Abstract
Bone disease is the leading cause of morbidity associated with multiple myeloma (MM). Lytic bone lesions have been detected in 90% of patients diagnosed with MM and present a great therapeutic challenge. After the removal of the tumor burden, the bone lesions persist and the bone remodeling homeostasis is not restored even in patients in clinical remission. To determine whether systemic factors generated by malignant MM cells can skew the osteoblast (OB) differentiation program of normal mesenchymal stem cells (MSCs), we generated an immortalized bone marrow MSC line (hTERT-MSC). The hTERT-MSCs were exposed to plasma from healthy donors and patients with MM. Cells grown in media supplemented with plasma from MM patients failed to differentiate into OBs, while the hTERT-MSCs grown in the presence of normal human plasma generated OB clusters that mineralized calcium, expressed Runx2, and were positive for alkaline phosphatase, fibronectin, collagen I, osteocalcin, and osteopontin. Blocking Dickkopf-1 (Dkk-1) and interleukin-7 (IL-7) in MM plasma restored proper OB differentiation of hTERT-MSCs. Finally, we show that hTERT-MSCs cultured in the presence of MM plasma adopt a cancer-associated stroma phenotype. Thus, we show, that systemic factors present in the plasma of patients with MM affect the behavior of non-malignant MSCs and contribute to the sustained bone disease reported in MM.
Keywords: Multiple myeloma, lytic bone lesions, osteoblast differentiation, Dkk-1, IL-7
Introduction
Multiple myeloma (MM), the second most common hematological malignancy in the US, remains fatal, with the median survival-rates of 5-years for patients with stage I and 2.5-years for those diagnosed with stage III disease [1]. MM is characterized by an overabundance of clonotypic plasma cells in the bone marrow and secretion of monoclonal immunoglobulin [2]. Osteolytic bone disease is the most common symptom in MM characterized by hypercalcemia, pathologic fractures, spinal cord compression, and bone pain [3]. At the core of bone disease are lytic bone lesions that occur in 90% of patients and arise as a result of the disruption of the bone remodeling homeostasis [4]. The pathogenesis of lytic lesions is driven by the loss of balance between the osteoblast (OB)-mediated bone formation and osteoclast-mediated bone resorption, which leads to the excessive loss of bone [5,6]. Even after successful treatment, bone lesions persist through complete remission [7].
Malignant plasma cells secrete osteoclast-activating factors, which are thought to mediate an increase in osteoclast activity, and thus cause bone resorption [8,9]. Bone loss is further exacerbated by the diminished activity and a decrease in overall numbers of OBs. Interaction between MM cells and OBs has been reported to induce apoptosis in OBs, thus depleting the population of mature bone-forming cells [10,11]. Additionally, a number of factors have been identified that prevent OB differentiation from mesenchymal stem cells (MSCs). Dickkopf-1 (Dkk-1), an inhibitor of Wnt signaling secreted by malignant plasma cells, inhibits OB differentiation. Elevated levels of Dkk-1 in MM patients have been correlated with osteolytic bone disease severity [9,12,13]. Additionally, interleukin-7 (IL-7), a cytokine secreted by bone marrow stromal cells, was shown to prevent OB formation by decreasing the activity of Runx2/Cbfa1, a transcription factor required for OB differentiation [14,15].
For many years, bisphosphonates have been the standard of care for patients with lytic bone lesions, however, while they reduce bone pain and other skeletal events, osteonecrosis of the jaw is a serious concern for patients receiving bisphosphonates [16]. Bortezomib has been shown to increase osteoblast activity and enhance bone formation, allowing the repair of osteolytic lesions [3]. Moreover, bortezomib has also been found to lower the levels of serum Dkk-1 and increase Runx2 activity [3,17].
Here we show that bone marrow MSCs robustly differentiated into OBs when cultured in the presence of plasma from healthy donors (i.e. normal human plasma, NHP). However, the OB differentiation program was inhibited when the MSCs were exposed to the plasma from patients with MM (MMP). Here we demonstrate that blocking Dkk-1 and IL-7 in MMP restored OB differentiation. Finally, we show that exposing non-malignant MSCs to the factors in MMP induced formation of cancer-associated stroma. Together with our previous finding that the levels of IL-7 are not restored to baseline levels in patients who are in remission from MM [18], the data presented here suggest that the altered systemic microenvironment contributes to bone disease in MM, preventing proper OB differentiation and restoration of bone homeostasis even after the removal or destruction of the malignant plasma cells.
Materials and methods
Ethics statement
Human bone marrow aspirates were collected and BM-MSCs were isolated after approval from the City of Hope Institutional Research Board and after written informed consent in accordance with the Declaration of Helsinki. Plasma from patients with MM was obtained at the time of a routine clinic visit after approval from the Health Research Board (University of Alberta), the Alberta Cancer Board, and Purdue Institutional Review Board and with signed informed consent in accordance with the Declaration of Helsinki. All human samples were anonymized; no donor-related information was stored by the investigators.
Materials
RPMI-1640 medium, Minimum Essential Medium Eagle with Alpha Modification medium (α-MEM), L-glutamine solution, penicillin-streptomycin solution, fetal bovine serum (FBS), CaCl2, sodium succinate, hydrocortisone, 0.25% Trypsin-EDTA solution, bovine serum albumin (BSA), β-mercaptoethanol, mouse anti-human alpha-smooth muscle actin (αSMA), Triton X-100, Alizarin Red S, SigmaFast BCIP/NBT tablets, and crystal violet were all purchased from Sigma. DAPI Nuclear Isolation and Staining Solution was from NPE Systems. TRIzol was obtained from Invitrogen. Human IL-7 receptor alpha (IL-7Rα), recombinant human IL-7, human fibronectin monoclonal antibody (MAb), human osteopontin MAb, donkey anti-mouse IgG NL557, donkey anti-mouse IgG NL493, donkey anti-rabbit IgG NL557 secondary antibodies were from R&D Systems. Human osteocalcin polyclonal antibody (PAb) was purchased from Santa Cruz Biotechnology. Human vimentin PAb and human caveolin-1 PAb were acquired from Cell Signaling Technology. Human collagen I MAb, Dkk-1 Inhibitor (WAY-262611) and recombinant human Dkk-1 were purchased from Millipore. Vectashield was obtained from Vector Laboratories. OneStep PCR Kit was from Qiagen. AccuScript high fidelity 1st strand cDNA synthesis kit was purchased from Agilent Technologies and DyNAmo HS SYBR Green qPCR Master Mix from Thermo Scientific. DNAse I was obtained from New England BioLabs. Human plasma from healthy donors (NHP, i.e. normal human plasma) was purchased from Equitech-Bio. Plasma from patients with MM (MMP, i.e. multiple myeloma plasma) (n = 16) was obtained at the time of a routine clinic visit.
Cell culture
Immortalized human bone marrow mesenchymal stem cells (hTERT-MSC) were used in all experiments. These cells were a developed by Dr. Carlotta Glackin (Beckman Research Institute, City of Hope National Medical Center). BM-MSCs were isolated from 15-week human fetal bone tissue and bone marrow was purified based on the expression of STRO-1bright/CD106+ or STRO-1bright/CD146+ expression [19,20]. This STRO-1bright/CD106+ or STRO-1bright/CD146+ cell population was then immortalized with hTERT in a pBABE retroviral insertion vector and stable clones were selected with puromycin, resulting in the creation of the hTERT-MSC cell line, which has been used to evaluate novel therapeutic agents in MM [21]. hTERT-MSCs were maintained in MSC growth medium (MSC-GM) (αMEM with 10% FBS, 1% L-glutamine, and 1% penicillin-streptomycin), and grown in a 37°C, 5% CO2 incubator. For all experiments cells were cultured in differentiation medium (RPMI-1640, 6.2 × 10-4 M CaCl2, 1 × 10-6 M sodium succinate, 1 × 10-6 M hydrocortisone, 1% penicillin-streptomycin supplemented with 20% FBS, normal human plasma (NHP), or plasma from patients with MM (MMP)). The differentiation medium with human plasma was sterile filtered through a 0.20 μm syringe filter.
Osteoblast differentiation
hTERT-MSC were plated in 500 μL MSC growth medium at 20,000 cells/well into a 48-well plate and grown until confluent. The growth medium was removed and replaced with 500 μL of differentiation medium (DM) with 20% FBS (negative control), 20% NHP (positive control), or 20% MMP (experimental, 2-3 individual plasma samples were mixed in each batch of MMP-DM to minimize patient-to-patient variation), FBS-DM, NHP-DM, MMP-DM respectively. Images were taken daily starting at day zero (when differentiation medium was added). After clusters formed in the positive control wells, samples were fixed in 10% neutral buffered formalin (NBF) for 15 min at room temperature (RT) and washed with 1X PBS for 5 min. Cells were stained using SigmaFast BCIP/NGT reagent to visualize alkaline phosphatase (ALP) per manufacturer’s instructions. Cells were incubated in 200 μL of the staining solution at RT until purple color began to develop (~30 min). The staining solution was removed and cells were washed with PBS. PBS (500 μl/well) was added to stained cells to prevent them from drying out during imaging.
Alizarin Red was used to stain for mineralized calcium deposited by osteogenic cells. Alizarin Red was dissolved in water, pH was adjusted to 4.1-4.3, and cells were stained per manufacturer’s instructions. Cells were incubated in the staining solution for 20 min and rinsed with PBS until it was clear when removed. Cells were kept in 500 μl/well of PBS during imaging. Images were taken with a Canon PowerShot A650IS digital camera on a Zeiss Axiovert 40 microscope.
Immunofluorescence
Cells were plated and cultured in differentiation medium as described above. At the end of the culture period cells were fixed in 10% NBF for 15 min at RT and permeabilized with 0.1% TritonX-100 in PBS for 10 minutes at room temperature. Non-specific antibody binding was blocked for 1hr in 1% BSA/PBS at RT. After a PBS wash, cells were incubated over night at 4°C at the following dilutions of primary antibodies made in 1% BSA/PBS: osteocalcin at 1:100, collagen I at 1:10, fibronectin and vimentin at 1:50, caveolin I at 1:400, and osteopontin and αSMA at 1:200. Subsequently, cells were washed with PBS and were incubated with a fluorophore conjugated donkey-anti-mouse or donkey-anti-rabbit secondary antibodies at 1:200 dilution for 1 hr at RT (secondary alone controls were included as negative controls). Nuclei were visualized with DAPI staining solution at 1:25 dilution stained for 5 min at RT. After a final PBS wash a drop of Vectashield mounting medium was added to each well to preserve fluorescence. All samples were imaged on a Zeiss AxioObserver fluorescent microscope.
RNA isolation and quantitative PCR
For RT-PCR experiments cells were plated in 6-well plates at 1 × 106 cells/well and cultured as described in the Osteoblast Differentiation section above in 4 ml/well of differentiation medium. Two wells from each condition, FBS, NHP, and MMP, were pooled together to isolate RNA for each biological replicate. Upon cluster formation in NHP plates, cells were then trypsinized, resuspended in 1 ml of TRIzol, and RNA was isolated according to the manufacturer’s instructions. RNA concentration was measured using a NanoDrop ND-1000 spectrophotometer and samples were stored at -80°C. Samples were treated with DNAse I to remove any DNA contamination. cDNA was prepared per instructions supplied with the AccuScript High Fidelity Reverse Transcriptase. Quantitative PCR (qPCR) was set-up using the procedure described for the DyNAmo HS SYBR Green Master Mix using the following primer sets: GAPDH forward 5’-GAGTCAACGGATTTGGTCGT-3’, GAPDH reverse 5’-GACAAGCTTCCCGTTCTCAG-3’; Runx2 forward 5’-GTGGACGAGGCAAGAGTTTCA-3’, Runx2 reverse 5’-CATCAAGCTTCTGTCTGTGCC-3’; and Twist forward 5’-TCTTACGAGGAGCTGCAGACGCA-3’, Twist reverse 5’-ATCTTGGAGTCCAGCTCGTCGCT-3’. As a negative control for each primer set, water was used instead of cDNA. Samples were run in the Applied Biosystems 7300 Real-Time PCR System using the following cycling conditions: 95°C/10 min; (40 cycles): 95°C/10 sec, 60°C/30 sec, 72°C/30 sec; 95°C/15 sec, 60°C/1 min, 95°C/15 sec, 60°C/15 sec; 72°C/10 min.
CFU-F
MSC cells were cultured in differentiation medium in 6-well plates as described above, trypsinized, and transferred into CFU-F medium (α-MEM with 20% FBS, 2 mM L-glutamine, 100 µM L-ascorbate-2-phosphate, 1% penicillin-streptomycin, and 5.0 × 10-5 M β-mercaptoethanol). A 1:5 serial dilution was set-up for each experimental condition in 5 ml/well of CFU-F medium in 6-well plates. Medium was changed every 3-4 days. On day 13 medium was removed, cells were rinsed 3x with PBS and fixed with 10% NBF for 10 min at RT. Cultures were washed 3x with PBS and air dried. Enough crystal violet staining solution (0.5% crystal violet in water) was added to cover the bottom of each well and plates were incubated for 10 min at RT. Crystal violet was washed out under running tap water until the water ran clear. Resultant colonies were counted and the data was plotted as percent CFU-F per experimental condition.
Dkk-1/IL-7 treatment
Plates were set-up as described in the Osteoblast Differentiation section above. Recombinant human Dkk-1 (20 nM) or IL-7 (30 pg/ml) were added to the NHP-containing differentiation medium, while the Dkk-1 inhibitor (0.5 μM) or anti-IL-7Rα antibody (1 μg/ml) were added to the MMP differentiation medium. Plates were incubated for 7 days and stained to detect the presence of ALP as described above.
Statistical analysis
Data were presented as mean ± s.e.m and statistical significance in all experiments was evaluated by a one-way ANOVA with Tukey’s post test using Prism 5 software (GraphPad Software, Inc) with p-values below 0.05 considered significant.
Results
Factors in the plasma of MM patients prevent OB differentiation
We have recently shown that expression levels of a number of cytokines do not return to the normal levels when a MM patient enters remission [18]. Therefore, we wanted to evaluate whether some of the soluble factors found in the plasma of MM patients could prevent osteoblast formation, and thus, contribute to the sustained bone disease prevalent in MM. To assess the effects of soluble factors present in the plasma of patients with MM on OB development, we determined the differentiation capacity of hTERT-MSC cells when exposed to plasma from healthy donors (NHP) and patients with MM (MMP). hTERT-MSC cell line is enriched in MSCs and is multipotent having the capacity to differentiate into the osteogenic, adipogenic, chondrocyte, myogenic, and neurogenic lineages. To set-up the differentiation assay, hTERT-MSC cells were seeded in mesenchymal stem cell growth medium (MSC-GM) and upon reaching confluency, MSC-GM was changed to differentiation medium supplemented with FBS, NHP, or MMP (FBS-DM, NHP-DM, or MMP-DM respectively) (Figure 1A). The cultures were observed for an additional 7-9 days and the capacity of hTERT-MSCs to differentiate, and thus lose the MSC phenotype, was evaluated as the ability of the cultured cells to form colonies in CFU-F assays. hTERT-MSC cells cultured in FBS-DM, NHP-DM, or MMP-DM displayed 30 ± 9%, 7.7 ± 2%, and 22 ± 5% CFU-F formation respectively. Therefore, we concluded that NHP-DM induced differentiation of hTERT-MSC cells with an observed decrease in CFU-F capacity (Figure 1B, 1C). While MMP-DM also induced a decrease in CFU-F forming units, the decrease was not statistically significant (Figure 1B, 1C). Thus, factors present in NHP induce differentiation of MSCs, and as a consequence, loss of CFU-F generating capacity.
Figure 1.
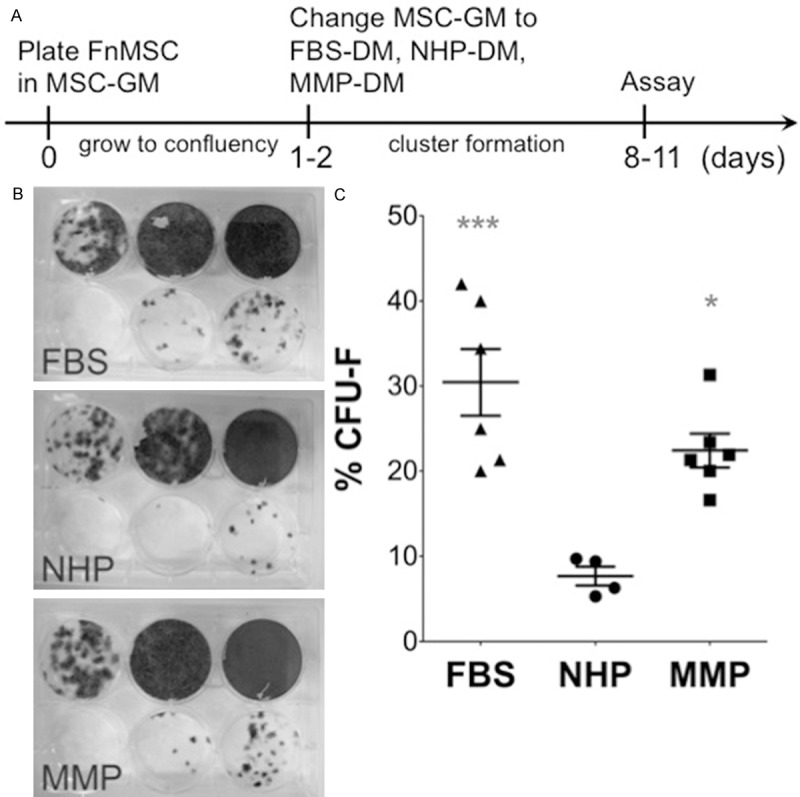
NHP-DM and MMP-DM induce a decrease in CFU-F forming capacity of hTERT-MSCs. A. Experimental set-up and timeline. B. hTERT-MSC cells were cultured in FBS-DM, NHP-DM, or MMP-DM, and subsequently, plated as a serial dilution (40,000; 8,000; 1,600; 320; 64; and 0 cells/well) into CFU-F assays. Resulting colonies were stained with crystal violet and counted. C. Quantification of the CFU-F assay was performed by counting the number of colonies in the 320 and 64 cells/well dilution as other concentrations resulted in too many colonies to count. Data is represented as % CFU-F = ((# colonies)/(# cells plated))*100 for each independent experiment (*p = 0.01, FBS vs. NHP; ***p = 0.0007, MMP vs. NHP; p > 0.05, FBS vs. MMP).
To determine whether NHP-DM induces OB phenotype, cultures were stained for the expression of alkaline phosphatase (ALP) and the ability to mineralize calcium. When grown in the presence of NHP, hTERT-MSC cells formed clusters that expressed high levels of ALP, a marker of mature OBs [3] (Figure 2A). On the other hand, cells cultured in FBS-DM or MMP-DM failed to form clusters with only a few ALP-positive cells visible (Figure 2A). Another characteristic of functional OBs is their ability to mineralize calcium measured by Alizarin Red staining [22]. Consistent with ALP expression, cultures grown in NHP-DM stained positive with Alizarin Red, while calcium mineralization was not observed in hTERT-MSC cultures exposed to MMP-DM or in FBS-DM cultured controls (Figure 2B). Next, we assessed the timing of OB differentiation. Cultures were observed daily and the cluster formation was plotted as a function of time (Figure 2C). While cells grown in NHP-DM exhibited early cluster formation (day 1: 3/8, day 2: 3/8, day 3: 2/8), majority of cells cultured in MMP-DM failed to form ALP-positive clusters (day 2: 1/8, day 3: 1/8, day 4: 1/8, never: 5/8).
Figure 2.
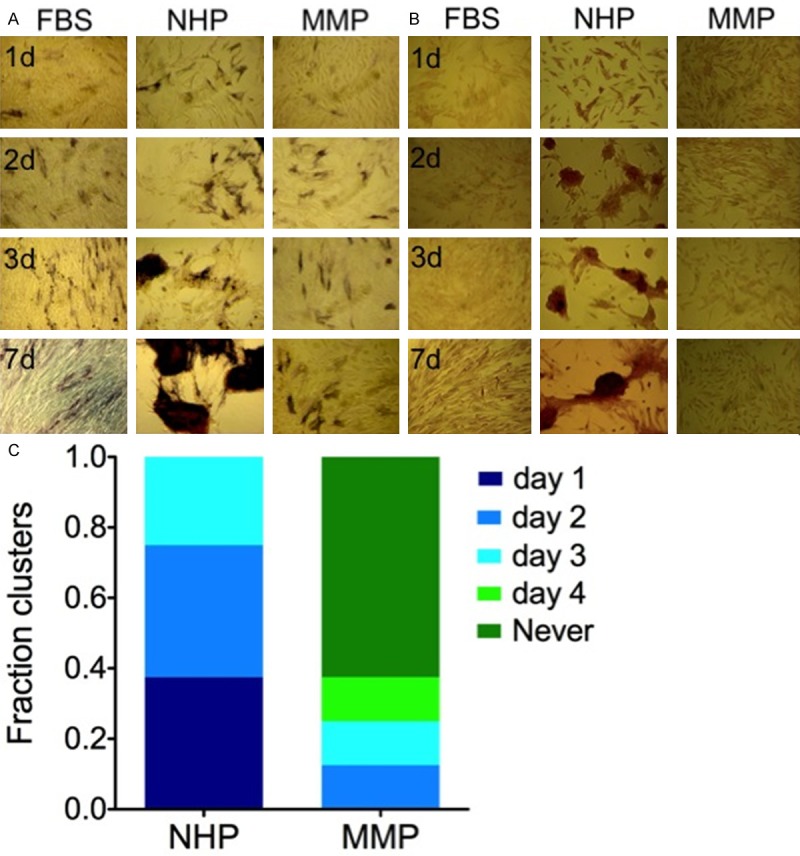
hTERT-MSCs cultured in MMP-DM fail to differentiate into OBs. hTERT-MSCs were cultured in FBS-DM, NHP-DM, or MMP-D. (A) OB formation was visualized by ALP staining (purple) and (B) calcium mineralization was detected using Alizarin Red staining (red). (C) Timing of OB differentiation was quantified based on the first day the cluster formation was evident in each condition (n = 8).
To evaluate the extent of the OB differentiation, we determined the expression of additional osteoblastic markers [23]. NHP induced expression of the extracellular matrix proteins fibronectin, collagen I, osteopontin, and osteocalcin, while MMP supported the expression of fibronectin, but not the other markers (Figure 3A). Inability of MMP to induce osteocalcin expression was further confirmed by RT-PCR (Supplementary Figure 1). Diminished expression of collagen I, osteopontin, and osteocalcin in MMP-DM grown hTERT-MSC cells, together with the failure of matrix maturation and mineralization phases of OB differentiation measured by ALP and Alizarin Red staining respectively suggested that either a loss of ‘differentiation-promoting’ factors or an overexpression of ‘differentiation-blocking’ factors in MMP was responsible for the observed defect in OB differentiation from hTERT-MSCs.
Figure 3.
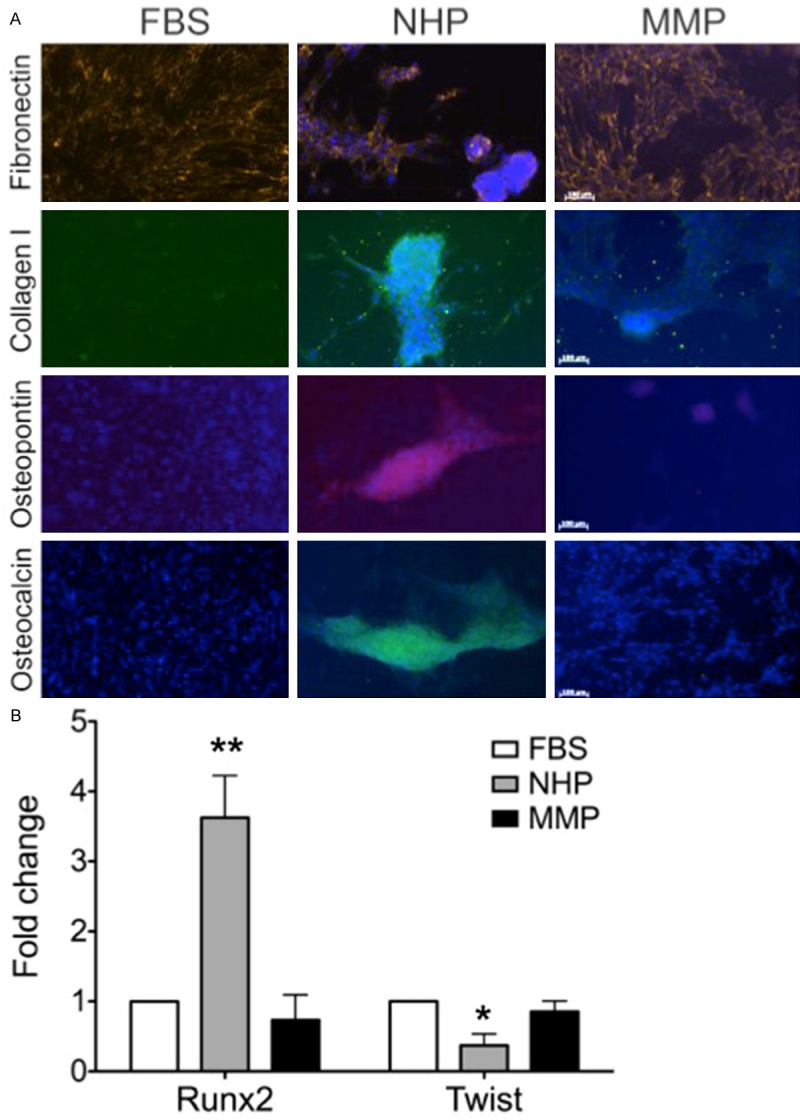
Expression of OB-specific markers is defective in hTERT-MSCs exposed to MMP. hTERT-MSCs were grown in FBS-DM, NHP-DM, or MMP-DM. A. Markers of OB differentiation were visualized by immunofluorescence (n = 3-6 independent experiments). B. Levels of Runx2 and Twist were measured for each condition by qPCR (**p-value = 0.004, FBS or MMP vs. NHP; *p-value = 0.03, FBS vs. NHP).
To further understand why hTERT-MSCs did not differentiate into OBs in the presence of plasma from MM patients, we looked upstream of the effector molecules (collagen, osteocalcin, etc.) at the level of transcription factors. Runx2 and Twist are the major transcription factors regulating the fate of MSCs and their ability to differentiate into OBs. Expression of Twist maintains the stem cell phenotype of the MSCs, while upregulation of Runx2 leads to OB differentiation and expression of Runx2-target genes, such as collagen I, osteocalcin, osteopontin, and ALP [23-25]. Confirming their OB phenotype, after 9 days in culture cells grown in NHP-DM exhibited a 5-times increase in the expression of Runx2 and a 2.3-times decrease in the levels of Twist compared to the MM-DM containing cultures (Figure 3B). The amount of Runx2 and Twist expressed by the MMP-DM cultures was not different from the levels of these molecules in undifferentiated hTERT-MSC cells grown in the presence of FBS (Figure 3B). Therefore, we established that the factors in the plasma from MM patients blocked OB differentiation of non-malignant MSCs by maintaining low levels Runx2, and thus, prevented the expression of proteins required for OB function.
Neutralizing Dkk-1 and IL-7 in the plasma of MM patients restores OB differentiation program of hTERT-MSCs
The data presented above and in other studies demonstrate that the state of malignancy can skew the stromal phenotype toward the emergence of a tumor-sustaining programming [26,27]. Therefore, we wanted to identify the factors in MMP responsible for the observed defect in OB differentiation and establish how the correct programing can be restored. Dkk-1 has been demonstrated to diminish the OB differentiation potential of MSCs [28] and an elevation of Dkk-1 levels has been detected in the bone marrow and blood sera from MM patients, which correlated with progressive bone disease [29,30]. IL-7 is another factor capable of attenuating OB differentiation [31]. We have recently shown that IL-7 levels are elevated in the circulatory environment of patients with MM [18]. When added to NHP-DM, Dkk-1 and IL-7 blocked OB differentiation of hTERT-MSCs. Compared to the control cells cultured in NHP-DM, hTERT-MSCs exposed to Dkk-1 or IL-7 failed to form ALP-positive clusters (Figure 4A). On the other hand, blocking Dkk-1 and IL-7 in MMP restored cluster formation and OB differentiation as measured by the appearance of ALP-positive cells in MMP-DM cultures supplemented with the Dkk-1 inhibitor or IL-7 receptor (IL-7R) neutralizing antibodies (Figure 4B). Therefore, we show that the disturbance of the systemic milieu in patients with MM affects the stromal environment contributing to the disruption of the bone remodeling homeostasis with the elevated levels of plasma Dkk-1 and IL-7 impeding OB differentiation and preventing the repair of bone lesions seen in MM patients.
Figure 4.
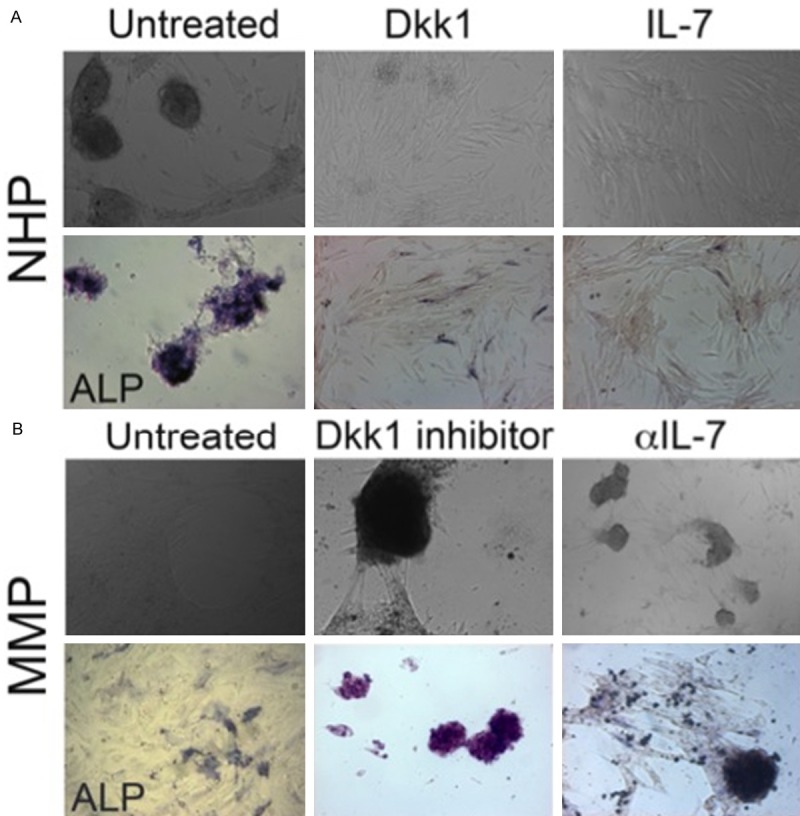
Dkk-1 and IL-7 block differentiation of hTERT-MSCs into OBs. A. hTERT-MSCs were cultured in NHP-DM with Dkk-1 or IL-7 added to the differentiation medium (n = 4 independent experiments). B. hTERT-MSCs were cultured in MMP-DM with Dkk-1 inhibitor or anti-IL-7 antibodies added to the differentiation medium (n = 4 independent experiments). Culture formation was visualized by brightfield microscopy (top panel) and OB differentiation was detected using ALP staining (bottom panel; purple).
Factors in the plasma of MM patients skew the differentiation of hTERT-MSCs toward the activated stroma phenotype
Since hTERT-MSCs cultured in MMP-DM fail to differentiate into OBs, we wanted to understand what happens to these cells upon exposure to MMP. Since fibronectin expression has been shown to upregulate osteogenesis [32], the inability of cells cultured in MMP-DM to differentiate into OB, even though they expressed fibronectin (Figure 3A), was puzzling. This prompted us to investigate the possibility that while MMP fails to induce OB differentiation of hTERT-MSCs, the factors found in MM plasma may force activation of an alternative program inducing a cancer-associated phenotype of MSCs [27]. Stromal cells in the proximity of the tumor acquire myofibroblastic characteristics and secrete cytokine and growth factors that promote tumor expansion and protect the growing tumor from the recognition by the host immune system [33]. Such MSC-derived stromal cells are referred to as cancer-associated fibroblasts or activated stroma [33,34]. Some of the features of the cancer-associated stroma are: the expression of vimentin, an intermediate filament, alpha-smooth muscle actin (αSMA), a type of actin identified in smooth muscle that is responsible for the contractile phenotype of stromal cells, and a downregulation of caveolin-1, a component of membrane caveolae [33,35-37].
Consistent with the cancer-associated stroma phenotype, increased expression of fibronectin, vimentin, and αSMA was detected in MMP-DM grown cells (Figures 3A and 5). Moreover, downregulation of caveolin-1 was also evident in MMP-DM cultures (Figure 5). Taken together, the elevated levels fibronectin, vimentin, αSMA, together with the loss of caveolin-1 expression suggest that MMP may induce differentiation of MSCs into cancer-associated stroma.
Figure 5.
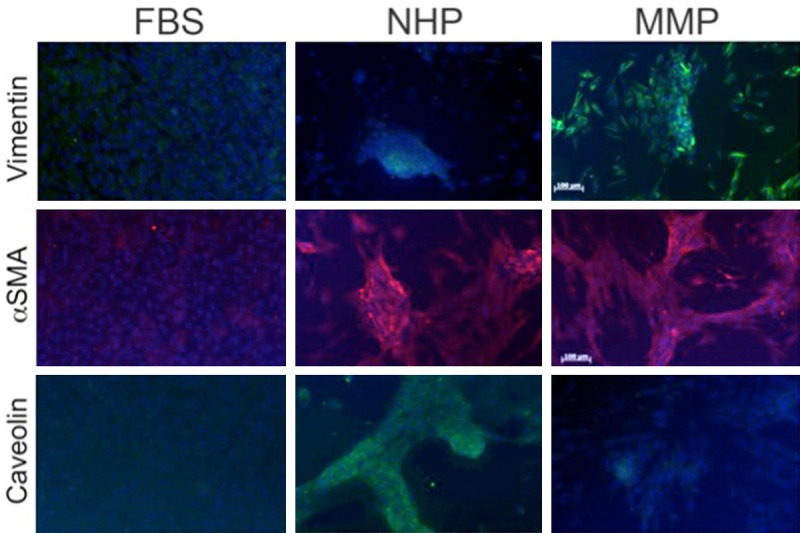
hTERT-MSCs cultured in MMP-DM acquire a cancer-associated stroma phenotype. hTERT-MSCs were grown in FBS-DM, NHP-DM, or MMP-DM. Markers of cancer-associated stroma were visualized by immunofluorescence (n = 3-6 independent experiments).
Discussion
The data presented here illustrates the importance of microenvironment in the progression of MM. We demonstrate that the alterations in the systemic microenvironment due to malignancy affected the differentiation of non-malignant MSCs into OBs contributing to the bone disease detected in 9 out of 10 myeloma patients [4]. Furthermore, we showed that correcting the systemic homeostasis by restoring the proper levels of cytokines and other secreted factors reestablished the normal differentiation program. Finally, our data suggested that instead of developing into OBs, the MSCs exposed to the circulatory factors in the plasma of MM patients converted to a cancer-associated stroma phenotype, likely contributing to sustained pro-tumorigenic microenvironment and the inevitable relapse of MM patients (Figure 6).
Figure 6.
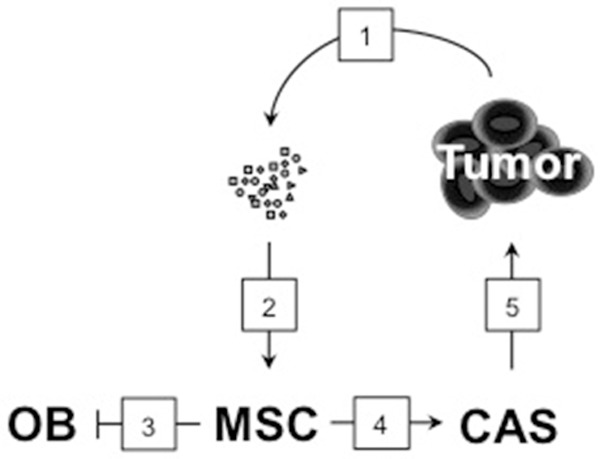
Systemic factors affect the fate of the MSCs. A proposed model of how plasma factors affect the differentiation potential of MSCs: (1) tumor cells secrete factors, cytokines, growth factors, hormones, into circulation; (2) these factors interact with MSCs, (3) blocking OB differentiation and (4) promoting the formation of cancer-associated stroma (CAS); (5) cancer-associated stroma produces tumor-promoting factors that contribute to the relapse of the malignancy.
We show that elevated levels of Dkk-1 and IL-7 in MMP were responsible for the observed defect in OB differentiation from hTERT-MSC cells. When grown in media supplemented with MMP, mRNA expression of Runx2, the osteoblast-specific transcription factor, was 5-times lower than expressed by hTERT-MSCs cultured in NHP-DM, while expression of Twist, transcription factor responsible for maintaining the stem cell phenotype of MSCs, was 2.3-times higher. MMP-grown hTERT-MSCs failed to mineralize calcium, as illustrated by the lack of the Alizarin Red-positive clusters. Furthermore, MMP-DM cultured cells did not express other markers of osteoblastogenesis and Runx2-target genes, e.g. collagen I, osteopontin, and osteocalcin. The defect in OB differentiation from MSCs was recreated by the addition of Dkk-1 or IL-7 to NHP-DM. Reducing the levels of Dkk-1 or IL-7 in MMP was sufficient to restore proper OB differentiation marked by the appearance of ALP-positive clusters. This suggested that the imbalance of either of these molecules may be enough to skew the differentiation potential of the MSCs.
Based on our previous findings that the levels of plasma cytokines are not restored to the pre-disease levels in patients in remission from MM [18], we hypothesized that instead of differentiating into OBs, MSC exposure to the soluble factors in MMP shifts the differentiation program toward the development of cancer-associated stroma. As discussed above, the hallmarks of activated stroma are the elevation of fibronectin, vimentin, αSMA, and a loss of caveolin-1 expression. It has been reported that fibronectin is produced by MSCs [38] and OBs during the normal course of differentiation [32], as well as by cancer-associated stroma, where expression has been correletaed with increased tumor growth and decreased survival [39]. We observed the presence of fibronectin in hTERT-MSC cultures grown in FBS-DM, NHP-DM, and MMP-DM. Vimentin has been detected in MMP-DM cultures, and it has been shown to suppress osteocalcin expression, and thus, OB differentiation [40]. Moreover, expression of vimentin by the activated stroma has been associated with shortened disease-free and overall survival rates [35]. αSMA, another marker of activated stroma [37] that is also present in OBs [41], was detected in both MMP-DM and NHP-DM cultures. Interestingly, while collagen I expression has been described in activated stroma, loss of collagen I in pancreatic adenocarcinoma was shown to correlate with worse prognosis and decreased survival [42], therefore, we attributed the low levels of collagen I in MMP-DM cultures to a similar phenomenon. Low expression of Runx2 and an elevated expression of Twist in MMP-DM cultures compared to NHP-DM cultures were also consistent with a cancer-associated stroma phenotype since elevated levels of Twist have been reported in cancer-associated stroma and are associated with poor prognosis [43]. Since Twist is also expressed by MSCs, it was not surprising that its levels were similar to those detected in FBS-DM cultured cells. A surprising finding was the detectable, but not statistically significant decrease in CFU-F forming ability of hTERT-MSCs grown in MMP-DM compared to FBS-DM controls (95% confidence intervals of 20.35-40.55% CFU-F formation and 17.25-27.58% for FBS-DM and MM-DM respectively). It is possible that the cells with the cancer-associated stroma phenotype differentiate from the hTERT-MSC and are responsible for the observed 8% decrease in the CFU-F forming ability of MMP-DM cultures. However, it is also possible the fraction of cells with the cancer-associated stroma phenotype retain the ability to form CFU-F.
Taken together, our data show that the systemic elevation of Dkk-1 and IL-7 results in the decrease in production of healthy OBs and contributes to the prolonged bone disease detected in MM patients. Furthermore, our findings imply that the acquisition of the cancer-associated stroma phenotype by MSCs may be responsible for producing tumor-promoting factors, and thus, further exacerbating the disease leading to the relapse of MM (Figure 6). Further studies will focus on isolating the factors responsible for conferring the activated stroma phenotype and identifying the means to block their effects on MSCs that have not been exposed to malignancy.
Acknowledgements
The authors would like to thank Dr. Dilini Gunasekera and Mukti Parikh for assisting with experimental design and set-up.
Abbreviations
- BM
bone marrow
- BM-MSC
bone marrow mesenchymal stem cell
- Dkk-1
Dickkopf-1
- FBS
fetal bovine serum
- hTERT-MSC
h-TERT immortalized bone marrow mesenchymal stem cells
- GM
growth medium
- IL-7
interleukin-7
- IL-7R
interleukin-7 receptor
- MM
multiple myeloma’
- MMP
multiple myeloma plasma
- MSC
meshenchymal stem cell
- NHP
normal human plasma
- OB
osteoblast
- Runx2
Runt-related transcription factor 2
Supporting Information
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Dimopoulos MA, San-Miguel JF, Anderson KC. Emerging therapies for the treatment of relapsed or refractory multiple myeloma. Eur J Haematol. 2011;86:1–15. doi: 10.1111/j.1600-0609.2010.01542.x. [DOI] [PubMed] [Google Scholar]
- 3.Zangari M, Terpos E, Zhan F, Tricot G. Impact of bortezomib on bone health in myeloma: A review of current evidence. Cancer Treatment Reviews. 2012;38:968–980. doi: 10.1016/j.ctrv.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 4.Roodman GD. Pathogenesis of myeloma bone disease. Leukemia. 2009;23:435–441. doi: 10.1038/leu.2008.336. [DOI] [PubMed] [Google Scholar]
- 5.Xu S, Evans H, Buckle C, Veirman KD, Hu J, Xu D, Menu E, Becker AD, Broek IV, Leleu X, Camp BV, Croucher P, Vanderkerken K, Riet IV. Impaired osteogenic differentiation of mesenchymal stem cells derived from multiple myeloma patients is associated with a blockade in the deactivation of the Notch signaling pathway. Leukemia. 2012;26:2546–2549. doi: 10.1038/leu.2012.126. [DOI] [PubMed] [Google Scholar]
- 6.Zannettino ACW, Farrugia AN, Kortesidis A, Manavis J, To LB, Martin SK, Diamond P, Tamamura H, Lapidot T, Fujii N, Gronthos S. Elevated Serum Levels of Stromal-Derived Factor-1α Are Associated with Increased Osteoclast Activity and Osteolytic Bone Disease in Multiple Myeloma Patients. Cancer Res. 2005;65:1700–1709. doi: 10.1158/0008-5472.CAN-04-1687. [DOI] [PubMed] [Google Scholar]
- 7.Dimopoulos M, Terpos E, Comenzo RL, Tosi P, Beksac M, Sezer O, Siegel D, Lokhorst H, Kumar S, Rajkumar SV, Niesvizky R, Moulopoulos LA, Durie BG Imwg. International myeloma working group consensus statement and guidelines regarding the current role of imaging techniques in the diagnosis and monitoring of multiple Myeloma. Leukemia. 2009;23:1545–1556. doi: 10.1038/leu.2009.89. [DOI] [PubMed] [Google Scholar]
- 8.Roodman GD. Biology of Osteoclast Activation in Cancer. J. Clin. Oncol. 2001;19:3562–3571. doi: 10.1200/JCO.2001.19.15.3562. [DOI] [PubMed] [Google Scholar]
- 9.Terpos E, Dimopoulos MA. Myeloma bone disease: pathophysiology and management. Ann Oncol. 2005;16:1223–1231. doi: 10.1093/annonc/mdi235. [DOI] [PubMed] [Google Scholar]
- 10.Silvestris F, Cafforio P, Calvani N, Dammacco F. Impaired osteoblastogenesis in myeloma bone disease: role of upregulated apoptosis by cytokines and malignant plasma cells. Br J Haematol. 2004;126:475–486. doi: 10.1111/j.1365-2141.2004.05084.x. [DOI] [PubMed] [Google Scholar]
- 11.Silvestris F, Cafforio P, Tucci M, Grinello D, Dammacco F. Upregulation of osteoblast apoptosis by malignant plasma cells: a role in myeloma bone disease. Br J Haematol. 2003;122:39–52. doi: 10.1046/j.1365-2141.2003.04374.x. [DOI] [PubMed] [Google Scholar]
- 12.Tian E, Zhan F, Walker R, Rasmussen E, Ma Y, Barlogie B, Shaughnessy JD. The Role of the Wnt-Signaling Antagonist DKK1 in the Development of Osteolytic Lesions in Multiple Myeloma. N Engl J of Med. 2003;349:2483–2494. doi: 10.1056/NEJMoa030847. [DOI] [PubMed] [Google Scholar]
- 13.Qian J, Xie J, Hong S, Yang J, Zhang L, Han X, Wang M, Zhan F, Shaughnessy JD, Epstein J, Kwak LW, Yi Q. Dickkopf-1 (DKK1) is a widely expressed and potent tumor-associated antigen in multiple myeloma. Blood. 2007;110:1587–1594. doi: 10.1182/blood-2007-03-082529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giuliani N, Rizzoli V, Roodman GD. Multiple myeloma bone disease: pathophysiology of osteoblast inhibition. Blood. 2006;108:3992–3996. doi: 10.1182/blood-2006-05-026112. [DOI] [PubMed] [Google Scholar]
- 15.Silbermann R, Roodman GD. Myeloma bone disease: Pathophysiology and management. Journal of Bone Oncology. 2013;2:59–69. doi: 10.1016/j.jbo.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berenson JR, Lichtenstein A, Porter L, Dimopoulos MA, Bordoni R, George S, Lipton A, Keller A, Ballester O, Kovacs M, Blacklock H, Bell R, Simeone JF, Reitsma DJ, Heffernan M, Seaman J, Knight RD. Long-term pamidronate treatment of advanced multiple myeloma patients reduces skeletal events. Myeloma Aredia Study Group. J. Clin. Oncol. 1998;16:593–602. doi: 10.1200/JCO.1998.16.2.593. [DOI] [PubMed] [Google Scholar]
- 17.Qiang YW, Hu B, Chen Y, Zhong Y, Shi B, Barlogie B, Shaughnessy JD. Bortezomib induces osteoblast differentiation via Wnt-independent activation of β-catenin/TCF signaling. Blood. 2009;113:4319–4330. doi: 10.1182/blood-2008-08-174300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zheng MM, Zhang Z, Bemis K, Belch AR, Pilarski LM, Shively JE, Kirshner J. The Systemic Cytokine Environment Is Permanently Altered in Multiple Myeloma. PLoS One. 2013;8:e58504. doi: 10.1371/journal.pone.0058504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gronthos S, Zannettino AC, Hay SJ, Shi S, Graves SE, Kortesidis A, Simmons PJ. Molecular and cellular characterisation of highly purified stromal stem cells derived from human bone marrow. J Cell Sci. 2003;116:1827–1835. doi: 10.1242/jcs.00369. [DOI] [PubMed] [Google Scholar]
- 20.Simmons PJ, Torok-Storb B. Identification of stromal cell precursors in human bone marrow by a novel monoclonal antibody, STRO-1. Blood. 1991;78:55–62. [PubMed] [Google Scholar]
- 21.Gunn EJ, Williams JT, Huynh DT, Iannotti MJ, Han C, Barrios FJ, Kendall S, Glackin CA, Colby DA, Kirshner J. The natural products parthenolide and andrographolide exhibit anti-cancer stem cell activity in multiple myeloma. Leuk Lymphoma. 2011;52:1085–1097. doi: 10.3109/10428194.2011.555891. [DOI] [PubMed] [Google Scholar]
- 22.Giuliani N, Bataille R, Mancini C, Lazzaretti M, Barille S. Myeloma cells induce imbalance in the osteoprotegerin/osteoprotegerin ligand system in the human bone marrow environment. Blood. 2001;98:3527–3533. doi: 10.1182/blood.v98.13.3527. [DOI] [PubMed] [Google Scholar]
- 23.Stein GS, Lian JB, van Wijnen AJ, Stein JL, Montecino M, Javed A, Zaidi SK, Young DW, Choi JY, Pockwinse SM. Runx2 control of organization, assembly and activity of the regulatory machinery for skeletal gene expression. Oncogene. 2004;23:4315–4329. doi: 10.1038/sj.onc.1207676. [DOI] [PubMed] [Google Scholar]
- 24.Cakouros D, Raices RM, Gronthos S, Glackin CA. Twist-ing cell fate: mechanistic insights into the role of twist in lineage specification/differentiation and tumorigenesis. J Cell Biochem. 2010;110:1288–1298. doi: 10.1002/jcb.22651. [DOI] [PubMed] [Google Scholar]
- 25.Isenmann S, Arthur A, Zannettino AC, Turner JL, Shi S, Glackin CA, Gronthos S. TWIST family of basic helix-loop-helix transcription factors mediate human mesenchymal stem cell growth and commitment. Stem Cells. 2009;27:2457–2468. doi: 10.1002/stem.181. [DOI] [PubMed] [Google Scholar]
- 26.Sounni NE, Noel A. Targeting the tumor microenvironment for cancer therapy. Clin Chem. 2013;59:85–93. doi: 10.1373/clinchem.2012.185363. [DOI] [PubMed] [Google Scholar]
- 27.Markovina S, Callander NS, O’Connor SL, Xu G, Shi Y, Leith CP, Kim K, Trivedi P, Kim J, Hematti P, Miyamoto S. Bone marrow stromal cells from multiple myeloma patients uniquely induce bortezomib resistant NF-kappaB activity in myeloma cells. Mol Cancer. 2010;9:176. doi: 10.1186/1476-4598-9-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fujita K, Janz S. Attenuation of WNT signaling by DKK-1 and -2 regulates BMP2-induced osteoblast differentiation and expression of OPG, RANKL and M-CSF. Mol Cancer. 2007;6:71. doi: 10.1186/1476-4598-6-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qiang YW, Chen Y, Stephens O, Brown N, Chen B, Epstein J, Barlogie B, Shaughnessy JD Jr. Myeloma-derived Dickkopf-1 disrupts Wnt-regulated osteoprotegerin and RANKL production by osteoblasts: a potential mechanism underlying osteolytic bone lesions in multiple myeloma. Blood. 2008;112:196–207. doi: 10.1182/blood-2008-01-132134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaiser M, Mieth M, Liebisch P, Oberlander R, Rademacher J, Jakob C, Kleeberg L, Fleissner C, Braendle E, Peters M, Stover D, Sezer O, Heider U. Serum concentrations of DKK-1 correlate with the extent of bone disease in patients with multiple myeloma. Eur J Haematol. 2008;80:490–494. doi: 10.1111/j.1600-0609.2008.01065.x. [DOI] [PubMed] [Google Scholar]
- 31.Giuliani N, Colla S, Morandi F, Lazzaretti M, Sala R, Bonomini S, Grano M, Colucci S, Svaldi M, Rizzoli V. Myeloma cells block RUNX2/CBFA1 activity in human bone marrow osteoblast progenitors and inhibit osteoblast formation and differentiation. Blood. 2005;106:2472–2483. doi: 10.1182/blood-2004-12-4986. [DOI] [PubMed] [Google Scholar]
- 32.Li B, Moshfegh C, Lin Z, Albuschies J, Vogel V. Mesenchymal stem cells exploit extracellular matrix as mechanotransducer. Sci Rep. 2013;3:2425. doi: 10.1038/srep02425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Polanska UM, Orimo A. Carcinoma-associated fibroblasts: non-neoplastic tumour-promoting mesenchymal cells. J Cell Physiol. 2013;228:1651–1657. doi: 10.1002/jcp.24347. [DOI] [PubMed] [Google Scholar]
- 34.Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL, Weinberg RA. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 35.Ngan CY, Yamamoto H, Seshimo I, Tsujino T, Man-i M, Ikeda JI, Konishi K, Takemasa I, Ikeda M, Sekimoto M, Matsuura N, Monden M. Quantitative evaluation of vimentin expression in tumour stroma of colorectal cancer. Br J Cancer. 2007;96:986–992. doi: 10.1038/sj.bjc.6603651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sotgia F, Martinez-Outschoorn UE, Pavlides S, Howell A, Pestell RG, Lisanti MP. Understanding the Warburg effect and the prognostic value of stromal caveolin-1 as a marker of a lethal tumor microenvironment. Breast Cancer Res. 2011;13:213. doi: 10.1186/bcr2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fujita H, Ohuchida K, Mizumoto K, Nakata K, Yu J, Kayashima T, Cui L, Manabe T, Ohtsuka T, Tanaka M. alpha-Smooth Muscle Actin Expressing Stroma Promotes an Aggressive Tumor Biology in Pancreatic Ductal Adenocarcinoma. Pancreas. 2010 doi: 10.1097/MPA.0b013e3181dbf647. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 38.Singh P, Schwarzbauer JE. Fibronectin and stem cell differentiation - lessons from chondrogenesis. J Cell Sci. 2012;125:3703–3712. doi: 10.1242/jcs.095786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ioachim E, Charchanti A, Briasoulis E, Karavasilis V, Tsanou H, Arvanitis DL, Agnantis NJ, Pavlidis N. Immunohistochemical expression of extracellular matrix components tenascin, fibronectin, collagen type IV and laminin in breast cancer: their prognostic value and role in tumour invasion and progression. Eur J Cancer. 2002;38:2362–2370. doi: 10.1016/s0959-8049(02)00210-1. [DOI] [PubMed] [Google Scholar]
- 40.Lian N, Wang W, Li L, Elefteriou F, Yang X. Vimentin inhibits ATF4-mediated osteocalcin transcription and osteoblast differentiation. J Biol Chem. 2009;284:30518–30525. doi: 10.1074/jbc.M109.052373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kinner B, Spector M. Expression of smooth muscle actin in osteoblasts in human bone. J Orthop Res. 2002;20:622–632. doi: 10.1016/S0736-0266(01)00145-0. [DOI] [PubMed] [Google Scholar]
- 42.Erkan M, Michalski CW, Rieder S, Reiser-Erkan C, Abiatari I, Kolb A, Giese NA, Esposito I, Friess H, Kleeff J. The activated stroma index is a novel and independent prognostic marker in pancreatic ductal adenocarcinoma. Clin Gastroenterol Hepatol. 2008;6:1155–1161. doi: 10.1016/j.cgh.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 43.Riaz M, Sieuwerts AM, Look MP, Timmermans MA, Smid M, Foekens JA, Martens JW. High TWIST1 mRNA expression is associated with poor prognosis in lymph node-negative and estrogen receptor-positive human breast cancer and is co-expressed with stromal as well as ECM related genes. Breast Cancer Res. 2012;14:R123. doi: 10.1186/bcr3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


