likely through AP2a-mediated negative regulation of ethylene biosynthesis and signaling (Manning et al., 2006; Karlova et al., 2011). Recently, at least 241 direct RIN targets, including CNR, NOR, and TDR4/SlFUL1, have been identified (Martel et al., 2011; Fujisawa et al., 2013). Besides ethylene and the above-mentioned regulators, ABA also is implicated to play a role in the fruit ripening of tomato and other species, including nonclimacteric fruit crops such as strawberry (Fragaria ananassa) and grape (Vitis vinifera; Jia et al., 2011; Seymour et al., 2013), although the mechanism remains unclear. In tomato, repression of LeNCED1 decreases the expression of several genes encoding ripening-associated cell wall enzymes and delays fruit ripening, suggesting that ABA promotes ripening (Sun et al., 2012b).
C2H2 zinc finger proteins (ZFPs) containing one or more zinc finger motifs constitute a large gene family, and members in this family are transcription factors involved in the transcriptional regulation of diverse biological processes (Englbrecht et al., 2004). Several ZFPs with a single C2H2 zinc finger motif in the C1-1i subfamily regulate flowering time, trichome development, and floral organ formation. For example, SUPERMAN regulates Arabidopsis stamen development (Sakai et al., 1995) and LATE FLOWERING acts as a floral repressor (Weingartner et al., 2011). Arabidopsis trichome development requires several single finger ZFPs, including GLABROUS INFLORESCENCE STEMS (GIS), GIS2, AtZFP5, AtZFP6, and AtZFP8; they likely act on the GA pathway (Gan et al., 2006, 2007; Zhou et al., 2011, 2013). Overexpression in Arabidopsis of the senescence-induced zinc finger gene AtZFP2 leads to delayed floral organ abscission (Cai and Lashbrook, 2008). Some two-fingered ZFPs in the C1-2i subfamily are known for their roles in stress responses. For example, SALT TOLERANCE ZINC FINGER/ZINC FINGER OF ARABIDOPSIS10 (STZ/SAT10) responds to salt, drought, cold, and ABA treatments, and its high expression enhances drought tolerance in Arabidopsis (Sakamoto et al., 2004). Like STZ/SAT10, the expression of ARABIDOPSIS ZINC FINGER PROTEIN1 (AZF1) and AZF2 also is induced by different stresses and ABA (Sakamoto et al., 2000). Overexpression of AZF1 and AZF2 represses a subset of genes regulated by osmotic stress and ABA and also several auxin-responsive genes, indicating that the two genes function as transcriptional repressors to inhibit plant growth under stress conditions (Kodaira et al., 2011). Although many ZFP transcription factors have been identified, DNA-binding sequences have been identified for only a few of them. It has been shown that AZFs and STZ bind to the sequences containing the A(G/C)T motif (Sakamoto et al., 2004), whereas DROUGHT AND SALT TOLERANCE from rice recognizes a cis-element containing TGCTANNATTG found in the promoters of PEROXIDASE24 PRECURSOR and the glutathione S-transferase OsGSTU2 (Huang et al., 2009).
There are at least 116 C2H2 zinc finger transcription factors in tomato (Tomato Genome Consortium, 2012), but few have been characterized molecularly. In this study, we characterized the role of the single zinc finger gene SlZFP2 in plant development. Using a reverse genetics approach, we show that SlZFP2 negatively regulates fruit ripening and also plays important roles in seed development and seed germination. Furthermore, through biochemical analysis and transcriptome profiling by RNA sequencing (RNA-seq), we identified the (A/T)(G/C)TT motif as the core binding site of SlZFP2 and at least 199 genes as its direct targets during early fruit growth. Our results demonstrate that SlZFP2 negatively regulates ABA biosynthesis through the direct suppression of several ABA biosynthetic genes and delays ripening through the down-regulation of the ripening regulator CNR.
RESULTS
SlZFP2 Is Expressed Mainly in Developing Fruits
Previous microarray analysis of tomato flowers and early-developing fruits revealed that a subset of transcription factors was preferentially expressed in developing fruits at 5 d post anthesis (DPA; Xiao et al., 2009). One of these was the transcription factor SlZFP2, named for its high similarity with the Arabidopsis zinc finger protein AtZFP2 (Supplemental Fig. S1). Consistent with the microarray results, semiquantitative reverse transcription (RT) and quantitative reverse transcription (qRT)-PCR analysis showed that SlZFP2 was expressed mainly from anthesis to fruit ripening; no or very limited expression was detected in vegetative tissues of tomato ‘M82’ and its wild relative Solanum pimpinellifolium LA1589 (Supplemental Fig. S2, A–D). Publicly available RNA-seq data also confirmed high SlZFP2 expression in fruits during fruit growth and ripening and further revealed its very low expression in roots, cotyledons, young leaves, and vegetative shoots (Supplemental Fig. S2, E and F).
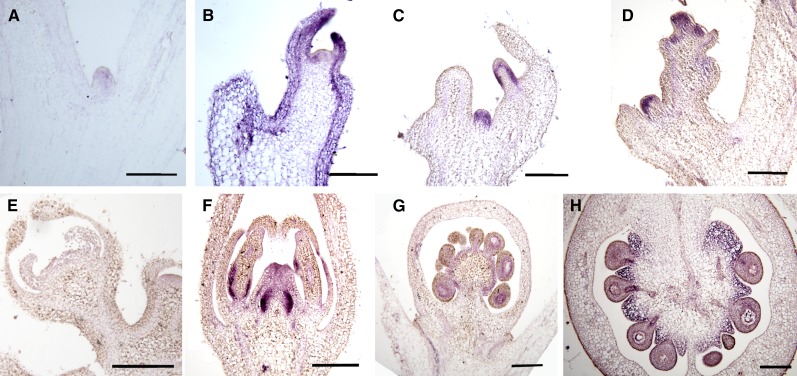
As shown in Figure 1, in situ hybridization analysis conducted on LA1589 revealed that, during vegetative growth, SlZFP2 was weakly expressed in shoot meristem but highly expressed in axillary buds and young leaves. Later during reproductive development, SlZFP2 expression was detected in floral meristems, ovule primordia and anthers, and particularly in ovules/seeds and their connective placental regions of anthesis flowers and 5-DPA fruits. This expression pattern suggests that SlZFP2 may play a role in bud growth and fruit development.
Figure 1.
SlZFP2 is expressed in both vegetative and reproductive tissues. In situ hybridization using sense (A and E) and antisense (B–D and F–H) probes of SlZFP2 was performed on apical meristems and young leaves (B), axillary buds (A and C), floral meristems (D), flower buds (E and F), ovaries at anthesis (G), and developing fruits (H) at 5 DPA from LA1589 plants. Bars = 200 μm.
SlZFP2 Regulates Fruit Ripening and Seed Development
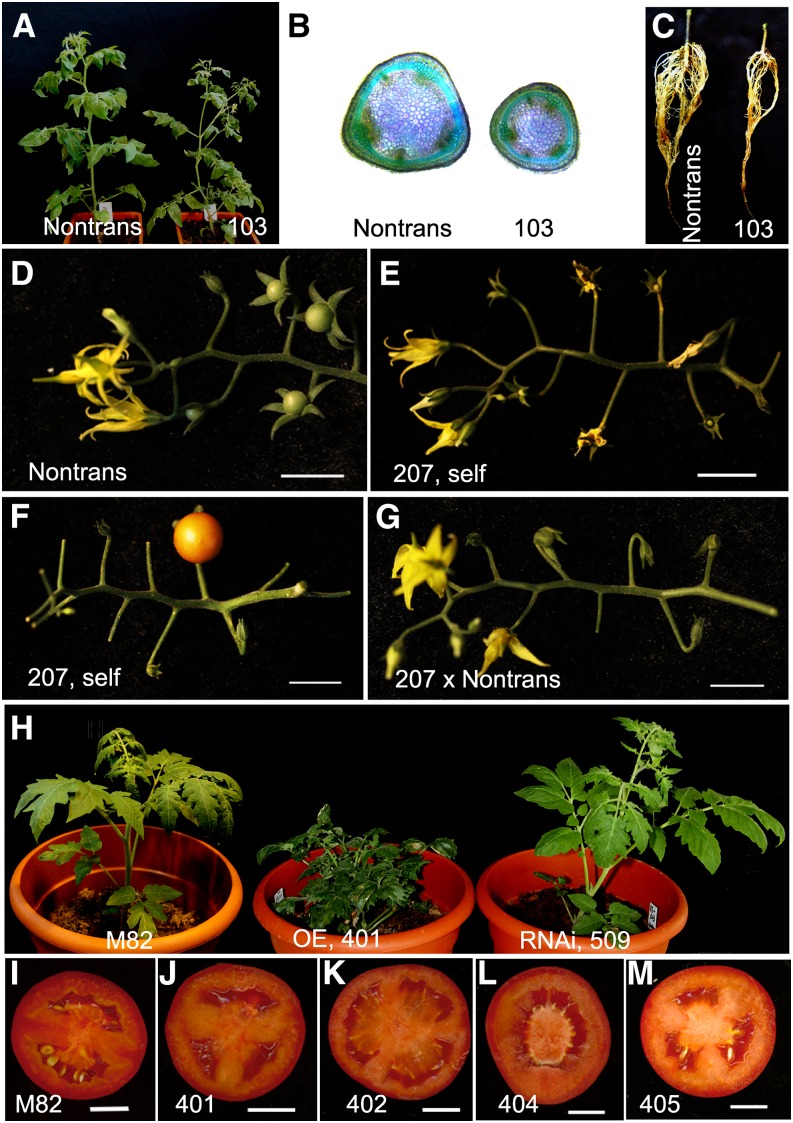
To investigate the role of SlZFP2 in fruit development, we generated its overexpression and RNA interference (RNAi) lines in cv M82 and LA1589. Compared with their corresponding nontransgenic plants, overexpression of the SlZFP2 coding sequence fused to the epitope tag hemagglutinin (HA-SlZFP2) driven by the cauliflower mosaic virus 35S promoter in cv M82 and LA1589 led to increased branching, shorter plants, and early flowering (Fig. 2, A and H). We also found that the HA-SlZFP2 overexpression lines from LA1589 had thinner stems and fewer roots (Fig. 2, B and C), while the SlZFP2 RNAi lines of LA1589 and cv M82 grew normally during the vegetative phase. However, the RNAi lines of LA1589 barely set fruits when pollinated with their own pollen, while those from cv M82 set fruits normally (Fig. 2, D–F). The problematic fruit set in these RNAi lines of LA1589 was improved by pollination with nontransgenic pollen (Fig. 2G). During fruit development, fruit weight and seed number were apparently not affected in the HA-SlZFP2 overexpression lines of LA1589, although some lines produced smaller fruits, but their seed weights were consistently reduced (Supplemental Fig. S3). More severely, overexpression of HA-SlZFP2 in cv M82 produced fruits with very few and even no seeds (Fig. 2, I–M). Thus, overexpression of HA-SlZFP2 affects vegetative growth and seed weight, whereas down-regulation of SlZFP2 by RNAi impacts fruit set.
Figure 2.
Phenotypes of SlZFP2 overexpression and RNAi lines. Representative transgenic lines from LA1589 (A–G) and cv M82 (H–M) show multiple phenotypes during vegetative growth and fruit development. Overexpression of HA-SlZFP2 in both LA1589 (A–C) and cv M82 (F and G) displayed increased branching, shorter plant stature, and early flowering (A and H) and also led to thinner stems (B) and few roots (C). Overexpression of HA-SlZFP2 in cv M82 severely affected seed development (I–M), whereas the SlZFP2 RNAi line of LA1589 showed severely affected fruit set (E and F) compared with its nontransgenic sibling (D). Fruit set in the RNAi line was rescued by pollination with nontransgenic pollen (G). The fruit images of cv M82 overexpression lines (J–M) were from primary (T0) transgenic plants; others were taken from T2 plants. Nontrans, Nontransgenic siblings; OE, overexpression line. Bars = 1 cm.
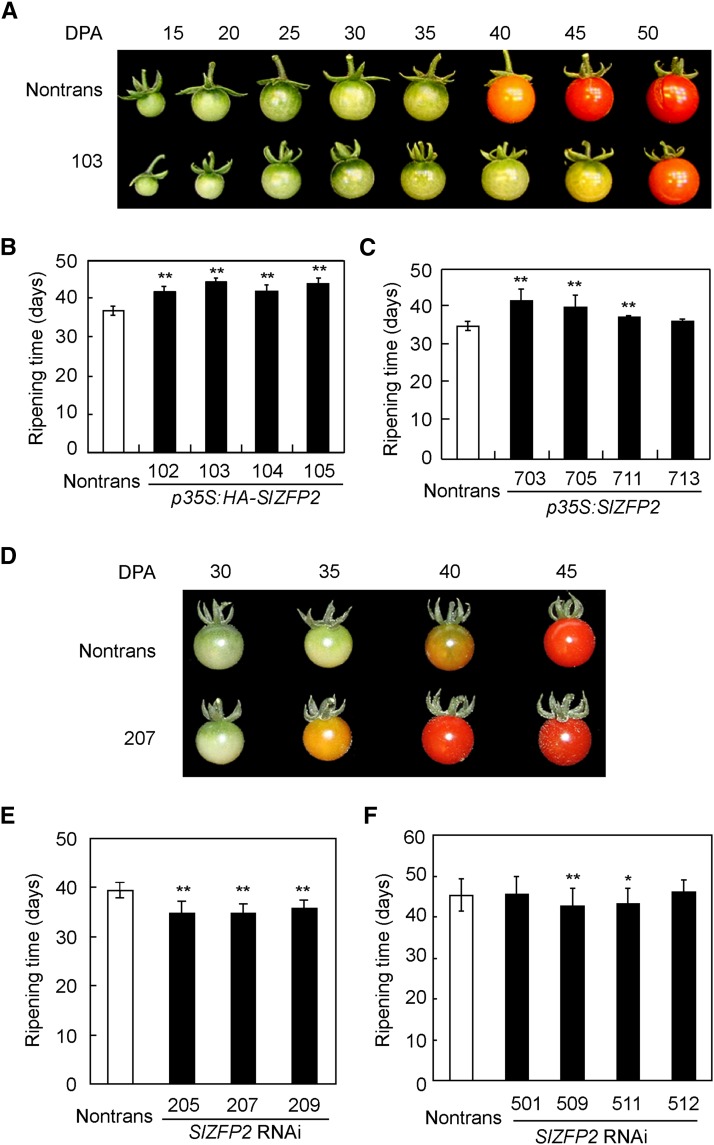
Given its high expression at later stages of fruit development, we investigated whether SlZFP2 plays a role in the regulation of fruit ripening. Indeed, fruit ripening was significantly delayed by 5 to 7 d in four homozygous HA-SlZFP2 overexpression lines of LA1589 compared with their nontransgenic siblings (Fig. 3, A and B). To determine whether the delay could be attributed to SlZFP2 expression, we further generated and analyzed transgenic lines of LA1589 constitutively expressed SlZFP2 alone under the control of the 35S promoter (p35S:SlZFP2 lines). We observed that the p35S:SlZFP2 fruits also required more days from anthesis to the turning stage, confirming that high SlZFP2 expression delays ripening (Fig. 3C). Due to the defective seed development of the HA-SlZFP2 overexpression lines from cv M82, their fruit ripening was not recorded. Although we failed to obtain homozygous plants for all SlZFP2 RNAi lines from LA1589, fruit ripening was accelerated in the heterozygous plants of the three lines investigated (Fig. 3, D and E). In addition, fruit ripening was significantly shortened in two of four cv M82 homozygous RNAi lines (Fig. 3F). Taken together, these results suggest that high SlZFP2 expression delays fruit ripening, whereas down-regulation of its expression promotes ripening.
Figure 3.
Fruit ripening of SlZFP2 overexpression and RNAi lines. A, Fruit ripening process of a representative HA-SlZFP2 overexpression line (103) compared with its nontransgenic sibling (Nontrans). B, Quantification of fruit ripening by days to reach the turning stage in four HA-SlZFP2 overexpression lines of LA1589. Fifteen to 50 fruits per plant from a total of three to five plants for each line were used to quantify ripening time. C, Quantification of fruit ripening in four SlZFP2 overexpression lines of LA1589. D, Fruit ripening of a representative SlZFP2 RNAi line (207) compared with its nontransgenic sibling. E, Quantification of fruit ripening in three SlZFP2 RNAi lines of LA1589. F, Quantification of fruit ripening in four SlZFP2 RNAi lines of cv M82. For cv M82 transgenic lines, five to 10 fruits were assessed. Statistical significance was based on Student’s t test: *, P < 0.05; and **, P < 0.01. Data are means ± sd; n = 45 to 100.
SlZFP2 Regulates Fruit ABA Production and Seed Germination
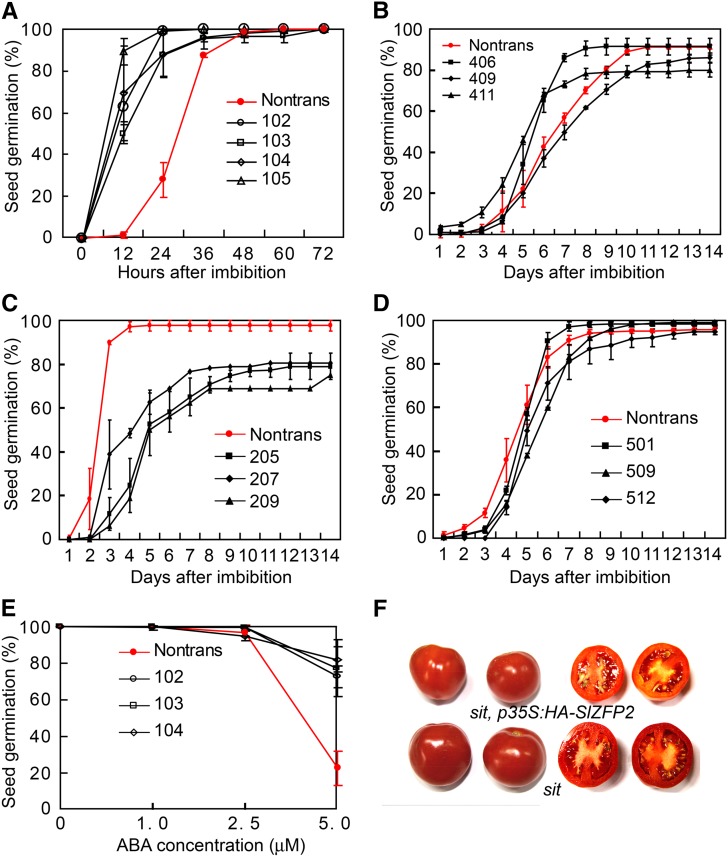
Since overexpression of SlZFP2 affected seed development, we further tested whether seed germination was impacted in SlZFP2 overexpression and RNAi lines. Freshly harvested seeds, and dry seeds stored for 5 d and 1 month under room temperature from four HA-SlZFP2 overexpression lines, germinated 12 to 24 h earlier than their nontransgenic siblings (Fig. 4A; Supplemental Fig. S4, A and B). Similarly, three of four LA1589 lines overexpressing SlZFP2 alone also had faster seed germination (Supplemental Fig. S4C). In addition, two of three HA-SlZFP2 overexpression lines of cv M82 showed early seed germination (Fig. 4B). In contrast, seeds from all SlZFP2 RNAi lines except one cv M82 line germinated slower (Fig. 4, C and D). For those SlZFP2 RNAi lines of LA1589, only 70% to 80% of seeds germinated after 2 weeks, contrasting with the nearly 100% germination rate of their nontransgenic siblings. These results indicate that high SlZFP2 expression promotes seed germination, while down-regulation of its expression inhibits germination.
Figure 4.
SlZFP2 promotes seed germination. A, Seed germination of four HA-SlZFP2 overexpression lines of LA1589 and their nontransgenic siblings (Nontrans). Germination based on radicle emergence was determined on seeds harvested from plants grown in the same seasons. B, Seed germination of three HA-SlZFP2 overexpression lines from cv M82 and their nontransgenic siblings. C, Seed germination of three SlZFP2 RNAi lines from LA1589 and their nontransgenic siblings. D, Seed germination of three SlZFP2 RNAi lines from cv M82 and their nontransgenic siblings. E, ABA sensitivity of three HA-SlZFP2 overexpression lines from LA1589 during seed germination. Seeds harvested from the same batch of plants were germinated on one-half-strength Murashige and Skoog medium with ABA supplemented at different concentrations as indicated. F, Fruits of the sit mutant with or without the HA-SlZFP2 transgene. Except for RNAi seeds from line 209, for which fewer than 50 seeds were assayed, at least 150 seeds were tested in triplicate. Seeds used for germination assays were dried for 5 d at room temperature. Data are means ± sd.
To test whether the sensitivity of seed germination to ABA was affected in SlZFP2 overexpression lines, we germinated seeds from three LA1589 lines overexpressing HA-SlZFP2 and their nontransgenic siblings on one-half-strength Murashige and Skoog medium supplemented with 0, 1, 2.5, and 5 μm ABA. The seed germination of the HA-SlZFP2 overexpression lines and their nontransgenic siblings was not substantially impaired by 1 and 2.5 μm ABA, but 5 μm ABA reduced the germination rate of nontransgenic seeds to about 20%, whereas more than 70% of seeds of the three HA-SlZFP2 overexpression lines still germinated (Fig. 4E). When constitutively expressing HA-SlZFP2 in the ABA-deficient mutant sit, viviparous seeds within fruits were more frequently observed (Fig. 4F). This further suggests that ABA signaling can be impaired by overexpression of HA-SlZFP2. A defect in ABA signaling resulting from high SlZFP2 expression also was suggested by the observation that the leaf stomata of the HA-SlZFP2 overexpression lines opened wider and were less sensitive to 1 and 5 μm ABA (Supplemental Fig. S5). Thus, ABA biosynthesis and/or signaling can be inhibited by high SlZFP2 expression.
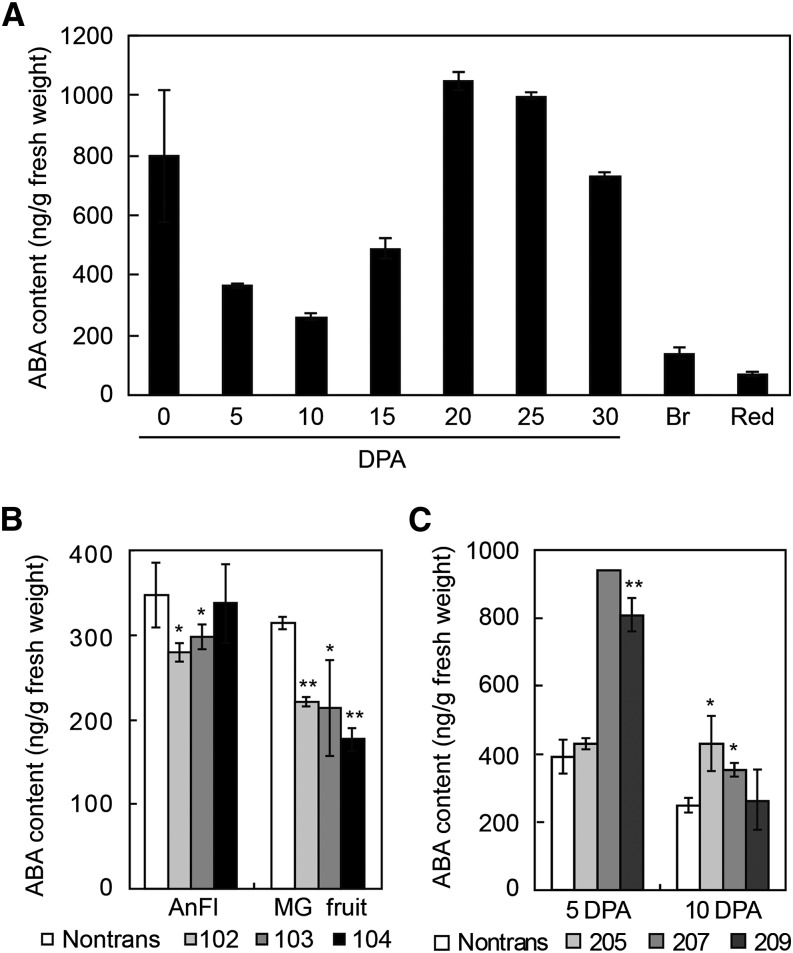
ABA levels in fruits change dynamically after anthesis (Buta and Spaulding, 1994). In agreement with their results, we also found that ABA production decreased after pollination and then increased after 10 DPA, reaching their maxima at 20 DPA, in both the small fruits of LA1589 and the relatively large ones of cv M82 (Fig. 5A; Supplemental Fig. S6). When overexpressing HA-SlZFP2 in LA1589, ABA levels in anthesis flowers and mature green (MG) fruits were lower, especially the latter, which contained only about two-thirds the amount of ABA in their nontransgenic siblings (Fig. 5B). Overexpression of HA-SlZFP2 also repressed ABA biosynthesis in leaves, especially in mature and old leaves (Supplemental Fig. S7A). If SlZFP2 is required for the suppression of ABA biosynthesis during fruit development, it would be expected that more ABA was accumulated in young fruits of its RNAi lines due to the released suppression. Indeed, down-regulation of SlZFP2 expression in LA1589 caused higher ABA levels to be accumulated in fruits at 5 and 10 DPA (Fig. 5C). These results confirm that SlZFP2 is required to repress ABA biosynthesis during early fruit growth.
Figure 5.
ABA levels in SlZFP2 overexpression and RNAi lines. A, Dynamic changes of ABA levels in the fruits of LA1589 after anthesis. Red, Red ripe. B, ABA levels in anthesis flowers (AnFl) and MG fruits from three HA-SlZFP2 overexpression lines and their nontransgenic siblings (Nontrans). C, ABA levels in the fruits at 5 and 10 DPA of three SlZFP2 RNAi lines and nontransgenic siblings. The three HA-SlZFP2 overexpression lines used for ABA measurements were homozygous T2 plants, while the three RNAi lines were T2 heterozygous plants. Three biological replicates were conducted, except for the measurement in the 5-DPA fruits of the RNAi line 207, which was done on the pooled samples from three replicates due to few fruits being obtained. Data represent means ± sd. Statistical significance was based on Student’s t test: *, P < 0.05; and **, P < 0.01.
Since ABA is derived from apocarotenoids, impairing ABA biosynthesis often affects fruit carotenoid accumulation, as shown in the fruits of the ABA-deficient mutant hp-3 (Galpaz et al., 2008). Similarly, fruits overexpressing HA-SlZFP2 also accumulated more β-carotene and lycopene, whereas SlZFP2 RNAi flowers contained less of these metabolites, further indicating a role of SlZFP2 in the regulation of the ABA biosynthesis pathway (Supplemental Table S1). Collectively, the results suggest that SlZFP2 negatively regulates ABA biosynthesis in tomato during fruit development.
SlZFP2 Negatively Regulates the Transcription of ABA Biosynthetic Genes
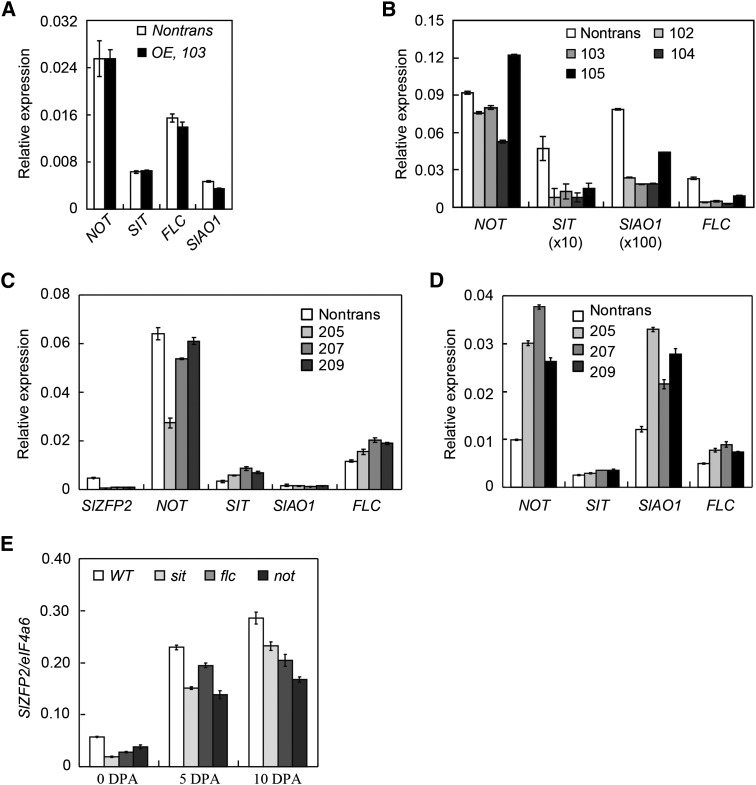
To understand the mechanism whereby SlZFP2 regulates ABA biosynthesis, we tested the possibility that it represses the transcription of ABA biosynthetic genes. Consistent with the ABA levels in nontransgenic leaves at different developmental stages, the ABA biosynthetic genes NOT, SIT, SlAO1, and FLC showed age-dependent expression patterns, as revealed by qRT-PCR; they all had higher expression in mature leaves (Supplemental Fig. S7B). In a representative HA-SlZFP2 overexpression line (line 103) of LA1589, these ABA biosynthetic genes were repressed in mature leaves to varying degrees (Supplemental Fig. S7B). Although in anthesis flowers, overexpression of HA-SlZFP2 did not affect the transcription of the ABA biosynthetic genes analyzed, SIT, FLC, and SlAO1 were substantially down-regulated in MG fruits from the four HA-SlZFP2 overexpression lines of LA1589 investigated (Fig. 6, A and B). NOT expression also was repressed in three of the four overexpression lines investigated.
Figure 6.
SlZFP2 regulates the expression of ABA biosynthetic genes during fruit development. A, Transcript levels of ABA biosynthetic genes in anthesis flowers of a representative HA-SlZFP2 overexpression line (103) and its nontransgenic sibling (Nontrans). B, Transcript levels of ABA biosynthetic genes in mature green fruits of four HA-SlZFP2 overexpression lines and their nontransgenic siblings. C, Transcript levels of ABA biosynthetic genes in anthesis flowers of three SlZFP2 RNAi lines and their nontransgenic siblings. D, Transcript levels of ABA biosynthetic genes in 2-DPA fruits of three SlZFP2 RNAi lines and their nontransgenic siblings. E, SlZFP2 expression in developing fruits of the ABA-deficient mutants not, sit, and flc. WT, Wild type. Data represent means ± sd; n = 3.
During fruit development, ABA is synthesized in both seeds and their surrounding parental tissues (Frey et al., 2004; Nambara and Marion-Poll, 2005). We then assessed the expression of the above-mentioned ABA biosynthetic genes in seeds extracted from fruits at MG, breaker (Br), and red ripe (B10, breaker plus 10 d) stages of the four HA-SlZFP2 overexpression lines in the LA1589 background. NOT and FLC were repressed in MG and Br seeds by overexpression of HA-SlZFP2 in LA1589, whereas SIT expression was elevated (Supplemental Fig. S8). Compared with their transcript levels in leaves, SIT and SlAO1 were either not expressed or expressed at very low levels in fruits and seeds at MG and later stages (Fig. 6B; Supplemental Fig. S8), suggesting that the two genes play minor roles in ABA production during the fruit-ripening process. Overall, the results indicate that overexpression of HA-SlZFP2 also represses the expression of ABA biosynthetic genes in MG or Br seeds. Thus, overexpression of HA-SlZFP2 can repress the transcription of several ABA biosynthetic genes during fruit development.
Since down-regulation of SlZFP2 expression in LA1589 led to more ABA accumulated in young fruits, we also investigated the transcript levels of these ABA biosynthetic genes in anthesis flowers and early developing fruits. SIT and FLC were slightly up-regulated in anthesis flowers of the three SlZFP2 RNAi lines investigated, but expression of NOT and SlAO1 was less affected (Fig. 6C). Furthermore, by analysis of their expression in 2-DPA fruits, we showed that all four ABA biosynthetic genes were up-regulated and that the transcript levels of NOT and SlAO1 were increased by more than 2-fold in the three RNAi lines compared with their nontransgenic siblings (Fig. 6D).
In addition, we found that SlZFP2 was positively regulated by ABA, because its expression was consistently lower in ovaries at anthesis and fruits at 5 and 10 DPA of the three ABA-deficient mutants not, sit, and flc compared with the wild type (Fig. 6E). This suggests that ABA activates SlZFP2 and the latter in turn represses ABA biosynthesis during fruit development.
SlZFP2 Regulates Fruit Ripening through CNR
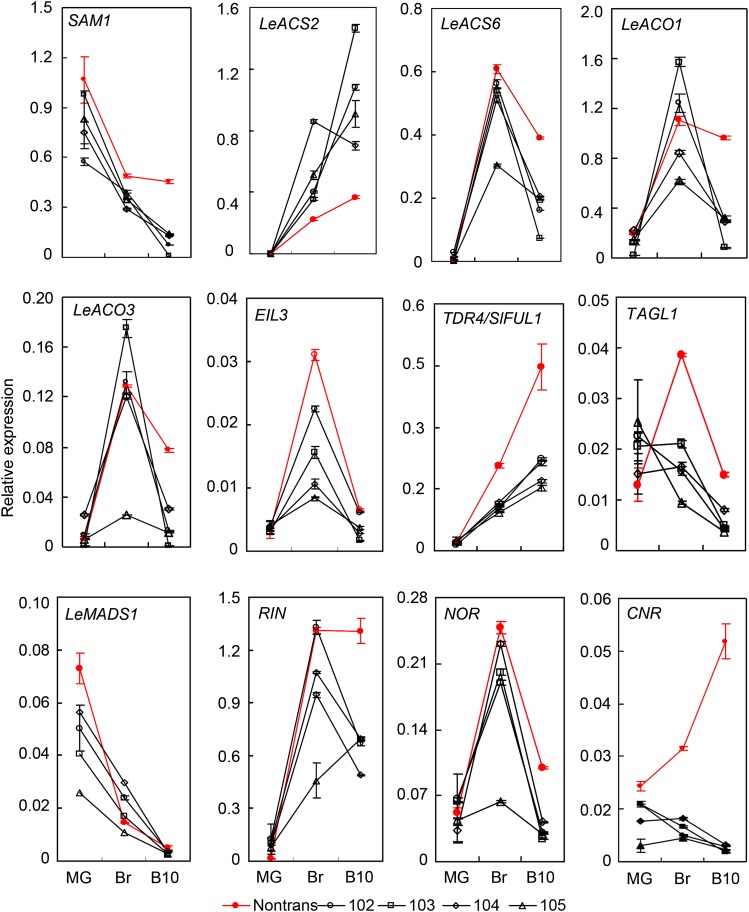
In addition to its role in seed germination, SlZFP2 also regulates fruit ripening (Fig. 3). To understand how this transcription factor regulates ripening, we analyzed the transcript levels of several ethylene biosynthetic genes and ripening regulators in SlZFP2 transgenic lines of LA1589 by qRT-PCR. Other than LeACS2, which was up-regulated in Br and B10 fruits, other ethylene biosynthetic genes, S-ADENOSYL-L-METHIONINE SYNTHETASE1 (SAM1), LeACS6, LeACO1, and LeACO3, were only down-regulated in B10 fruits by overexpression of HA-SlZFP2 (Fig. 7). Expression of the ethylene signaling component ETHYLENE INSENSITIVE3-LIKE3 (EIL3) was not affected. This indicates that SlZFP2 may not directly regulate ethylene production at the onset of ripening. Moreover, fruit ripening in tomato also is regulated by several transcription factors that act upstream of ethylene production (Vrebalov et al., 2002; Giovannoni, 2004; Itkin et al., 2009; Chung et al., 2010; Bemer et al., 2012; Seymour et al., 2013). Compared with their nontransgenic siblings, the ripening regulator CNR was consistently repressed in MG, Br, and B10 fruits by overexpression of HA-SlZFP2, whereas RIN and NOR were mainly repressed in B10 fruits (Fig. 7). In addition, TDR4/SlFUL1 and TAGL1 were down-regulated in Br and B10 fruits of the four HA-SlZFP2 overexpression lines. Recently, LeMADS1, which can form heterodimers in vitro with RIN, has been implicated to weaken the latter’s action on ripening regulation (Dong et al., 2013). However, LeMADS1 expression was only slightly down-regulated in MG fruits and not affected at the Br and B10 stages (Fig. 7).
Figure 7.
Transcript levels of fruit-ripening genes in the HA-SlZFP2 overexpression lines of LA1589. Total RNA was isolated from whole fruits at the MG, Br, and B10 stages of the HA-SlZFP2 overexpression lines and their nontransgenic siblings (Nontrans). Expression levels relative to tomato eukaryotic initiation factor 4A6 (SleIF4a6) were determined for each ripening-related gene by qRT-PCR in three technical replicates (three pooled samples from the same set of plants). Data are means ± sd; n = 3.
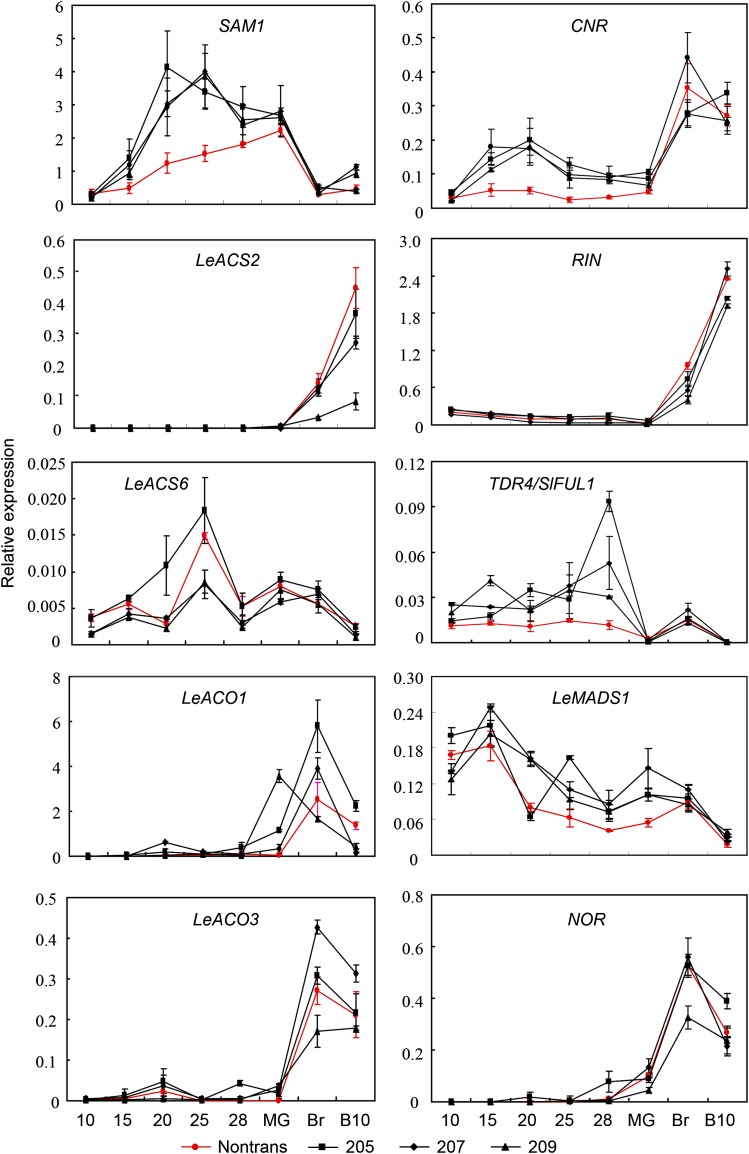
Because fruit ripening was shortened in SlZFP2 RNAi lines, we also monitored the transcription of the above-mentioned ripening genes in three RNAi lines from LA1589. In addition to the three ripening stages (MG, Br, and B10), more time points (10, 15, 20, 25, and 28 DPA) were included because of the high SlZFP2 expression throughout the entire fruit development and its repressive role on the expression of several fruit-ripening genes. For the ethylene biosynthetic genes, SAM1 expression was enhanced in the RNAi fruits from 15 DPA until the MG stage. Expression of the two ACC oxidase genes, LeACO1 and LeACO3, was increased in the MG fruits of the three SlZFP2 RNAi lines, but the two ACC synthase genes, LeACS2 and LeACS6, were not affected. However, the ripening regulators CNR and TDR4/SlFUL1 were activated much earlier; their expression started to increase as early as 15 DPA, in contrast to very low expression before the MG stage in their nontransgenic lines (Fig. 8). This indicates that SlZFP2 represses the expression of CNR and TDR4/SlFUL1 in developing fruits before entering the ripening process. In addition, decrease in LeMADS1 expression during fruit development was delayed in these RNAi fruits, whereas RIN and NOR were not impacted by the down-regulation of SlZFP2 expression. Taken together, these results suggest that SlZFP2 regulates fruit ripening through the modulation of CNR expression.
Figure 8.
Transcript levels of fruit-ripening genes in the SlZFP2 RNAi lines of LA1589. Total RNA was isolated from whole fruits at stages 10, 15, 20, 25, and 28 DPA, MG, Br, and B10 of three SlZFP2 RNAi lines and their nontransgenic siblings (Nontrans). Expression levels relative to SleIF4a6 were determined for each ripening-related gene by qRT-PCR in three technical replicates (three pooled samples from the same set of plants). Data are means ± sd; n = 3.
SlZFP2 Binds to (A/T)(G/C)TT-Containing Sequences
We investigated the subcellular localization of SlZFP2 to confirm its presence in the nucleus, using a transient expression assay in Nicotiana benthamiana leaves. Fluorescent signals were detected only in the nuclei of leaf epidermal cells transiently expressing an SlZFP2-YFP (for yellow fluorescent protein) fusion protein driven by the 35S promoter (Supplemental Fig. S9), indicating that SlZFP2 is likely a nucleus-localized protein. Then, two approaches were applied to identify the SlZFP2-binding sequences: the selected amplification and binding (SAAB) assay and a bacterial one-hybrid (B1H) screen. Following six rounds of selection, as part of the SAAB assay using a purified GST-SlZFP2 fusion protein expressed in Escherichia coli, together with a synthetic random 14-mer oligonucleotide library, we recovered 89 DNA fragments. Of these, 64 unique sequences were identified; all of these contained one to four AGTT/AACT repeats, while one-third contained one or two extra ACTT/AAGT and a few contained TCTT/AAGA or TGTT/AACA (Table I). As a complement to this approach, B1H screening resulted in the identification of 24 unique sequences from a total of 63 clones. Except for one clone that contained a partial TCTT/AAGA sequence, the others contained at least one of the four repeats identified by SAAB. For the 24 unique sequences, 14 contained one or two TCTT/AAGA repeats, and 10 and seven had AGTT/AACT and ACTT/AAGT, respectively (Table I). These results indicate that SlZFP2 binds in vitro to DNA sequences containing (A/T)(G/C)TT.
Table I. DNA-binding motifs of SlZFP2 identified by SAAB and B1H.
| Method | Frequency of DNA Repeats Found in Sequenced Clones |
|||||
|---|---|---|---|---|---|---|
| AGTT/AACT | TGTT/AACA | ACTT/AAGT | TCTT/AAGA | Total | No. of Clones | |
| SAABa | 161 | 2 | 34 | 2 | 199 | 64 |
| B1Hb | 24 (10) | 21 (7) | 3 (3) | 60 (14) | 108 (34) | 63 (24) |
All oligonucleotides enriched by SAAB contain at least two repeats of the four kinds of tetramer. bThe numbers of unique clones sequenced are indicated in parentheses.
To identify putative SlZFP2 targets in the tomato genome, we performed BLAST searches against a database made from 1.5-kb upstream sequences of the predicted tomato coding sequences retrieved from the Sol Genomics Network database (version SL2.4) using the 24 unique DNA sequences from the B1H assay as queries. DNA sequences from the SAAB screening were not included in the BLAST queries because all these sequences contain multiple (A/T)(G/C)TT repeats. After manual removal of sequences with mismatches on the (A/T)(G/C)TT core sequences, we identified 2,338 genes containing at least one binding site in their 1.5-kb promoter regions. Although CNR and the ABA biosynthetic genes NOT, SIT, FLC, and SlAO1 regulated by SlZFP2 are not in the list, by manual check we identified multiple (A/T)(G/C)TT motifs within their 1-kb promoter regions (Supplemental Fig. S10). In addition, SlZFP2-binding sites also were found in the SlAO2 promoter. Thus, there are at least 2,344 genes potentially targeted by SlZFP2 in the tomato genome.
Direct Target Genes of the Transcription Factor SlZFP2
We then applied RNA-seq to investigate how many of these putative target genes in the list were differentially expressed in 2-DPA fruits between a representative RNAi line from LA1589 (line 207) and its nontransgenic sibling. Reads from three biological replicates were mapped to the tomato genome sequences (version 2.4) by Tophat (Trapnell et al., 2009). Then, differentially expressed genes were picked by Cufflinks (Trapnell et al., 2010). In total, 2,722 differentially expressed genes (adjusted P < 0.05) were identified (Supplemental Table S2). A total of 193 out of these differentially expressed genes are in the list of putative SlZFP2 target genes identified by the above-mentioned BLAST search (Supplemental Table S3). Expression of the ABA biosynthetic gene SlAO2 was increased significantly in the 2-DPA fruits of the SlZFP2 RNAi line 207. Expression of other ABA biosynthetic genes, NOT, SIT, and SlAO1, also was increased by more than 2-fold in the SlZFP2 RNAi line 207, although not selected by Cufflinks (Supplemental Table S3). FLC did not show differential expression by RNA-seq, but its slightly elevated expression was independently detected by qRT-PCR in all three SlZFP2 RNAi lines of LA1589 (Fig. 6D). CNR expression was detected in 2-DPA fruits by RNA-seq and was increased slightly by the down-regulation of SlZFP2 expression. Therefore, there are at least 199 genes in total directly targeted by the transcription factor SlZFP2.
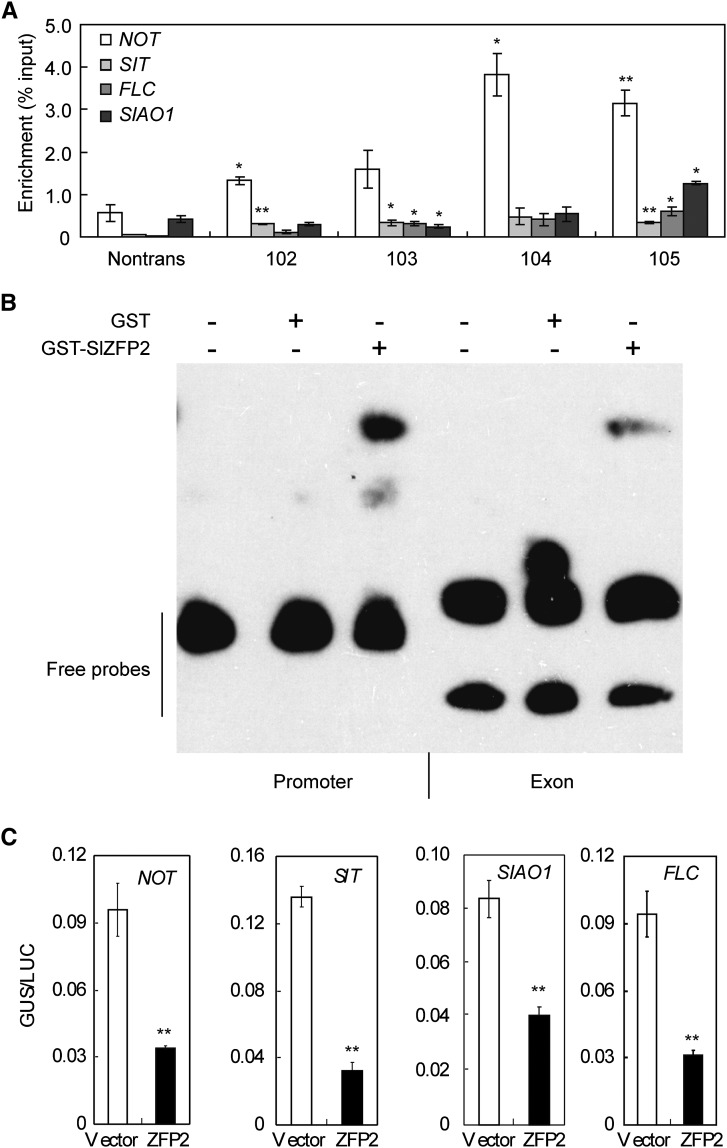
To verify the binding ability of SlZFP2 to the promoters of these ABA biosynthetic genes, we performed chromatin immunoprecipitation (ChIP) assays on four HA-SlZFP2 overexpression lines and their nontransgenic siblings. Quantitative PCR (qPCR) quantification of the precipitated chromatin DNA fragments by HA antibody revealed that the NOT, SIT, and FLC promoter regions containing multiple clusters of (A/T)(G/C)TT motifs were highly enriched in leaf samples from the four HA-SlZFP2 overexpression lines of LA1589 (Fig. 9A). There also was an obvious enrichment of the SlAO1 promoter in line 105. As verification of the ChIP-qPCR results, binding to the SlAO1 promoter was further confirmed by electrophoretic mobility shift assay (EMSA) using the GST-SlZFP2 fusion protein expressed in E. coli (Fig. 9B). Furthermore, using a transient expression assay with protoplasts isolated from Arabidopsis leaves, we demonstrated that SlZFP2 functions as a repressor to inhibit the expression of the ABA biosynthetic genes NOT, SIT, SlAO1, and FLC. The transient expression assay was conducted in three biological replicates, using the GUS reporter gene driven by each promoter (0.8–2 kb) of the four ABA biosynthetic genes as reporters and the p35S:SlZFP2 construct as an effector. The expression of the luciferase (LUC) reporter gene driven by the 35S promoter was used as an internal control. Compared with empty vector controls, cotransformation with the effector plasmid significantly decreased the GUS expression driven by the four gene promoters in protoplasts based on the GUS activities relative to LUC activity (Fig. 9C). We conclude that SlZFP2 represses the expression of ABA biosynthetic genes through direct binding to their promoters.
Figure 9.
SlZFP2 binds directly to the promoters of ABA biosynthetic genes. A, Promoter enrichment of ABA biosynthetic genes by ChIP assay. Enrichments relative to input were determined by qPCR in triplicate. Nontrans, Nontransgenic siblings. B, In vitro binding of GST-SlZFP2 to the SlAO1 promoter using EMSA. The probes were amplified by PCR from genomic DNA using the same sets of primers used for the ChIP assay. C, Transient expression analysis of SlZFP2 binding to the promoters of ABA biosynthetic genes. The GUS reporters were driven by the promoters of the ABA biosynthetic genes NOT (1,856 bp), SIT (1,699 bp), FLC (1,959 bp), and SlAO1 (838 bp). After cotransformation with the internal control p35:LUC and the effector p35:SlZFP2 or pUC118 (empty vector control), GUS activity normalized to LUC activity (GUS/LUC) of each reporter was compared between the effector (p35S:SlZFP2) and the empty vector control (vector). Statistical significance was based on Student’s t test: *, P < 0.05; and **, P < 0.01. Data are means ± sd; n = 3.
DISCUSSION
ABA facilitates plant adaptation to biotic and abiotic stresses and plays indispensable roles in many developmental processes. Accordingly, several ABA biosynthetic genes have been shown to be up-regulated transcriptionally under stress conditions (Nambara and Marion-Poll, 2005). Less is known about how ABA biosynthesis is regulated developmentally. Recently, ABA was implicated in fruit set and ripening in tomato (McAtee et al., 2013), but the underlying molecular mechanism remained elusive. In this study, we identified a tomato transcription factor, SlZFP2, that was up-regulated after anthesis. By analysis of transgenic lines constitutively expressing or suppressing SlZFP2, we demonstrated that SlZFP2 is responsible for the negative regulation of ABA biosynthesis during fruit development through direct binding to promoters of several ABA biosynthetic genes containing (A/T)(G/C)TT motifs. We also showed that SlZFP2 delays the onset of fruit ripening mainly through transcriptional repression of the ripening regulator CNR.
SlZFP2 Regulates Fruit and Seed Development in Two Tomato Genetic Backgrounds
Overall, altering SlZFP2 expression in LA1589 and cv M82 showed similar phenotypes; in both genetic backgrounds, overexpression lines showed increased branching, earlier flowering, and defective seed development, whereas down-regulation of its expression accelerated fruit ripening and inhibited seed germination. Variation in phenotypic severity was observed mainly on fruit set between RNAi lines from LA1589 and cv M82; the LA1589 lines set fruits poorly, whereas fruit set in the M82 lines was largely unaffected. Overexpression of HA-SlZFP2 in cv M82 had a more severe effect on seed development, in which no seed was formed in fruits of several lines. In contrast, when HA-SlZFP2 was overexpressed in LA1589, only the seed weight was affected. The difference in phenotypic severity is likely due to variation in endogenous hormone levels between the two genotypes, because fruit ABA accumulation after anthesis was slightly different between the current tomato LA1589 and cv M82; LA1589 had less ABA per fruit due to its smaller size (approximately 1 g per fruit for LA1589 compared with approximately 50–60 g per fruit for M82 in our growth conditions), and its ABA levels dropped faster after anthesis. It has been shown that auxin and ABA levels as well as their dynamic changes during fruit growth may vary even between cv Pik-red and cv Ailsa Craig (Buta and Spaulding, 1994). It is also likely caused by slightly different expression patterns of SlZFP2 in the two genetic backgrounds during flower and fruit development. SlZFP2 was not expressed in LA1589 flower buds; on the contrary, it was weakly expressed at that time in cv M82 (Supplemental Fig. S2, C and D). Another possibility we cannot rule out is the impact on plant growth by the self-pruning (sp) mutation in cv M82, although this is less likely. SP is involved in shoot sympodial development and also interacts with SINGLE FLOWER TRUSS to regulate the transition to flowering and yield heterosis in tomato (Pnueli et al., 1998; Krieger et al., 2010; Lifschitz et al., 2014). Because overexpression of SlZFP2 accelerates flowering, loss of SP activity may have a substantial effect on plant development also regulated by SlZFP2.
Fruit set in tomato is mainly regulated by an interplay between auxin, cytokinin, and GA (Gillaspy et al., 1993; Mariotti et al., 2011; McAtee et al., 2013). Accumulating data from tomato and other plant species has suggested that ethylene and ABA might play a role in fruit set, because their production decreases dramatically right after pollination (Vriezen et al., 2008; Nitsch et al., 2009; Pascual et al., 2009; Wang et al., 2009; Carbonell-Bejerano et al., 2011; Martínez et al., 2013). Our RNA-seq data suggested that the problematic fruit set in the SlZFP2 RNAi lines seems to result from the disturbed action of multiple hormones, because the expression of many genes involved in the biosynthesis and/or perception of auxin, cytokinin, GA, ABA, and ethylene was affected. In general, the action of auxin and GA, two major hormones that promote fruit set, was attenuated, while the action of ABA and ethylene was enhanced, which is required to be limited for successful fruit set (Supplemental Table S4). The majority of differentially expressed genes involved in ABA and ethylene pathways were activated by down-regulation of SlZFP2 expression, suggesting that the transcription factor functions as a repressor to inhibit the two pathways during early fruit development. The altered gene expression involved in auxin and GA pathways may explain the defective seed development observed in the SlZFP2 transgenic lines, at least in part.
Transcriptional Regulation of ABA Biosynthesis during Fruit Development by SlZFP2
The mechanisms by which stresses regulate the transcription of ABA biosynthetic genes are known to be highly complex (Seo and Koshiba, 2002). Our results provide several lines of evidence to show that SlZFP2 is a transcriptional repressor to limit ABA production during fruit development in tomato. First, SlZFP2 overexpression lines displayed typical ABA-deficient phenotypes of faster seed germination and altered carotenoid composition. The viviparity frequently observed in the ABA-deficient sit mutant constitutively expressing HA-SlZFP2 further suggests that ABA biosynthesis and/or signaling can be attenuated by high SlZFP2 expression. Consistently, SlZFP2 RNAi seeds germinated slower. Second, ABA production is negatively correlated to altered SlZFP2 expression: lower in MG fruits with high HA-SlZFP2 expression, while higher in 2-DPA fruits with down-regulated SlZFP2 expression. Third, the transcription of several ABA biosynthetic genes is regulated by SlZFP2 during fruit development.
Our analysis of ABA production and the expression of ABA biosynthetic genes in different tissues suggests that SlZFP2 mainly fine-tunes ABA biosynthesis during fruit development. Unlike the ABA-deficient mutants not, sit, and flc, both SlZFP2 overexpression and RNAi lines did not show a wilty phenotype under normal growth conditions, suggesting that SlZFP2 has minor roles, if any, in ABA biosynthesis during vegetative growth. This notion is further supported by the fact that high SlZFP2 expression represses ABA production mainly in mature leaves but not in young leaves. Furthermore, the MG fruits of the HA-SlZFP2 overexpression lines, in which ABA production was most affected, still contained two-thirds the amount of ABA of the wild type (their nontransgenic siblings).
Furthermore, it has been shown that AtZFP2 is induced by ABA in Arabidopsis seedlings using massive parallel sequencing, although no phenotypes in seedlings have been described (Hoth et al., 2002). We also found that SlZFP2 was down-regulated in anthesis flowers and young fruits of the three ABA-deficient mutants, not, sit, and flc, suggesting that SlZFP2 is involved in the feedback regulation of ABA biosynthesis during fruit development.
SlZFP2 Negatively Regulates Fruit Ripening through Modulating CNR Transcription
Fruit ripening in tomato is known to be influenced by transcription factors acting upstream of ethylene production that constitute a complex regulatory network operating through both ethylene-dependent and independent pathways. Among them, RIN is a master regulator that is required for activation of the developmentally controlled transcription of LeACS1A and LeACS4, which in turn contributes to the initiation of ripening-related ethylene production (Barry et al., 2000). RIN can form heterodimers with TAGL1 and TAG1 and binds to the cis-elements of NOR and TDR4/SlFUL1, depending on CNR activity (Martel et al., 2011; Fujisawa et al., 2013). Because the rin mutation did not abolish CNR expression during fruit ripening (Martel et al., 2011), there may be different regulatory mechanisms controlling CNR and RIN transcription. Our phenotypic and transcriptional analysis demonstrated that SlZFP2 is a regulatory component of fruit ripening in tomato through the regulation of CNR expression, independent of RIN. This notion is supported by the fact that CNR expression was dramatically repressed by the overexpression of HA-SlZFP2 from MG to ripe and was activated much earlier in the fruits of the SlZFP2 RNAi lines. Furthermore, TDR4/SlFUL1, acting downstream of CNR, showed a similar expression pattern to CNR in both the SlZFP2 overexpression and RNAi lines. RIN and its direct target genes NOR and TAGL1 were only down-regulated in ripe fruits of the HA-SlZFP2 overexpression lines but were not affected by the down-regulation of SlZFP2 (Figs. 7 and 8), suggesting that SlZFP2 acts independently of RIN.
Because expression of the ethylene biosynthetic genes LeACS6, LeACO1, and LeACO3 was not affected before the ripe stage by altered SlZFP2 expression, SlZFP2 may not directly regulate ethylene production during the ripening process. The repressed expression of the ethylene-related genes LeACS6, LeACO1, LeACO3, and EIL3 in ripe fruits by the overexpression of HA-SlZFP2 may be explained by the down-regulated RIN or CNR transcription at this stage. However, the reason for the observed increase in LeACS2 expression in the HA-SlZFP2 overexpression lines at the Br and ripe stages remains to be determined. One possibility is that the autocatalytic ethylene biosynthesis was less affected by high SlZFP2 expression, because, unlike the greenish fruits produced by rin, cnr, and nor mutants (Vrebalov et al., 2002; Manning et al., 2006; Giovannoni, 2007), SlZFP2 overexpression and RNAi lines produced morphologically red ripe fruits (Fig. 3). Thus, SlZFP2 also function as a transcription repressor to delay the onset of ripening.
Several recent studies indicate that ABA promotes fruit ripening in tomato, strawberry, and other species (Böttcher et al., 2010; Jia et al., 2011; Sun et al., 2012b). Our results support this hypothesis, because suppression of ABA biosynthesis as a consequence of elevated levels of SlZFP2 expression results in delayed ripening. However, the mechanism by which ABA regulates ripening remains unclear. One possibility is that ABA promotes ethylene production during fruit ripening, an idea that is supported by the observation that exogenous application of ABA to tomato MG fruits resulted in increased LeACO1 and LeACS2 expression (Zhang et al., 2009; Sun et al., 2012a). Interestingly, silencing of SlNECD1 caused the down-regulation of several genes involved in ripening-associated cell metabolism, although higher ethylene production was observed in Br and later fruits (Sun et al., 2012b), suggesting that ABA-regulated ripening may be ethylene independent. It has been shown that ABA also functions as a ripening promoter in the nonclimacteric fruit strawberry, where ethylene is not the primary factor triggering ripening (Jia et al., 2011). In our study, except for LeACS2, other ethylene biosynthetic genes were not affected at the Br stage in these ABA-deficient lines when overexpressing HA-SlZFP2. Therefore, we hypothesize that ripening regulation by ABA in tomato might be perceived through an ethylene-independent pathway that is mediated by SlZFP2. Further investigation of the molecular mechanisms underlying the regulation of CNR transcription by SlZFP2 and ABA signaling may shed light on the role of ABA in ripening regulation in tomato and other fruits.
Direct Target Genes of SlZFP2
SlZFP2 belongs to the TFIIIA-type C2H2 zinc finger protein family, where DNA binding sequences have been identified for a few members. The stress- and ABA-inducible proteins STZ and AZFs bind to A(G/C)T repeats in vitro (Sakamoto et al., 2004), and this element has been found in promoters of AZF1- and AZF2-regulated genes (Kodaira et al., 2011). Using a SAAB assay and B1H screening, we identified the (A/T)(G/C)TT motif as the core SlZFP2-binding sequence. Together with transcriptome analysis, we prove that NOT, SlAO1, SIT, and FLC are direct targets of SlZFP2 based on the fact that multiple (A/T)(G/C)TT repeats are present in the promoters of the four ABA biosynthetic genes and the confirmed binding ability in vivo and in vitro. SlAO2 also is likely the direct target of SlZFP2, because its promoter contains the core (A/T)(G/C)TT-binding motifs and its expression was regulated by this transcription factor. Further supportive evidence comes from the similar expression patterns between SlZFP2 and the ABA biosynthetic genes NOT, SIT, and FLC in 5-DPA fruits by in situ hybridization; they all were expressed in the funiculus and placenta regions (Supplemental Fig. S11). Moreover, SlZFP2 shares a similar expression pattern with the tomato ABA 8′-hydroxylase gene SlCYP707A1, which is responsible for the reduction of ABA levels in pollinated ovary and is predominantly expressed in the ovules and placenta of anthesis flowers (Nitsch et al., 2009). Arabidopsis NCED3, ABA2, and AAO3 have been shown to be expressed in the vascular systems of roots, leaves, stems, and inflorescences (Koiwai et al., 2004; Endo et al., 2008). Although the detailed expression patterns in fruits have not yet been determined for most ABA biosynthetic genes, the Arabidopsis ABA2 gene is primarily expressed in the funiculus and placenta of silique (Cheng et al., 2002). Thus, SlZFP2 is expressed in regions with high ABA levels, so we reason that NOT, SlAO1, SIT, and FLC are direct targets of SlZFP2 during fruit development.
Other than SlZFP2, several transcription factors have been shown to directly target the ABA biosynthetic genes NCEDs and AAO3 in rice and Arabidopsis, respectively (Yaish et al., 2010; Jiang et al., 2012; Jensen et al., 2013; Je et al., 2014; Yang et al., 2014). Recently, a tomato C1-2i member of the C2H2-type zinc finger proteins SlZF2 also was shown to regulate ABA biosynthesis because of increased ABA production in its overexpression lines (Hichri et al., 2014). But it remains to be determined whether SlZF2 directly targets any ABA biosynthetic genes. All the above-mentioned transcription factors are positive regulators of ABA biosynthesis. On the contrary, SlZFP2 functions as a repressor to fine-tune ABA production during fruit development. Thus, SlZFP2 represents a new player in the regulation of ABA biosynthesis.
In addition to the above-mentioned ABA biosynthetic genes, the CNR promoter also contains SlZFP2-binding sites, and its expression is regulated by this transcription factor. Therefore, CNR is very likely the direct target gene of SlZFP2 to control fruit ripening. In addition to the above-mentioned six genes, we identified more than 2,000 genes containing SlZFP2-binding sequences in their 1.5-kb promoters, and among them, the transcription of 193 genes was differentially expressed in 2-DPA fruits of a representative SlZFP2 RNAi line (line 207) and its nontransgenic sibling; they are likely the direct target genes of SlZFP2 at the early fruit growth stage (Supplemental Table S3). Notably, more than half of the 193 target genes were repressed in 2-DPA fruits by down-regulation of SlZFP2 expression, and many are putatively involved in several cellular processes, including chlorophyll and heme synthesis by chlorophyllase2 (Solyc12g005300), protochlorophyllide reductase (Solyc12g013710), and porphobilinogen deasminase (Solyc07g066470). This indicates that SlZFP2 not only functions as a repressor to inhibit ABA biosynthesis and fruit ripening but also as a transcription activator or coactivator to promote gene expression involved in other aspects of plant development. It is possible that SlZFP2 targets genes involved in diverse pathways, a hypothesis that will require further investigation.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
The wild relative of tomato (Solanum lycopersicum), Solanum pimpinellifolium LA1589, the ABA-deficient mutants sit (LA0574), not (LA0617), and flc (LA0673), and cv Rheinland Ruhm (LA0535) were obtained from the Tomato Genetics Resource Center at the University of California. The tomato ‘M82’ was provided by Dr. Daniel Zamir at the Hebrew University of Jerusalem. Plants, including transgenic lines, were grown in phytotrons at 20°C to 25°C under a humidity of 70% to 80%, with illumination for 16 h daily by 150 mE m–2 s–1 light from metal halide and high-pressure sodium lamps. Plants were fertilized weekly with all-purpose fertilizer and watered as needed. For the ABA-deficient mutants, 50 μm ABA was foliar sprayed weekly until most of the fruits reached the ripe stage.
Generation of Transgenic Lines
SlZFP2 (Solyc07g006880, unigene Sol Genomics Network-U576689) was identified previously as a differentially expressed gene during floral and early fruit development (TC128959 on the microarray chips; Xiao et al., 2009). The SlZFP2 complementary DNA (cDNA) was isolated from LA1589 by RT-PCR using primers XP0034 and XP0036 and cloned into the pGEM T-Easy vector (Promega). Information on the primers used in this study is provided in Supplemental Table S5. To overexpress SlZFP2, the p35S:SlZFP2 and p35S:HA-SlZFP2 constructs were made by placing the full-length SlZFP2 cDNA with or without an HA tag fused between the cauliflower mosaic virus 35S promoter and the NOS terminator of the binary vector pHX20 derived from pZH01 (Xiao et al., 2003). The HA-SlZFP2 fusion was made by PCR-based manipulation with primers XP0035 and XP0036. The forward primer XP0035 contains a Kozak cassette followed by the HA coding sequence added in frame to the N terminus of SlZFP2. For construction of the SlZFP2 RNAi vector, the last 274 bp of the SlZFP2 coding sequence plus the 127-bp 3′ untranslated region were amplified using primers XP0028 and XP0029 and cloned into the binary vector pFGC5941 in both the sense and antisense directions (Kerschen et al., 2004). The plasmids were then introduced into the Agrobacterium tumefaciens strain GV3101. The p35S:HA-SlZFP2 and SlZFP2 RNAi constructs were used to transform LA1589 and cv M82, and p35S:SlZFP2 was transformed into LA1589, as described previously (McCormick, 1991).
Phenotypic Analysis of Transgenic Lines
All the analyses in this study were conducted on homozygous plants of overexpression and RNAi lines using their respective pooled nontransgenic siblings as controls, except RNAi lines of LA1589, for which heterozygous plants were used because of the failure to obtain their homozygous plants. For the selection of homozygous plants of the SlZFP2 overexpression lines, 50 to 100 5-d-old seedlings from individual T2 plants genotyped were tested for their resistance to 30 mg L–1 hygromycin B (Roche). Similarly, 50 to 100 2-week-old seedlings grown in phytotrons were sprayed with 0.02% (v/v) Basta to select homozygous RNAi plants (Shanghai Sangon), and the resistance was recorded when the nontransgenic seedlings were dead. For those segregating transgenic lines, transgenic plants were selected and verified by genotyping with transgene-specific primers: Hygromycin B resistance gene-specific primers XP0515 and XP0516 for overexpression lines and herbicide resistance gene-specific primers XP0517 and XP0518 for RNAi plants.
Leaf stomata sizes of the HA-SlZFP2 overexpression lines from LA1589 were measured and analyzed using ImageJ (http://rsbweb.nih.gov/ij/) based on scanning electron microscopy images taken from plastic replicas of abaxial leaf surfaces. Except for some RNAi lines with limited seeds available, seed germination based on radicle emergence was monitored at 25°C in 12-h intervals or daily by placing at least 150 seeds in three replicates on filter paper moistened with distilled water. For the ABA sensitivity assay, seed germination was performed on filter paper moistened with distilled water supplemented with 0, 1, 2.5, and 5 μm ABA. ABA was dissolved in absolute methanol, and the final concentration of methanol was 0.027% (v/v) in all ABA treatments and the mock solution.
Quantification of ABA Content
ABA extraction from leaves, flowers, and fruits was conducted as described previously (Pan et al., 2010). Briefly, samples were ground into a fine powder in liquid nitrogen. For each sample, 50 or 100 mg of ground tissue was extracted twice with 10 volumes of extraction buffer containing 2-propanol:water:concentrated HCl (2:1:0.002, v/v/v) for 30 min at 4°C, followed by two extractions with dichloromethane. After centrifugation, the chloroform phases containing ABA were combined and concentrated using a nitrogen evaporator. Pellets were redissolved in 100 μL of methanol. Before extraction, 100 ng of [2H6]ABA (Icon Isotopes; catalog no. A101-169-2) was added as an internal standard. The samples were then analyzed on an Agilent liquid chromatography-tandem mass spectrometry device (1200/6520) system equipped with a ZORBAX Eclipse XDB-C18 column as described previously (Yano et al., 2009). ABA levels were determined using the MassHunter qualitative software (Agilent; version B.03.01) based on responsive signals of the internal standard and the sample ABA.
Quantification of Carotenoids
Carotenoids were extracted from 500 mg of fresh fruits and analyzed by HPLC as described previously (Fu et al., 2012). HPLC analysis was carried out using a Waters Alliance 2695 system consisting of a 2695 module and a 2996 photodiode array detector, equipped with a 250- × 4.6-mm i.d., 5-μm, YMC reverse-phase C30 column and a 20- × 4.6-mm i.d., YMC C30 guard column.
RNA in Situ Hybridization
Shoot, flower buds, ovaries at anthesis, and 5-DPA fruits were collected from LA1589 plants. Tissue fixation, sectioning, and hybridization with digoxigenin-labeled sense and antisense probes were performed as described previously (Coen et al., 1990). For SlZFP2, a 401-bp fragment (the same region used for the RNAi construct) was used for the probe template. Probe templates of three ABA biosynthetic genes also were made by PCR amplification using the following primer sets: XP2247 and XP2248 (NOT; nucleotides 1,492–1,869), XP2249 and XP2250 (SIT; nucleotides 3,200–3,907), and XP2245 and XP2246 (FLC; nucleotides 2,324–2,805).
Real-Time qRT-PCR
Total RNA was extracted from various tomato tissues with Trizol reagent (Invitrogen) based on previously described methods (Xiao et al., 2009). Residual genomic DNA in the RNA samples was removed by RNase-free DNase according to the manufacturer’s protocol (New England Biolabs). One microgram of DNase-treated total RNA was used to synthesize first-strand cDNA using the First Strand cDNA Synthesis Kit (Thermo Fisher Scientific), and real-time RT-PCR was performed with three technical replicates using SYBR Premix ExTaq (Takara Biotech) on an ABI Applied Biosystems StepOnePlus machine (Life Technologies). Transcript levels were calculated as relative expression to SleIF4α6 (Xiao et al., 2008).
Gene Expression Profiling by RNA-seq
High-throughput RNA-seq was used to quantify genome-wide gene expression regulated by SlZFP2. Total RNA was extracted by Trizol reagent (Invitrogen) from 2-DPA fruits of the RNAi line 207 and its nontransgenic sibling (207N) as described previously (Xiao et al., 2009). Paired-end sequencing libraries were created using the TruSeq stranded mRNA kit (RS-122-2101; Illumina) and sequenced on Illumina’s Miseq system using the 500-cycles Miseq reagent kit (MS-102-2003). The 250-bp paired-end reads were mapped to the tomato genome using the Tophat program version 2.0.12 (Trapnell et al., 2009). Three biological replicates were conducted. In total, the numbers of read pairs mapped for each replicate were as follows: 1,497,635 (207N, replicate 1; 80.7% mapped), 1,191,334 (207N, replicate 2; 80.6%), 1,279,658 (207N, replicate 3; 83.2%), 573,982 (207, replicate 1; 80.2%), 749,210 (207, replicate 2; 80.2%), and 1,158,542 (207, replicate 3; 80.5%). Then, differentially expressed genes (adjusted P of 0.05 or less) were identified by Cufflinks version 2.2.1 (Trapnell et al., 2010). A total of 2,722 differentially expressed genes were identified, and the data set (Supplemental Table S2) was further compared with the list of genes containing (A/T)(G/C)TT motifs in their promoters to select genes directly targeted by SlZFP2 at the early fruit growth stage (Supplemental Table S3). The raw reads and gene expression data have been deposited in the National Center for Biotechnology Information (accession no. GSE63838).
Identification of DNA-Binding Sequences Recognized by SlZFP2
Two approaches were used to identify DNA-binding sequences of SlZFP2: SAAB assay and B1H screening. SAAB was conducted essentially as described previously (Peng et al., 2002). Briefly, a 14-bp randomized oligonucleotide library was prepared by annealing two synthesized oligonucleotides, 5′-GGGAAGACGGATCCATTGCA-N14-CTGTAGGAATTCGGACCCT-3′ and 5′-AGGGTCCGAATTCCTACAG-3′, followed by primer extension at 72°C. The full-length SlZFP2 coding sequence was amplified by PCR using primers XP0662 and XP0684 and cloned into the pGEX-4T-3 vector. After transformation into Escherichia coli, the GST-SlZFP2 fusion protein was expressed and purified using Glutathione Sepharose 4B beads according to the manufacturer’s instructions (GE Healthcare Life Sciences). Two micrograms of purified GST-SlZFP2 protein bound to the beads was incubated on ice for 30 min with 0.8 ng of library DNA in a binding buffer containing 20 mm HEPES (pH 7.9), 100 mm KCl, 20 mm EDTA, 20 mm EGTA, 1% (v/v) Nonidet P-40, 1 mm benzamidine, 0.5 mm phenylmethanesulfonyl fluoride (PMSF), 0.5 mm dithiothreitol, 20% (v/v) glycerol, 50 ng mL−1 bovine serum albumin, and 10 ng μL−1 poly(dI∙dC). After three washes with the binding buffer without bovine serum albumin, bound DNA was recovered from beads by incubation at 45°C for 1 h with 50 mm Tris-HCl (pH 8), 5 mm EDTA, 100 mm sodium acetate, and 0.5% (w/v) SDS. Then, the selected DNA was amplified by PCR using the PCR primers 5′-GGGAAGACGGATCCATTGCA-3′ and 5′-AGGGTCCGAATTCCTACAG-3′, and the PCR products were further purified by native PAGE. The selection was repeated another five times with a reduced library aliquot down to 0.2 ng of DNA, and the final selected DNA fragments were cloned into the pMD18-T vector (Takara Biotech). In total, 120 clones were picked for sequencing, and 89 sequences of high quality were obtained.
For the B1H assay, the full-length SlZFP2 sequence was cloned into the pB1H1 vector, and an 18-bp randomized oligonucleotide library was cloned into the pH3U3 vector (Addgene; http://www.addgene.org) as described previously (Meng et al., 2005). Self-activation of the HIS3 and URA3 reporter genes in the primary library containing an estimated 2 × 107 clones was avoided by selection with 2 mm 5-fluoroorotic acid. The resulting library DNA was purified and transformed into US0hisB−pyrF− electrocompetent cells containing the bait plasmid pB1H1 with SlZFP2 coding sequence. Approximately 1 × 108 cells containing the bait and prey library were plated on HIS− selective minimal medium supplemented with 4 mm 3-amino-1,2,4-triazole at 37°C for 24 h. Plasmid DNA from the surviving colonies was digested with XmnI to remove the bait plasmid. The prey DNA was then purified by the QIAquick PCR purification column (Qiagen) and transformed into the selection strain US0hisB−pyrF− for counterselection with 2 mm 5-fluoroorotic acid. After incubation at 37°C for 24 h, individual colonies were selected for sequencing.
Subcellular Localization of SlZFP2-YFP
The SlZFP2-YFP fusion was made by ligation of a PCR-amplified full-length SlZFP2 fragment, made by using primers XP0210 and XP0211, to the YFP coding sequence (Clontech). This cassette was cloned into the pHX20 vector. The A. tumefaciens GV3101 strain containing p35S:YFP or p35S:SlZFP2-YFP was infiltrated into Nicotiana benthamiana leaves, and transient expression was monitored using a Zeiss LSM510 Meta confocal scanning microscope.
ChIP Assay
ChIP assays were performed on four homozygous HA-SlZFP2 overexpression lines (102–105) using their nontransgenic siblings as controls. Tissue fixation, cross-linking, and chromatin isolation were performed as described previously (Ito et al., 2012), with minor modifications. Young leaves from 45-d-old plants, including apical meristems, were ground into a fine powder in liquid nitrogen. A total of 1.5 g of powder was suspended in 25 mL of nuclear isolation buffer A (10 mm Tris-HCl [pH 8], 0.4 m Suc, 5 mm KCl, 5 mm MgCl2, 5 mm EDTA, 1% [v/v] formaldehyde, 0.05% [v/v] Triton X-100, and 1 mm PMSF). After 10 min of incubation at room temperature, cross-linking was stopped with Gly at a final concentration of 0.125 m. Lysates were filtered through two layers of Miracloth (Millipore) and cleared by centrifugation at 845g for 10 min. The pellets were then washed with ice-cold nuclear isolation buffer B (10 mm Tris-HCl [pH 8], 0.4 m Suc, 5 mm KCl, 5 mm MgCl2, 5 mm EDTA, 5 mm β-mercaptoethanol, and complete Roche Protease inhibitor tablets). The nuclei were lysed in nuclei lysis buffer containing 50 mm Tris-HCl (pH 8), 10 mm EDTA, 1% (w/v) SDS, 1 mm PMSF, 50 μm MG-132 (Sigma-Aldrich), and complete Roche Protease inhibitor tablets. Chromatin DNA was sheared to 500 to 1,000 bp using a 130-W Ultrasonic Processor VXC-130 (Sonics). After removing cell debris by centrifugation, the solution containing chromatin was diluted with ChIP dilution buffer (16.7 mm Tris-HCl [pH 8], 167 mm NaCl, 1.1% [w/v] Triton X-100, 1.2 mm EDTA, 1 mm PMSF, 50 μm MG-132, and complete protease inhibitor mixture tablets), followed by overnight incubation on ice with Dynabeads Protein G (Invitrogen) coupled to HA monoclonal antibody (Sigma-Aldrich). Beads were washed three times with ice-cold low-salt buffer (20 mm Tris-HCl [pH 8], 150 mm NaCl, 0.2% [w/v] SDS, 0.5% [v/v] Triton X-100, and 2 mm EDTA) and three times with ice-cold Tris-EDTA buffer (10 mm Tris-HCl [pH 8] and 1 mm EDTA). DNA subjected to ChIP was eluted with elution buffer (1% [w/v] SDS with freshly added 0.168 g of NaHCO3 per 20 mL) and reverse cross-linked overnight at 65°C by adding 5 m NaCl to a final concentration of 0.2 m. Eluted DNA samples were treated with Protease K (Sigma-Aldrich) at 50°C for 2 h, and DNA pellets were dissolved in 100 μL of Tris-EDTA buffer after further purification by phenol/chloroform extraction and precipitation. Aliquots of 2 μL were used for qPCR.
EMSA
EMSA was performed using E. coli-expressed GST-SlZFP2 protein and biotin-labeled DNA fragments containing putative binding sites for SlZFP2 (Thermo Fisher Scientific). The same primers used for the ChIP-qPCR analysis were used for preparing probe templates by PCR. Binding reactions were conducted at room temperature for 20 min in 20 μL of binding buffer containing 10 mm Tris (pH 7.5), 50 mm KCl, 1 mm dithiothreitol, 2.5% (v/v) glycerol, 0.05% (v/v) Nonidet P-40, 5 mm MgCl2, 0.5 mm EDTA, 50 ng mL−1 poly(dI∙dC), 1.5 μg of purified GST-SlZFP2, and 50 fmol biotin-labeled PCR fragments. Protein-DNA complexes were separated on a 6% (w/v) native polyacrylamide gel in 0.5× Tris-borate/EDTA buffer. After electrophoresis, protein-DNA complexes were transferred onto Hybond-N+ nylon membranes (GE Healthcare Life Sciences). The protein-DNA binding interaction was detected using the Light Shift Chemiluminescent EMSA Kit (Thermo Fisher Scientific).
Transient Expression Assay in Arabidopsis Protoplasts
The transient expression assay was conducted according to the protocol described by Yoo et al. (2007). A pUC118-based SlZFP2 expression cassette driven by the cauliflower mosaic virus 35S promoter (effector) or pUC118 (vector control) was cointroduced into protoplasts isolated from Arabidopsis (Arabidopsis thaliana) leaves with GUS reporter plasmids, of which 0.8- to 2-kb promoters of the ABA biosynthetic genes NOT, SIT, FLC, and SlAO1 were placed upstream of the GUS coding sequences, together with the cauliflower mosaic virus 35S:LUC plasmid as an internal control. The reporter and internal control plasmids were also pUC118 based. For each assay, three biological replicates were performed, and GUS activity was normalized to the internal control LUC activity measured by the Thermo Scientific Varioskan Flash Multimode Reader (Thermo Fisher Scientific).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. SlZFP2 encodes a C2H2-type zinc finger protein mainly expressed in fruits.
Supplemental Figure S2. SlZFP2 expression in various tissues.
Supplemental Figure S3. Fruit and seed morphology of HA-SlZFP2 overexpression lines in LA1589 background.
Supplemental Figure S4. Seed germination of SlZFP2 overexpression lines.
Supplemental Figure S5. Overexpression of HA-SlZFP2 affects stomata aperture.
Supplemental Figure S6. Changes of ABA levels in cv M82 fruits after pollination.
Supplemental Figure S7. Repressed ABA biosynthesis in p35::HA-SlZFP2 leaves.
Supplemental Figure S8. Transcript levels of ABA biosynthetic genes in p35::HA-SlZFP2 seeds.
Supplemental Figure S9. Subcellular localization of SlZFP2 protein.
Supplemental Figure S10. SlZFP2 binding sites found in the promoters of NOT, SIT, SlAO1, SlAO2, FLC, and CNR.
Supplemental Figure S11. Expression of SlZFP2 and the ABA biosynthetic genes by in situ hybridization.
Supplemental Table S1. Carotenoid accumulation in anthesis flowers and fruits of SlZFP2 overexpression and RNAi lines.
Supplemental Table S2. Differentially expressed genes in 2-DPA fruits of SlZFP2 RNAi line 207 identified by RNA-seq.
Supplemental Table S3. Expression levels (fragments per kilobase of transcripts per million mapped fragments) of SlZFP2 target genes.
Supplemental Table S4. Differentially expressed genes involved in hormone biosynthesis and signaling in 2-DPA fruits of the SlZFP2 RNAi line 207.
Supplemental Table S5. Primer information used in the study.
Supplementary Material
Acknowledgments
We thank the Tomato Genetic Resource Center (tgrc.ucdavis.edu/) and Daniel Zamir for providing the tomato seeds used in the study, Hongwei Xue for help with the generation of some HA-SlZFP2 transgenic plants, Xuan Li for retrieving promoter sequences, Meng Li for taking care of plants, and Ao Li, Lulu Bi, and Jun Yang for technical help with plasmid construction and in situ hybridization.
Glossary
- ABA
abscisic acid
- ACC
1-amino-1-carboxylic acid
- RNA-seq
RNA sequencing
- RT
reverse transcription
- qRT
quantitative reverse transcription
- RNAi
RNA interference
- MG
mature green
- Br
breaker
- B10
breaker plus 10 days
- SAAB
selected amplification and binding
- B1H
bacterial one-hybrid
- ChIP
chromatin immunoprecipitation
- qPCR
quantitative PCR
- EMSA
electrophoretic mobility shift assay
- cDNA
complementary DNA
- PMSF
phenylmethanesulfonyl fluoride
Footnotes
This work was supported by the Ministry of Science and Technology (grant nos. 2012AA100105 and 2012CB113900), the Shanghai Committee of Science and Technology (grant no. 11PJ1410900), and the Chinese Academy of Sciences (grant no. 2009OHTP07).
References
- Barrero JM, Rodríguez PL, Quesada V, Piqueras P, Ponce MR, Micol JL (2006) Both abscisic acid (ABA)-dependent and ABA-independent pathways govern the induction of NCED3, AAO3 and ABA1 in response to salt stress. Plant Cell Environ 29: 2000–2008 [DOI] [PubMed] [Google Scholar]
- Barry CS, Llop-Tous MI, Grierson D (2000) The regulation of 1-aminocyclopropane-1-carboxylic acid synthase gene expression during the transition from system-1 to system-2 ethylene synthesis in tomato. Plant Physiol 123: 979–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bemer M, Karlova R, Ballester AR, Tikunov YM, Bovy AG, Wolters-Arts M, Rossetto PdeB, Angenent GC, de Maagd RA (2012) The tomato FRUITFULL homologs TDR4/FUL1 and MBP7/FUL2 regulate ethylene-independent aspects of fruit ripening. Plant Cell 24: 4437–4451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böttcher C, Keyzers RA, Boss PK, Davies C (2010) Sequestration of auxin by the indole-3-acetic acid-amido synthetase GH3-1 in grape berry (Vitis vinifera L.) and the proposed role of auxin conjugation during ripening. J Exp Bot 61: 3615–3625 [DOI] [PubMed] [Google Scholar]
- Burbidge A, Grieve TM, Jackson A, Thompson A, McCarty DR, Taylor IB (1999) Characterization of the ABA-deficient tomato mutant notabilis and its relationship with maize Vp14. Plant J 17: 427–431 [DOI] [PubMed] [Google Scholar]
- Buta JG, Spaulding DW (1994) Changes in indole-3-acetic acid and abscisic acid levels during tomato (Lycopersicon esculentum Mill.) fruit development and ripening. J Plant Growth Regul 13: 163–166 [Google Scholar]
- Cai S, Lashbrook CC (2008) Stamen abscission zone transcriptome profiling reveals new candidates for abscission control: enhanced retention of floral organs in transgenic plants overexpressing Arabidopsis ZINC FINGER PROTEIN2. Plant Physiol 146: 1305–1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbonell-Bejerano P, Urbez C, Granell A, Carbonell J, Perez-Amador MA (2011) Ethylene is involved in pistil fate by modulating the onset of ovule senescence and the GA-mediated fruit set in Arabidopsis. BMC Plant Biol 11: 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng WH, Endo A, Zhou L, Penney J, Chen HC, Arroyo A, Leon P, Nambara E, Asami T, Seo M, et al. (2002) A unique short-chain dehydrogenase/reductase in Arabidopsis glucose signaling and abscisic acid biosynthesis and functions. Plant Cell 14: 2723–2743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung MY, Vrebalov J, Alba R, Lee J, McQuinn R, Chung JD, Klein P, Giovannoni J (2010) A tomato (Solanum lycopersicum) APETALA2/ERF gene, SlAP2a, is a negative regulator of fruit ripening. Plant J 64: 936–947 [DOI] [PubMed] [Google Scholar]
- Coen ES, Romero JM, Doyle S, Elliott R, Murphy G, Carpenter R (1990) floricaula: a homeotic gene required for flower development in Antirrhinum majus. Cell 63: 1311–1322 [DOI] [PubMed] [Google Scholar]
- Dong T, Hu Z, Deng L, Wang Y, Zhu M, Zhang J, Chen G (2013) A tomato MADS-box transcription factor, SlMADS1, acts as a negative regulator of fruit ripening. Plant Physiol 163: 1026–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo A, Sawada Y, Takahashi H, Okamoto M, Ikegami K, Koiwai H, Seo M, Toyomasu T, Mitsuhashi W, Shinozaki K, et al. (2008) Drought induction of Arabidopsis 9-cis-epoxycarotenoid dioxygenase occurs in vascular parenchyma cells. Plant Physiol 147: 1984–1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englbrecht CC, Schoof H, Böhm S (2004) Conservation, diversification and expansion of C2H2 zinc finger proteins in the Arabidopsis thaliana genome. BMC Genomics 5: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feurtado JA, Huang D, Wicki-Stordeur L, Hemstock LE, Potentier MS, Tsang EW, Cutler AJ (2011) The Arabidopsis C2H2 zinc finger INDETERMINATE DOMAIN1/ENHYDROUS promotes the transition to germination by regulating light and hormonal signaling during seed maturation. Plant Cell 23: 1772–1794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey A, Effroy D, Lefebvre V, Seo M, Perreau F, Berger A, Sechet J, To A, North HM, Marion-Poll A (2012) Epoxycarotenoid cleavage by NCED5 fine-tunes ABA accumulation and affects seed dormancy and drought tolerance with other NCED family members. Plant J 70: 501–512 [DOI] [PubMed] [Google Scholar]
- Frey A, Godin B, Bonnet M, Sotta B, Marion-Poll A (2004) Maternal synthesis of abscisic acid controls seed development and yield in Nicotiana plumbaginifolia. Planta 218: 958–964 [DOI] [PubMed] [Google Scholar]
- Fu X, Kong W, Peng G, Zhou J, Azam M, Xu C, Grierson D, Chen K (2012) Plastid structure and carotenogenic gene expression in red- and white-fleshed loquat (Eriobotrya japonica) fruits. J Exp Bot 63: 341–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa M, Nakano T, Shima Y, Ito Y (2013) A large-scale identification of direct targets of the tomato MADS box transcription factor RIPENING INHIBITOR reveals the regulation of fruit ripening. Plant Cell 25: 371–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galpaz N, Wang Q, Menda N, Zamir D, Hirschberg J (2008) Abscisic acid deficiency in the tomato mutant high-pigment 3 leading to increased plastid number and higher fruit lycopene content. Plant J 53: 717–730 [DOI] [PubMed] [Google Scholar]
- Gan Y, Kumimoto R, Liu C, Ratcliffe O, Yu H, Broun P (2006) GLABROUS INFLORESCENCE STEMS modulates the regulation by gibberellins of epidermal differentiation and shoot maturation in Arabidopsis. Plant Cell 18: 1383–1395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan Y, Liu C, Yu H, Broun P (2007) Integration of cytokinin and gibberellin signalling by Arabidopsis transcription factors GIS, ZFP8 and GIS2 in the regulation of epidermal cell fate. Development 134: 2073–2081 [DOI] [PubMed] [Google Scholar]
- Gillaspy G, Ben-David H, Gruissem W (1993) Fruits: a developmental perspective. Plant Cell 5: 1439–1451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannoni JJ. (2004) Genetic regulation of fruit development and ripening. Plant Cell (Suppl) 16: S170–S180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannoni JJ. (2007) Fruit ripening mutants yield insights into ripening control. Curr Opin Plant Biol 10: 283–289 [DOI] [PubMed] [Google Scholar]
- Harrison E, Burbidge A, Okyere JP, Thompson AJ, Taylor IB (2011) Identification of the tomato ABA-deficient mutant sitiens as a member of the ABA-aldehyde oxidase gene family using genetic and genomic analysis. Plant Growth Regul 64: 301–309 [Google Scholar]
- Hauser F, Waadt R, Schroeder JI (2011) Evolution of abscisic acid synthesis and signaling mechanisms. Curr Biol 21: R346–R355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hichri I, Muhovski Y, Žižkova E, Dobrev PI, Franco-Zorrilla JM, Solano R, Lopez-Vidriero I, Motyka V, Lutts S (2014) The Solanum lycopersicum Zinc Finger2 cysteine-2/histidine-2 repressor-like transcription factor regulates development and tolerance to salinity in tomato and Arabidopsis. Plant Physiol 164: 1967–1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoth S, Morgante M, Sanchez JP, Hanafey MK, Tingey SV, Chua NH (2002) Genome-wide gene expression profiling in Arabidopsis thaliana reveals new targets of abscisic acid and largely impaired gene regulation in the abi1-1 mutant. J Cell Sci 115: 4891–4900 [DOI] [PubMed] [Google Scholar]
- Huang XY, Chao DY, Gao JP, Zhu MZ, Shi M, Lin HX (2009) A previously unknown zinc finger protein, DST, regulates drought and salt tolerance in rice via stomatal aperture control. Genes Dev 23: 1805–1817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itkin M, Seybold H, Breitel D, Rogachev I, Meir S, Aharoni A (2009) TOMATO AGAMOUS-LIKE 1 is a component of the fruit ripening regulatory network. Plant J 60: 1081–1095 [DOI] [PubMed] [Google Scholar]
- Ito S, Song YH, Josephson-Day AR, Miller RJ, Breton G, Olmstead RG, Imaizumi T (2012) FLOWERING BHLH transcriptional activators control expression of the photoperiodic flowering regulator CONSTANS in Arabidopsis. Proc Natl Acad Sci USA 109: 3582–3587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Je J, Chen H, Song C, Lim CO (2014) Arabidopsis DREB2C modulates ABA biosynthesis during germination. Biochem Biophys Res Commun 452: 91–98 [DOI] [PubMed] [Google Scholar]
- Jensen MK, Lindemose S, de Masi F, Reimer JJ, Nielsen M, Perera V, Workman CT, Turck F, Grant MR, Mundy J, et al. (2013) ATAF1 transcription factor directly regulates abscisic acid biosynthetic gene NCED3 in Arabidopsis thaliana. FEBS Open Bio 3: 321–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia HF, Chai YM, Li CL, Lu D, Luo JJ, Qin L, Shen YY (2011) Abscisic acid plays an important role in the regulation of strawberry fruit ripening. Plant Physiol 157: 188–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Liang G, Yu D (2012) Activated expression of WRKY57 confers drought tolerance in Arabidopsis. Mol Plant 5: 1375–1388 [DOI] [PubMed] [Google Scholar]
- Karlova R, Rosin FM, Busscher-Lange J, Parapunova V, Do PT, Fernie AR, Fraser PD, Baxter C, Angenent GC, de Maagd RA (2011) Transcriptome and metabolite profiling show that APETALA2a is a major regulator of tomato fruit ripening. Plant Cell 23: 923–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerschen A, Napoli CA, Jorgensen RA, Müller AE (2004) Effectiveness of RNA interference in transgenic plants. FEBS Lett 566: 223–228 [DOI] [PubMed] [Google Scholar]
- Kim DH, Yamaguchi S, Lim S, Oh E, Park J, Hanada A, Kamiya Y, Choi G (2008) SOMNUS, a CCCH-type zinc finger protein in Arabidopsis, negatively regulates light-dependent seed germination downstream of PIL5. Plant Cell 20: 1260–1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klee HJ, Giovannoni JJ (2011) Genetics and control of tomato fruit ripening and quality attributes. Annu Rev Genet 45: 41–59 [DOI] [PubMed] [Google Scholar]
- Ko JH, Yang SH, Han KH (2006) Upregulation of an Arabidopsis RING-H2 gene, XERICO, confers drought tolerance through increased abscisic acid biosynthesis. Plant J 47: 343–355 [DOI] [PubMed] [Google Scholar]
- Kodaira KS, Qin F, Tran LS, Maruyama K, Kidokoro S, Fujita Y, Shinozaki K, Yamaguchi-Shinozaki K (2011) Arabidopsis Cys2/His2 zinc-finger proteins AZF1 and AZF2 negatively regulate abscisic acid-repressive and auxin-inducible genes under abiotic stress conditions. Plant Physiol 157: 742–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koiwai H, Nakaminami K, Seo M, Mitsuhashi W, Toyomasu T, Koshiba T (2004) Tissue-specific localization of an abscisic acid biosynthetic enzyme, AAO3, in Arabidopsis. Plant Physiol 134: 1697–1707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger U, Lippman ZB, Zamir D (2010) The flowering gene SINGLE FLOWER TRUSS drives heterosis for yield in tomato. Nat Genet 42: 459–463 [DOI] [PubMed] [Google Scholar]
- Lifschitz E, Ayre BG, Eshed Y (2014) Florigen and anti-florigen: a systemic mechanism for coordinating growth and termination in flowering plants. Front Plant Sci 5: 465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning K, Tör M, Poole M, Hong Y, Thompson AJ, King GJ, Giovannoni JJ, Seymour GB (2006) A naturally occurring epigenetic mutation in a gene encoding an SBP-box transcription factor inhibits tomato fruit ripening. Nat Genet 38: 948–952 [DOI] [PubMed] [Google Scholar]
- Mariotti L, Picciarelli P, Lombardi L, Ceccarelli N (2011) Fruit-set and early fruit growth in tomato are associated with increases in indoleacetic acid, cytokinin, and bioactive gibberellin contents. J Plant Growth Regul 30: 405–415 [Google Scholar]
- Martel C, Vrebalov J, Tafelmeyer P, Giovannoni JJ (2011) The tomato MADS-box transcription factor RIPENING INHIBITOR interacts with promoters involved in numerous ripening processes in a COLORLESS NONRIPENING-dependent manner. Plant Physiol 157: 1568–1579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez C, Manzano S, Megías Z, Garrido D, Picó B, Jamilena M (2013) Involvement of ethylene biosynthesis and signalling in fruit set and early fruit development in zucchini squash (Cucurbita pepo L.). BMC Plant Biol 13: 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAtee P, Karim S, Schaffer R, David K (2013) A dynamic interplay between phytohormones is required for fruit development, maturation, and ripening. Front Plant Sci 4: 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick S. (1991) Transformation of tomato with Agrobacterium tumefaciens. InLindsey K, ed, Plant Tissue Culture Manual, Vol 6. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 1–9 [Google Scholar]
- Meng X, Brodsky MH, Wolfe SA (2005) A bacterial one-hybrid system for determining the DNA-binding specificity of transcription factors. Nat Biotechnol 23: 988–994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambara E, Marion-Poll A (2005) Abscisic acid biosynthesis and catabolism. Annu Rev Plant Biol 56: 165–185 [DOI] [PubMed] [Google Scholar]
- Nitsch LM, Oplaat C, Feron R, Ma Q, Wolters-Arts M, Hedden P, Mariani C, Vriezen WH (2009) Abscisic acid levels in tomato ovaries are regulated by LeNCED1 and SlCYP707A1. Planta 229: 1335–1346 [DOI] [PubMed] [Google Scholar]
- Pan X, Welti R, Wang X (2010) Quantitative analysis of major plant hormones in crude plant extracts by high-performance liquid chromatography-mass spectrometry. Nat Protoc 5: 986–992 [DOI] [PubMed] [Google Scholar]
- Pascual L, Blanca JM, Cañizares J, Nuez F (2009) Transcriptomic analysis of tomato carpel development reveals alterations in ethylene and gibberellin synthesis during pat3/pat4 parthenocarpic fruit set. BMC Plant Biol 9: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng H, Zheng L, Lee WH, Rux JJ, Rauscher FJ III (2002) A common DNA-binding site for SZF1 and the BRCA1-associated zinc finger protein, ZBRK1. Cancer Res 62: 3773–3781 [PubMed] [Google Scholar]
- Pnueli L, Carmel-Goren L, Hareven D, Gutfinger T, Alvarez J, Ganal M, Zamir D, Lifschitz E (1998) The SELF-PRUNING gene of tomato regulates vegetative to reproductive switching of sympodial meristems and is the ortholog of CEN and TFL1. Development 125: 1979–1989 [DOI] [PubMed] [Google Scholar]
- Sagi M, Scazzocchio C, Fluhr R (2002) The absence of molybdenum cofactor sulfuration is the primary cause of the flacca phenotype in tomato plants. Plant J 31: 305–317 [DOI] [PubMed] [Google Scholar]
- Sakai H, Medrano LJ, Meyerowitz EM (1995) Role of SUPERMAN in maintaining Arabidopsis floral whorl boundaries. Nature 378: 199–203 [DOI] [PubMed] [Google Scholar]
- Sakamoto H, Araki T, Meshi T, Iwabuchi M (2000) Expression of a subset of the Arabidopsis Cys(2)/His(2)-type zinc-finger protein gene family under water stress. Gene 248: 23–32 [DOI] [PubMed] [Google Scholar]
- Sakamoto H, Maruyama K, Sakuma Y, Meshi T, Iwabuchi M, Shinozaki K, Yamaguchi-Shinozaki K (2004) Arabidopsis Cys2/His2-type zinc-finger proteins function as transcription repressors under drought, cold, and high-salinity stress conditions. Plant Physiol 136: 2734–2746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo M, Koshiba T (2002) Complex regulation of ABA biosynthesis in plants. Trends Plant Sci 7: 41–48 [DOI] [PubMed] [Google Scholar]
- Seymour GB, Østergaard L, Chapman NH, Knapp S, Martin C (2013) Fruit development and ripening. Annu Rev Plant Biol 64: 219–241 [DOI] [PubMed] [Google Scholar]
- Sun L, Sun Y, Zhang M, Wang L, Ren J, Cui M, Wang Y, Ji K, Li P, Li Q, et al. (2012a) Suppression of 9-cis-epoxycarotenoid dioxygenase, which encodes a key enzyme in abscisic acid biosynthesis, alters fruit texture in transgenic tomato. Plant Physiol 158: 283–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Yuan B, Zhang M, Wang L, Cui M, Wang Q, Leng P (2012b) Fruit-specific RNAi-mediated suppression of SlNCED1 increases both lycopene and β-carotene contents in tomato fruit. J Exp Bot 63: 3097–3108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AJ, Jackson AC, Parker RA, Morpeth DR, Burbidge A, Taylor IB (2000) Abscisic acid biosynthesis in tomato: regulation of zeaxanthin epoxidase and 9-cis-epoxycarotenoid dioxygenase mRNAs by light/dark cycles, water stress and abscisic acid. Plant Mol Biol 42: 833–845 [DOI] [PubMed] [Google Scholar]
- Toh S, Imamura A, Watanabe A, Nakabayashi K, Okamoto M, Jikumaru Y, Hanada A, Aso Y, Ishiyama K, Tamura N, et al. (2008) High temperature-induced abscisic acid biosynthesis and its role in the inhibition of gibberellin action in Arabidopsis seeds. Plant Physiol 146: 1368–1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomato Genome Consortium (2012) The tomato genome sequence provides insights into fleshy fruit evolution. Nature 485: 635–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C, Pachter L, Salzberg SL (2009) TopHat: discovering splice junctions with RNA-Seq. Bioinformatics 25: 1105–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, van Baren MJ, Salzberg SL, Wold BJ, Pachter L (2010) Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol 28: 511–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrebalov J, Pan IL, Arroyo AJ, McQuinn R, Chung M, Poole M, Rose J, Seymour G, Grandillo S, Giovannoni J, et al. (2009) Fleshy fruit expansion and ripening are regulated by the tomato SHATTERPROOF gene TAGL1. Plant Cell 21: 3041–3062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrebalov J, Ruezinsky D, Padmanabhan V, White R, Medrano D, Drake R, Schuch W, Giovannoni J (2002) A MADS-box gene necessary for fruit ripening at the tomato ripening-inhibitor (rin) locus. Science 296: 343–346 [DOI] [PubMed] [Google Scholar]
- Vriezen WH, Feron R, Maretto F, Keijman J, Mariani C (2008) Changes in tomato ovary transcriptome demonstrate complex hormonal regulation of fruit set. New Phytol 177: 60–76 [DOI] [PubMed] [Google Scholar]
- Wang H, Schauer N, Usadel B, Frasse P, Zouine M, Hernould M, Latché A, Pech JC, Fernie AR, Bouzayen M (2009) Regulatory features underlying pollination-dependent and -independent tomato fruit set revealed by transcript and primary metabolite profiling. Plant Cell 21: 1428–1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasilewska A, Vlad F, Sirichandra C, Redko Y, Jammes F, Valon C, Frei dit Frey N, Leung J (2008) An update on abscisic acid signaling in plants and more.... Mol Plant 1: 198–217 [DOI] [PubMed] [Google Scholar]
- Weingartner M, Subert C, Sauer N (2011) LATE, a C(2)H(2) zinc-finger protein that acts as floral repressor. Plant J 68: 681–692 [DOI] [PubMed] [Google Scholar]
- Xiao H, Jiang N, Schaffner E, Stockinger EJ, van der Knaap E (2008) A retrotransposon-mediated gene duplication underlies morphological variation of tomato fruit. Science 319: 1527–1530 [DOI] [PubMed] [Google Scholar]
- Xiao H, Radovich C, Welty N, Hsu J, Li D, Meulia T, van der Knaap E (2009) Integration of tomato reproductive developmental landmarks and expression profiles, and the effect of SUN on fruit shape. BMC Plant Biol 9: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao H, Wang Y, Liu D, Wang W, Li X, Zhao X, Xu J, Zhai W, Zhu L (2003) Functional analysis of the rice AP3 homologue OsMADS16 by RNA interference. Plant Mol Biol 52: 957–966 [DOI] [PubMed] [Google Scholar]
- Xiong L, Gong Z, Rock CD, Subramanian S, Guo Y, Xu W, Galbraith D, Zhu JK (2001a) Modulation of abscisic acid signal transduction and biosynthesis by an Sm-like protein in Arabidopsis. Dev Cell 1: 771–781 [DOI] [PubMed] [Google Scholar]
- Xiong L, Ishitani M, Lee H, Zhu JK (2001b) The Arabidopsis LOS5/ABA3 locus encodes a molybdenum cofactor sulfurase and modulates cold stress- and osmotic stress-responsive gene expression. Plant Cell 13: 2063–2083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong L, Lee H, Ishitani M, Zhu JK (2002) Regulation of osmotic stress-responsive gene expression by the LOS6/ABA1 locus in Arabidopsis. J Biol Chem 277: 8588–8596 [DOI] [PubMed] [Google Scholar]
- Xiong L, Zhu JK (2003) Regulation of abscisic acid biosynthesis. Plant Physiol 133: 29–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaish MW, El-Kereamy A, Zhu T, Beatty PH, Good AG, Bi YM, Rothstein SJ (2010) The APETALA-2-like transcription factor OsAP2-39 controls key interactions between abscisic acid and gibberellin in rice. PLoS Genet 6: e1001098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Worley E, Udvardi M (2014) A NAP-AAO3 regulatory module promotes chlorophyll degradation via ABA biosynthesis in Arabidopsis leaves. Plant Cell 26: 4862–4874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Zhang B, Jia J, Yan C, Habaike A, Han Y (2013) RRP41L, a putative core subunit of the exosome, plays an important role in seed germination and early seedling growth in Arabidopsis. Plant Physiol 161: 165–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano R, Kanno Y, Jikumaru Y, Nakabayashi K, Kamiya Y, Nambara E (2009) CHOTTO1, a putative double APETALA2 repeat transcription factor, is involved in abscisic acid-mediated repression of gibberellin biosynthesis during seed germination in Arabidopsis. Plant Physiol 151: 641–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo SD, Cho YH, Sheen J (2007) Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat Protoc 2: 1565–1572 [DOI] [PubMed] [Google Scholar]
- Zhang M, Yuan B, Leng P (2009) The role of ABA in triggering ethylene biosynthesis and ripening of tomato fruit. J Exp Bot 60: 1579–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, An L, Sun L, Zhu S, Xi W, Broun P, Yu H, Gan Y (2011) Zinc Finger Protein5 is required for the control of trichome initiation by acting upstream of Zinc Finger Protein8 in Arabidopsis. Plant Physiol 157: 673–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Sun L, Zhao Y, An L, Yan A, Meng X, Gan Y (2013) Zinc Finger Protein 6 (ZFP6) regulates trichome initiation by integrating gibberellin and cytokinin signaling in Arabidopsis thaliana. New Phytol 198: 699–708 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.