Abstract
Leishmaniasis is spreading from mediterranean countries to the north of Europe. The Alps are not an endemic region and there are only few reports of sporadic cases. We report the case of a 72 year old male who presented after a syncope with fever, cough and a sacral skin rash. Clinical examination revealed splenomegaly, elevated liver enzymes and pancytopenia; differential diagnosis included myeloproliferative or lymphoproliferative disorders, infections and auto-immune conditions that cause enlargement of the spleen and liver diseases, however, all tests were negative. In 18FDG PET computerized tomography, pathological and diffuse uptake in the spleen was seen, with mild and homogeneous FDG uptake in the bone marrow and normal tracer uptake elsewhere in the body. Bone marrow aspiration revealed the presence of numerous intra- and extracellular Leishmania amastigotes. Travel history indicated that he had been in Sardinia for a 7-day vacation several months ago. The patient promptly responded to treatment with liposomal amphotericin B. Imported visceral leishmaniasis is likely to be seen more frequently in non-endemic regions and fever, pancytopenia and splenomegaly are diagnostic clues, whereas diagnostic confirmation may be done by detection of Leishmania spp. amastigotes in the bone marrow.
Keywords: Immunocompetent, imported, visceral leishmaniasis, imaging, hypersplenism
Introduction
Leishmaniasis is a common parasitic disease in developing countries [1] with a wide variability of clinical manifestations: it can be asymptomatic or manifest in its three clinical variants including visceral (VL, also known as kala-azar), cutaneous and mucosal leishmaniasis [2]. More than 17 different Leishmania species are known [3]. In the mediterranean region, Leishmania infantum is the major cause of disseminated visceral forms. VL is endemic in more than 60 countries worldwide, but 90% of cases are confined to just six countries: India, Bangladesh, Nepal, Brazil, Ethiopia, and Sudan; [3]. Leishmaniasis is the only tropical/subtropical vector-borne disease that has been endemic in Southern Europe [1].
Transmission may be zoonotic or anthroponotic, by the bite of female phlebotomite sand flies. In Mediterranean countries, dogs are the main reservoir of parasites, whereas the prevalence of canine Leishmaniasis is high as well (over 25%). The incubation period of the infection varies between one and twelve months, and VL is characterized by persistent fever or febrile episodes generally followed by phases of apyrexia. Fatigue, weight loss, hepato-splenomegaly, elevation of liver enzymes, anemia, leucopenia and thrombocytopenia are other signs and symptoms.
This case report emphasizes that diagnosis may be insidious when Leishmaniasis is imported into non-endemic regions. The first published case in South Tyrol is reported, an alpine province of Italy located in the upper north of the mediterranean country.
Case presentation
A 72-year-old German male with the past medical history of renal cell cancer that was cured by nephrectomy several years ago was admitted to our hospital because of “syncope in the course of febrile infection”. Upon arrival in the emergency department, the patient reported to have experienced palpitations and tachycardia for about two to three weeks. For this reason he had seen a cardiologist a week ago who, however, did not reveal anything abnormal. Then the patient noted the appearance of an erythematous-desquamative lesion at the level of the buttock that on advice of a dermatologist was successfully treated with an antifungal cream. Four days before admission he broke into shivers which were associated with dry cough but no fever leading to the prescription of azithromycin by his family physician. The four day course of antibiotic was without benefit and chills and cough persisted which were subsequently associated with fever. The night prior to admission, on his way to the bathroom, the patient collapsed without loss of consciousness. For this reason he decided to visit the regional hospital’s emergency room.
Upon arrival, the patient displayed heart rate and blood pressure within normal limits and without pathological orthostatic changes. Respiratory rate was normal and temperature was 37.5°C. He had not traveled in tropical or exotic areas and had no exposure to animals. His life style was without risky behavior.
Results of cardiovascular examinations were normal. A 12-lead electrocardiogram showed sinus rhythm without conduction abnormalities. Also the rest of physical and neurological examinations was normal including liver and spleen, the latter not being palpable.
Entrance laboratory examinations showed mild anemia (hemoglobin of 11.0 g/dl) and mild thrombocytopenia (137,000/μl); white blood cell count was 5,000/µl with 59.9% neutrophils, 25.9% lymphocytes, 12.3% monocytes, 1.2% eosinophils and 0.9% basophils; creatinine was increased (1.26 mg/dl) with an estimated glomerular filtraton rate of 56 ml/min.m2; liver enzymes were slightly elevated (alkaline phosphatase 165 U/l, upper range of normal < 40 U/l), serum gamma-glutamyl transferase 132 U/l, upper range of normal < 60 U/l) with alanine transaminase and aspartate transaminase being normal; C-reactive protein was increased (7.05 mg/dl, upper range of normal 0.5 mg/dl).
Diagnostic work-up explored for the type of syncope with unremarkable tests results including 48 hours of telemetric monitoring. Cerebral computerized tomography did not show focal brain lesions. An ultrasound examination of the abdomen showed mild splenomegaly (14.1 cm) in the absence of other pathological findings. On transthoracic echocardiography, slight thickening of the mitral leaflets in the absence of coarse vegetation was suspected but not confirmed in trans-esophageal echocardiography. Because of pancytopenia and splenomegaly, a bone marrow biopsy was performed, from which however no diagnostic clues were initially obtained. The patient was therefore subjected to whole body 18FDG-positron emission computerized tomography (18FDG –PET/CT) in order to identify candidate lymph nodes for eventual biopsy; results confirmed splenomegaly with pathological accumulation of the radiopharmaceutical with mild and homogeneous FDG uptake in the bone marrow, however, in the absence of further hypermetabolic findings (Figure 1).
Figure 1.
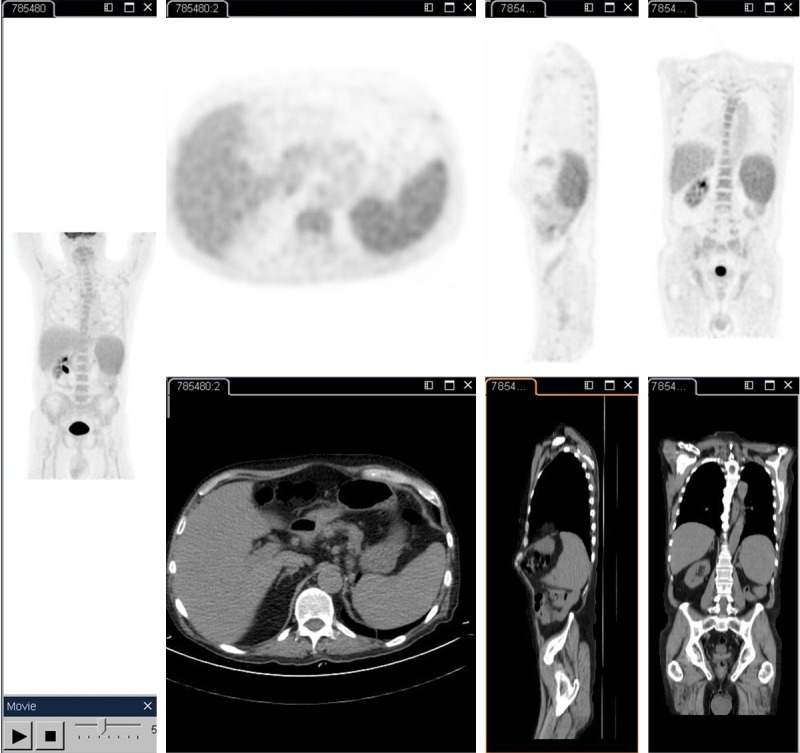
18FDG PET scan demonstrating hypermetabolic areas in spleen and bone marrow
Suspected fungal skin infection of the buttocks, which was treated topically by the family physician, was not considered as a cause of fever and systemic signs of inflammation. In addition, skin biopsy was avoided because of a keloid present after kidney surgery at the incision site. Microbiologic culture for fungi performed from a skin scrap from the suspected site upon admission was negative. Additionally, serial blood and stool cultures were performed, all negative. An oral swab culture was positive for Candida spp.. Systemic therapy with fluconazole was started at a daily dose of 400 mg.
Unremarkable serologic test results were obtained for antibodies to Cytomegaly and Ebstein Barr virus, Borrelia burgdorferi, Coxiella burneti, Mycoplasma pneumoniae, Chlamydia trachomatis; Brucella spp., Treponema pallidum, Bartonella henselae, Toxoplasma, and Interferon-gamma release assay (Quantiferon-TB) for latent tuberculosis infection; (1–3)-β-D-glucan assay, Widal–Wright test and HIV test were also negative.
Autoimmune diseases were searched with a series of autoantibody tests which showed only weak positivity for antibodies to smooth muscle (titer 1:40), anti-nuclear antibodies (1:160 with a nucleolar pattern) without concomitant extractable nuclear antigen, and a test for cryoglobulins was positive, the rest of screening tests being negative. The observed increase of liver enzymes remained unchanged during the diagnostic workup period and alpha 1 antitrypsin levels were increased as well.
Since microbiologic, hematologic and autoimmune tests were all not diagnostic, extended differential diagnoses were considered: for possible Salmonella-caused disease, empiric oral treatment with doxycyclin 200 mg/day was started, and for suspected visceral Leishmaniasis a fragment of the bone marrow sample was stained with May-Grünwald Giemsa. Microscopic examination turned out positive for the presence of numerous amastigotes of Leishmania spp. (Figure 2), and serology confirmed the diagnosis of visceral leishmaniasis (anti-Leishmania IgG titer 1:320).
Figure 2.
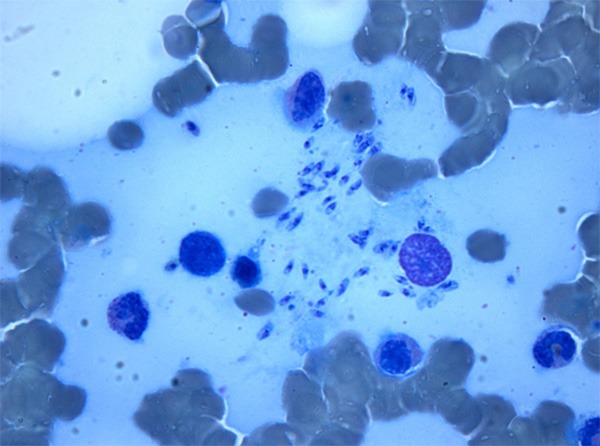
Histopathological examination of the bone marrow showing extracellular leishmania amastigotes; May-Grünwald Giemsa stain.
Therapy with liposomal amphotericin B at 3 mg/kg/day once daily at days 1-5 and days 14 and 21 with quick normalization of body temperature, subsequent decrease of indices of hepatic cytolysis and normalization of pancytopenia was observed along subjective clinical improvement. The patient was discharged after more than four weeks of hospitalization with the diagnosis of VL and its successful initial treatment that was tolerated without renal side effects.
Discussion
The present case study reflects the complexity of an extended diagnostic work-up in a patient who presented with fever, mild pancytopenia, elevated liver enzymes and splenomegaly. Typical causes for such combination of symptoms and findings turned out negative and empiric short-term antimicrobial therapies including macrolide antibiotic, tetracycline and fluconazole were not successful. Because of mild pancytopenia, diagnostic considerations included hematological diseases including lymphoma of the spleen and hairy cell leukemia. Negative peripheral blood smear and molecular diagnostic tests as well as the result of bone marrow biopsy examination made hematological diseases other than a rare primary lymphoma of the spleen very unlikely. The execution of bone marrow biopsy, however, was crucial because upon re-examination the diagnosis of a disseminated infection with Leishmania spp. was made only there, then confirmed by positive serology.
In non-endemic areas, surprising identification in bone marrow smears of Leishmania amastigotes both extra- and intracellularly within macrophages is being reported more frequently [4-6]. The diagnosis of VL by Leishmania infantum can then be supported by serology and polymerase chain reaction, and confirmed by clinical responsiveness to therapy with liposomal amphotericin B. This was so also in the case of our patient.
Splenomegaly without lymphadenopathy in not uncommon in VL and confirmation for this pattern comes from 18FDG –PET/CT findings [7-10]. Our related test result is in line with previous descriptions of FDG avid splenic accumulation due to parasite uptake into cells of the reticulo-endiothelial system to form nodular red pulp expansion. Lymphnodes were FDG negative and identification of Leishmania amastigotes in the bone marrow avoided splenectomy.
The travel history of our patient was unremarkable except a one-week vacation in Sardinia several months ago. In Italy canine leishmaniasis is most frequent in the areas of the Tyrrhenian, Ionian and Adriatic coasts in central and southern Italy, but also in Sicily, Sardinia and Elba, which are typical mediterranean climate zones [11,12]. Due to climatic changes seen over the last decade, an increase in the number of dogs infected with leishmaniasis has been observed in areas previously considered not at risk. The most likely place of infection in our case is Sardinia. However, imported VL in the Italian Alps has not been reported in the medical literature even though endemic mediterranean areas are frequently visited by inhabitants for vacations.
In severe immunodeficiency, VL is a well-known opportunistic infection particularly in areas where leishmaniasis is endemic. In non-endemic regions, it has rarely been seen in patients who are not immunosuppressed. Immunosuppression may lead to reactivation of a preexisting leishmania infection. Leishmania transmission may also occur with blood transfusions. However, none of these risk factors were present in our case.
In conclusion, imported VL is likely to be seen more frequently in non-endemic regions like central and northern Europe and fever, pancytopenia and splenomegaly are diagnostic clues. Diagnostic confirmation is provided by histologic bone marrow finding of Leishmania spp. amastigotes.
Disclosure of conflict of interest
None.
References
- 1.Guerin PJ, Olliaro P, Sundar S, Boelaert M, Croft SL, Desjeux P, Wasunna MK, Bryceson AD. Visceral leishmaniasis: current status of control, diagnosis, and treatment, and a proposed research and development agenda. Lancet Infect Dis. 2002;2:494–501. doi: 10.1016/s1473-3099(02)00347-x. [DOI] [PubMed] [Google Scholar]
- 2.Pavli A, Maltezou HC. Leishmaniasis, an emerging infection in travelers. Int J Infect Dis. 2010;14:e1032–e1039. doi: 10.1016/j.ijid.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 3.Maltezou H, Gikas A, editors. eds. Tropical and Emerging Infectious Diseases. Kerala, India: Research Signpost; 2010. pp. 163–185. [Google Scholar]
- 4.Drexler B, Holbro A. Unexpected bone marrow finding in a patient with pancytopenia after hematopoietic stem cell transplantation. Blood. 2014;124:678. doi: 10.1182/blood-2014-05-576769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mansueto P, Seidita A, Vitale G, Cascio A. Leishmaniasis in travelers: A literature review. Travel Med Infect Dis. 2014;12:563–581. doi: 10.1016/j.tmaid.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 6.Silva JM, Zacarias DA, de Figueirêdo LC, Soares MR, Ishikawa EA, Costa DL, Costa CH. Bone marrow parasite burden among patients with New World kala-azar is associated with disease severity. Am J Trop Med Hyg. 2014;90:621–626. doi: 10.4269/ajtmh.13-0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fuertes J, Garcia-Bennett JR, Iftimie S, Danús M, Abreu JA. Focal splenic FDG uptake in a patient with Kala-Azar (visceral leishmaniasis) Clin Nucl Med. 2014;39:387–90. doi: 10.1097/RLU.0000000000000369. [DOI] [PubMed] [Google Scholar]
- 8.Gibson GM, Arnold C, Ravi Kumar AS. 18F-FDG uptake in multiple splenic foci on PET/CT: an unusual case of visceral leishmaniasis. Clin Nucl Med. 2014;39:828–30. doi: 10.1097/RLU.0000000000000378. [DOI] [PubMed] [Google Scholar]
- 9.Kyrtatos PG, Debard A, Martin-Blondel G, Alvarez M, Delobel P, Marchou B, Massip P, Wagner T. FDG-PET/CT findings during immune reconstitution in an HIV-1 patient infected with visceral leishmaniasis. Infection. 2013;41:1017–9. doi: 10.1007/s15010-013-0459-2. [DOI] [PubMed] [Google Scholar]
- 10.Lupi A, Todeschini G, Zanco P. Diffuse metabolic activation of reticuloendothelium on F-18 FDG PET imaging in a case of visceral leishmania. Clin Nucl Med. 2006;31:34–6. doi: 10.1097/01.rlu.0000191574.30071.d7. [DOI] [PubMed] [Google Scholar]
- 11.Cascio A, Gradoni L, Scarlata F, Gramiccia M, Giordano S, Russo R, Scalone A, Camma C, Titone L. Epidemiologic surveillance of visceral leishmaniasis in Sicily, Italy. Am J Trop Med Hyg. 1997;57:75–78. doi: 10.4269/ajtmh.1997.57.75. [DOI] [PubMed] [Google Scholar]
- 12.Di Masi F, Ursini T, Iannece MD, Chianura L, Baldasso F, Foti G, Di Gregorio P, Casabianca A, Storaci N, Nigro L, Colomba C, Marazzi MG, Todaro G, Tordini G, Zanelli G, Cenderello G, Acone N, Polilli E, Migliore S, Almi P, Pizzigallo E, Sagnelli E, Mazzotta F, Russo R, Manzoli L, Parruti G. Five-year retrospective Italian multicenter study of visceral leishmaniasis treatment. Antimicrob Agents Chemother. 2014;58:414–418. doi: 10.1128/AAC.00840-13. [DOI] [PMC free article] [PubMed] [Google Scholar]


