Abstract
Progressive supranuclear palsy (PSP) is an atypical parkinsonism, which is the third most common geriatric neurodegenerative disease. We reported three pathology-confirmed Chinese PSP cases with special focus on the pathological accumulations of tau, a-synuclein and A-beta in the three PSP brains. Cases 1 and 2 initiated with extrapyramidal signs and gait disorders, while case 3 suffered behavioral abnormalities with cognitive decline at the beginning. In neuropathology, PSP-changes such as tau-positive tufed astrocytes, oligdendrocytes with the tau-positive coiled-body and threads and globose NFTs were widely seen in the basal ganglia, isocortex and allocortex, as well as in brainstem, cerebellum and spinal cord. In addition, numerous AGs were found in the hippocampus of cases 1 & 2, while Aβ amyloid depositions were found in hippocampus and leptomeningeal vessels of case 1 and in neocortex, entorhinal cortex, hippocampus, cingulate gyrus and amygdale of case 3. Vessel infarcts were observed in cases 1. Cortical laminar III necrosis in case 1 suggested the ischemic damage. Cervical spinal cords in cases 2 & 3 were obtained with tau-positive globose NFTs, tufted astrocytes and neuropil threads were respectively found in the neurons of anterior horn and surrounding white matters. In summary, pathological examination is crucial for the ambiguous cases to exclude other neurodegenerative diseases. Furthermore, cervical spinal cord should be routinely examined in the PSP autopsy.
Keywords: Progressive supranuclear palsy, autopsy, pathology, Alzheimer’s disease
Introduction
Progressive supranuclear palsy (PSP) is an atypical parkinsonism, which is the third most common geriatric neurodegenerative disease after Alzheimer’s disease (AD) and Parkinson’s disease (PD), with the prevalence of 6.4 per 100,000 [1]. PSP was first described by Steel, Richardson and Olszewski as a clinical disease in 1964 [2]. The clinical findings of PSP were mainly featured by akinesia, symmetric rigidity, vertical gaze palsy, postural instability and falls. The classic clinical type is called Richardson’s syndrome. While there are additional two types, i.e. PSP-parkinsonism (PSP-P) and pure akinesia with gait freezing (PAGF) [3]. Meantime, non-motor symptoms such as dementia, apraxia of speech and neuropsychiatric disorders are also common seen in PSP [4,5]. Based on the clinicopathological studies, several clinical diagnostic criteria for PSP have been raised, such as the National Institute of Neurological Disorders and Stroke and the Society for PSP (NINDS-SPSP) [6,7].
In neuropathology, PSP belongs to four-repeat tauopathy and neuronal loss, gliosis and neurofibrillary tangles have been found in the basal ganglia, diencephalon, brainstem, cerebellum and spinal cord, especially with Gallyas silver stains and immunohistochemistry of tau protein [8]. The globus pallidus, subthalamic nucleus and substantia nigra are the most affected, while the limbic lobe is generally thought to be less affected in PSP. However, SPECT studies illustrate that the hypoperfusion in anterior cingulate and midcingulate is also involved in PSP [9,10]. Tufted astrocytes are believed as a distinct pathological change in PSP, which can be usually found in the motor cortex and striatum [11]. Other than tau accumulation, Lewy body can be seen in about 10% of PSP cases, which is comparable to the proportion in normal elderly population [12]. Alzheimer type pathology like numerous senile plaques can also been observed in the cortex of PSP, but seems to reflect independent pathological processes [13].
In China, few pathologically confirmed PSP cases have been reported due to the very low rate of autopsy. Therefore, the clinical differential diagnosis from frontotemporal lobe dementia and corticobasal degeneration is crucial and sometimes difficulty for the atypical PSP cases. In this study, we reported three pathology-confirmed Chinese PSP cases collected from the brain bank of geriatric neurodegenerative diseases at the Chinese PLA general hospital [14]. Comparative clinicopathologic study was carried out with special focus on the pathological accumulations of tau, a-synuclein and A-beta in the three PSP brains. Furthermore, the clinical heterogeneity in PSP and atypical PSP cases can be better understood with the related neuropathological evidences.
Case report
Case 1 is a 79-year-old man with progressive dyskinesia in limbs, postural abnormality, recurrent falls and language disorders. Six years ago, he began to suffer impaired dexterity of limbs and was prone to falls. About 3 months later, he fell again with right femoral neck fracture. Since then, he presented non-fluent disturbance of speech. He was admitted to our hospital due to sudden weakness in left leg and walking difficulty at 75 years old. Neurological examinations showed masked face and dysarthria. Both of recent and distant memories were affected. He was even unable to make a simple calculation or answered a simple question. Ocular movements were normal. Muscle strength was decreased in left leg (4/5), but normal in other limbs (5/5). Resting and postural tremors in limbs were not observed. Limbs and axial rigidity was detected and alternative movement was awkward. Nuchal dystonia was found with retrocollis. Deep tendon reflexes in left were brisker than right. Left Babinski’s sign and bilateral Chadock’s sign were positive. Brain CT showed mild symmetric enlargement in the lateral ventricles. Multiple small hypodensity foci were also found in the periventricular areas. X-ray showed the stenosis of canalis vertebralis at the level of cervical vertebrae 4-5. Thus, he was diagnosed as multiple cerebral infarcts and cervical spondylotic myelopathy. However, treatment focusing on cerebral ischemia was of no benefits. One year later, he was again admitted to our hospital due to weakness in limbs and confusion of consciousness for one day. In neurological examinations, his speech was nonfluent with incoherent words. Ocular movements were not limited but slow in vertical direction. Muscle strength in lower limbs was decreased (4/5). Gait was unstable. Sensation was intact. Deep tendon reflexes in bilateral upper limbs were symmetric, but decreased in lower limbs. Bilateral palmomental, sucking reflexes, Babinski’s and Chadock’s sign were positive. There were no benefits in treatment with levodopa. His clinical diagnosis was multiple cerebral infarcts, vascular parkinsonism, and cervical spondylotic myelopathy. Later, his condition was deteriorated and finally became bedridden. At 79 year old, the patient died due to choking by feeding apple. The total duration of disease was 6 years. There was no family history of any neurological disease.
Case 2 is an 83-year-old man with 11-year history of progressive movement difficulties in limbs, falls, ocular movement disorders and postural abnormality. He began to experience the recurrent falls with postural abnormality characterized by leaning back up the stairs or forward down the stairs at 72 years old. He also presented bradykinesia in limbs, lack of spontanous speech and orolingual dyskinesia. One year later, he suffered prominent memory impairment. He was unable to write and dress. Second year after onset, he had right clavicle fracture resulted from falls. In neurological examinations, he demonstrated markedly decreased spontaneous speech, poor language comprehension, difficulty in language expression, and impaired calculation, recent and remote memories. The MMSE score was 10/30. Ocular movements were limited on up gaze. He had wriggling of the tongue. Muscle strength was normal. Muscular tension was normal in the limbs but with marked axial rigidity. Nuchal dystonia was found with retrocollis. The finger-nose test and knee-heel test were normal. Sensations were intact. Deep tendon reflex were lower but symmetrical. Babinski’s sign and Chaddock sign were negative. The clinical diagnosis was probable PSP. At 76 years old, he was back to our hospital. Neurological examinations showed limted vertical eye movement with clumsy rapid alternative movements in hands. Brain MRI showed atrophy in frontal lobe and brainstem. No benefits were found in treatment with levodopa. Subsequently, his condition deteriorated. At 78 years old, his speech became incomprehensible. Movement disorders in limbs progressed and he was bedridden. At 82 years old, he was hospitalized in our department again, because of a fever and drowsiness. Neurological examinations revealed severe dementia. There was marked axial and limbs rigidity. The decerebrated posture was elicited by pain stimulation. Deep tendon reflex in lower limbs was weak. Sucking reflexes were positive. Jaw reflex was brisk. Palmomental reflex was positive. Bilateral Chaddock’s sign was positive, but Babinski’s sign was negative. One year later, he died from pneumonia. There was no history of cerebral vascular disease or encephalitis.
Case 3 is an 83-year-old male patient with 9-year history of behavioral abnormalities with cognitive decline, and 4-year history of bradykinesia with postural abnormality. At 75 years old, he presented behavioral abnormality like greeting strangers and became irritable. At 77 years old, compulsive behaviors like hyperphagia were observed on him. At the same time, his language was incoherent and he was scatterbrained during the converation. He even got lose in the familiar place. At 78 years old, he was admitted into our department due to behaviroal abnormality and memory loss. Neurological examinations showed that he was euphoric. His language was lack of coherence. His memory was impaired and MMSE score was 22/30. Ocular movements were normal. Muscle strength and tension in the limbs was normal. Sensation was normal. Babinski’s sign was negative. The clinical diagnosis was probable early Alzheimer’s disease. At 79 years old, neurological examinations exhibited impaired vertical eye movements. Muscle strength and tension in the limbs was normal but gait was abnormal. He was loss of swinging in right arm and dragging in right leg during walking. Nuchal dystonia was found with retrocollis. Palmomental reflexe was positive. The clinical diagnosis was considered as probable AD or corticobasal degeneration (CBD). Donepezil seemed to be effective on him. At 80 years old, he was severe dementia with MMSE score 9/30. There was no spontaneous speech. Pupils were equal size and light reflex was normal. There was palsy of vertical eye movements but horizontal eye movements were normal. He suffered from dysarthria and dysphagia. Muscle strength in limbs was normal. Axial and upper limbs rigidity was observed. Deep tendon reflex in the right limbs were brisker than the left. At 82 years old, he became mutism. He had trismus. Muscle strength in bilateral lower limbs was weak (4/5). Hypertonia was more prominent in the right. Bilateral Babinski’s and Chadock’s signs were negative, but bilateral palmomental and sucking reflexes were positive. Brain MRI showed mild ventricle enlargement, marked atrophy in the left frontal and temporal lobes and midbrain, and ‘Hummingbird sign’ in the midbrain (Figure 1A). PET (18F-FDG) revealed hypometabolism in bilateral cerebral cortex and thalamus, with more prominent in left frontal, temporal and parietal cortex. He died at 83 years old. He had history of bilateral orchidectomy due to prostate cancer at 76 years old. There was no family history of neurological disease. The summary of clinical information was listed in Table 1.
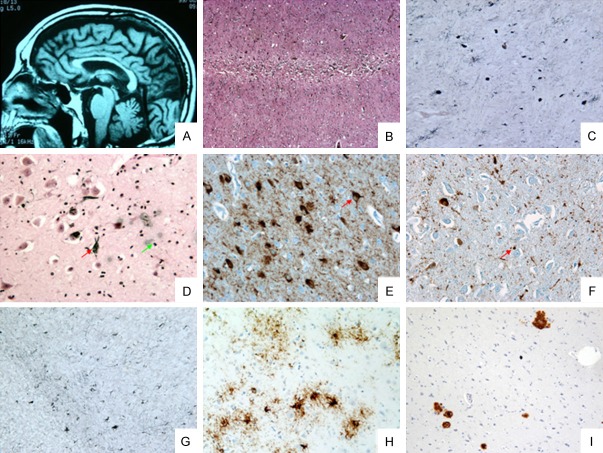
Figure 1.
(A) Hummingbird sign (case 3); (B) Cortical laminar necrosis (case 1); (C) Tufted astrocytes (case 1, red arrow) globous NETs and neuropil therads in globus pallidus; (D) Alzheimer’s disease-like neurofibrally tangles (NFTs) (case 1, red arrow) and argyrophilic grains (AGs) in CA1 (green arrow); (E) Globous NETs, pre-tangles (case 2, red arrow) and AGs in CA1; (F) CA4 was less affected than CA1 with obvious AGs (case 2, red arrow); (G) Tufted astrocytes and threads in cerebellar white matter (case 2); (H) Tufted astrocytes, coiled-bodies and threads in frontal cortex (case 3); (I) A-beta amyloid deposition in amygdala (case 3). HE stain (B), Gallyas-Braak stain (C, D & G), AT8 stain (E, F & H), A-beta stain (I); Scale bar: 500 μm in (B), 100 μm in (C-F, H & I), and 50 μm in (G).
Table 1.
Clinical summary of PSP patients
| ES | GP | MS | DTR | CN | SS | BS | SLNP | OD | Apraxia | Speech | GF | CDN | NI | LRP | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case 1 | ++ | + | + | + | - | - | + | + | - | - | + | ++ | - | + | - |
| Case 2 | ++ | + | - | + | - | - | - | + | + | + | + | ++ | + | + | - |
| Case 3 | + | + | - | + | - | - | - | + | - | - | + | + | ++ | + | NA |
BS: Babinski’s sign; CDN: cognitive disorders and other neuropsychiatric symptoms; CN: coordination; DTR: deep tendon reflex; ES: extrapyramidal signs; GF: gait disorders or falls; GP: gaze palsy; LRP: levodopa-responsive parkinsonism; MS: muscle strength; NA: not available; NI: neuroimage findings; OD: orofacial dyskinesia; SLNP: symptoms of lower cranial nerve palsy; SS: sensory; +: positive findings; ++: initial symptom; -: negative findings.
Pathological methods
The brains of the three cases and spinal cords of case 2 and 3 were obtained at autopsy within 24 hours after death. They were fixed in 10% formalin for 2 weeks. Tissue blocks were taken from the following brain regions: superior frontal gyrus, precentral gyrus, parietal lobe, middle temporal gyrus, occipital lobe, cingulate gyrus, hippocampus, amygdala, basal ganglia, thalamus, midbrain, pons, medulla, cerebellar vermis and hemisphere, and the cervical, thoracic and lumbar spinal cord. 8 μm thick, paraffin sections were stained by HE, LFB, Holzer, Congo-red, Bodian staining and Gallyas-Braak silver impregnation method. In addition, sections were also immunostained with anti-tau (Dako), anti-ubiquitin (Dako), anti-Aβ (Dako), anti-α-synuclein (Sigma), anti-GFAP (Dako) antibodies using EnVision system (Dako) and DAB coloration.
Pathological findings
The brain weight in case 1 was 1240 g. In macroscopic examination, atrophy of brain with more prominent in the right fronto-parietal region was found. Coronal sections showed moderate dilatation of the lateral ventricles. Mild atrophy was seen in striatum and thalamus. A few old lacunar infarcts were observed in basal ganglia, midbrain and pons. The substantia nigra and locus ceruleus showed depigmentation. Microscopic examination revealed neuronal loss and gliosis in frontal and parietal cortex, as well as in the striatum, substantia nigra and locus ceruleus. Globose inclusions could be seen in some neurons, and occupied a large part of cytoplasm. In addition, cortical laminar necrosis was seen, which is the typical pathological change of hypoxia (Figure 1B). Lewy bodies were not found. Modified Gallyas-Braak method and AT8 immunohistochemistry revealed various types of cytoskeletal abnormalities in neurons and glia. Many globose NFTs were distributed in globus pallidus (Figure 1C), subthalamic nucleus, putamen, substantia nigra, superior colliculus, locus coeruleus and other brainstem nuclei. The tau-positive tufed astrocytes were seen in frontal cortex, basal ganglion and brainstem. There were numerous tau-positive coiled bodies in cerebral white matter, basal ganglia, brainstem and cerebellum. Meanwhile, a few AD-like NFTs can be observed in frontal cortex and hippocampus by Modified Gallyas-Braak stains, while argyrophilic grains (AGs) can seen in the CA1 region (Figure 1D). In Bodian stains, amyloid depositions were observed in hippocampus and leptomeningeal vessels in frontal, parietal and temporal lobes. Therefore, the neuropathology diagnosis should be PSP, AD (Braak Stage V, Thal Stage 2) [15,16], argyrophilic grains disease (AGD) (Ferrer Stage II) [17] and cerebral infarcts.
The brain weight of case 2 was 1136 g. There was mild atrophy in frontal cortex, basal ganglia and thalamus, and moderate atrophy in brainstem and cerebellum. The lateral ventricles and mid-brain aquaeduct were enlarged. The caudate nucleus and globus pallidus were atrophic. The substantia nigra and locus ceruleus were pale. Microscopic examinations showed mild neuronal loss and gliosis in the cortex, and severe loss of neurons, gliosis and globose inclusions in the basal ganglion, substantia nigra, locus ceruleus and dentate nucleus. No lewy bodies were detected. Mild to moderate loss of myelin with gliosis could be observed in the white matter of brainstem and cerebellum. GFAP stains demonstrated obvious proliferation of astrocytes in the cerebral cortex, basal ganglia and brainstem. Bodian stains showed a few NFTs in the frontal, parietal and temporal neocortex and hippocampus. No senile plaques were seen in the cerebral cortex and hippocampus. Modified Gallyas-Braak method and AT8 stains showed many globose NFTs in the globus pallidus, putamen, substantia nigra, locus ceruleus, pontine nucleus, dentate nucleus and anterior horn of cervical cord. In CA1 region of hippocampus, more globose NFTs were observed than CA4 region and obvious AGs were found (Figure 1E, 1F). Tuft-shaped astrocytes, coiled-bodies and threads were demonstrated to coexist in the frontal and parietal cortex, cingulate gyrus, basal ganglia and cerebellar white matter (Figure 1G) in Gallyas-Braak and AT8 stains. In summary, the neuropathology diagnosis was PSP, AGD (Ferrer Stage II) [17] and lacunar infarcts.
In case 3, the brain weight was 1223 g and mild atrophy was found in the cerebrum and brainstem, with more prominent in the left frontal lobe. The left globus pallidus were also mild atrophic. The substantia nigra and locus ceruleus were pale. Microscopic examinations revealed severe neuronal loss with extensive gliosis in the second and third layers of the frontal, temporal and parietal lobes. Superficial spongiform changes were seen in the frontal, parietal and cingulated cortex. The ballooned neurons were not found in the parietal, insular and cingulated cortex. Severe neuronal loss and many ballooned neurons were also found in the amygdala. Mild to moderate loss of myelin was observed in the cerebral white matter, basal ganglia, brainstem and cerebellum. Pyramidal neurons in the hippocampus were slightly loss. A few NFTs in pyramidal neurons and granulous cells were observed by Bodian staining. Pick body and senile plaques were not seen. Neuronal loss and gliosis were more prominent in globus pallidus, thalamus, Meynert nucleus, mammillary body, substantia nigra, locus ceruleus, other gray matter of the brainstem, dentate nucleus and Purkinje cells of the cerebellum and motor neurons in the anterior horn of the spinal cord. Modified Gallyas-Braak staining also showed lots of tau-positive globose NFTs in the remaining neurons including the spinal motor neurons. Tufted-astrocytes were widespreadly observed in frontal, parietal and cingulate gyrus, thalamus, striatum, gray matter of brainstem, cerebellar cortex and white matter of cervical cord in Gallyas-Braak and AT8 stains. Tau-positive astrocytic plaques were not found. The oligdendrocytes with the tau-positive coiled-body and threads were widely distributed (Figure 1H), with tau positive AD-like NFTs in hippocampus and entorhinal cortex (Braak Stage II) [15] and Aβ amyloid depositions in neocortex, entorhinal cortex, hippocampus, cingulate gyrus and amygdala (Figure 1I) (Thal Phase 2) [16]. The neuropathology diagnosis was PSP with severe cortical involvement, predorminant senile plaques AD-like pathology. The information of pathological summary was provided in Table 2.
Table 2.
Pathological summary of PSP patients
| BW | DD | AA | CC | CWM | CG | HC | AD | AS | GP | SN | STN | LC | OBN | CB | SC | IHC | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case 1 | 1240 g | 6 yrs | 79 | + | + | + | + | NA | + | + | + | + | + | + | + | NA | AT8 (+) |
| a-syn (-) | |||||||||||||||||
| A-beta (+) | |||||||||||||||||
| Case 2 | 1136 g | 11 yrs | 83 | + | + | + | + | NA | + | + | + | + | + | + | + | + | AT8 (+) |
| a-syn (-) | |||||||||||||||||
| A-beta (-) | |||||||||||||||||
| Case 3 | 1223 g | 9 yrs | 83 | + | + | + | + | + | + | + | + | + | + | + | + | + | AT8 (+) |
| a-syn (-) | |||||||||||||||||
| A-beta (+) |
AA: Age at autopsy; AD: Amygdale; AS: Anterior striatum (caudate nucleus and putamen); a-syn: A-synuclein; A-beta: A-beta amyloid; BW: Brain weight (gram: g); CB: Cerebellum; CC: Cerebral cortex; CG: Cingulate gyrus; CWM: Cerebral/cerebellar white matter; DD: Duration of disease (years: yrs); GP: Globus pallidus; HC: Hippocampus; IHC: Immunohistochemistry; LC: Locus ceruleus; NA: Not available; OBN: Other brainstem nuclei; SC: Spinal cord; SN: Substantia nigra; STN: Subthalamic nucleus; +: positive findings (neuron loss, gliosis or pathological accumulation); -: negative findings.
Discussion
The three Chinese cases with pathological-confirmed PSP were heterogeneous in clinical manifestations. Cases 1 and 2 initiated with extrapyramidal signs and gait disorders, which is generally believed as the typical onset symptom in PSP. While case 3 suffered behavioral abnormalities with cognitive decline at the beginning. Asymmetric atrophy in the left cortex and pyramidal tract signs were also observed. Thus, the diagnosis of AD or CBD had ever been considered. Babinski’s sign was found in case 1, which is not a common sign in PSP and probably be attributed to vascular lesions [18]. In case 2, apraxia and orofacial dyskinesia were observed and might lead to potential clinical misdiagnosis. Both cases 1 and 2 were administrated with levodopa without any benefits, which may differentiate from PD and PSP-P. Actually, PSP-P is a clinical phenotype with early parkinsonism-dominated, an initial response to levodopa therapy and lower tau burden in basal ganglia. All these features are different from classic PSP (Richardson’s syndrome). Hummingbird sign as a hallmark for PSP was found in the atypical case [19], and suggested the possibility of PSP with consistency of future pathological findings.
With regard to the neuropathology of the three cases, PSP-changes such as tau-positive tufed astrocytes, oligdendrocytes with the tau-positive coiled-body and threads and globose NFTs were widely seen in the basal ganglia, isocortex and allocortex, as well as in brainstem, cerebellum and spinal cord. Basal ganglia are early affected in PSP, in which astrocytes are probably the primary targets of tau phosphorylation [20]. In addition, numerous AGs were found in the hippocampus of cases 1 & 2, while Aβ amyloid depositions were found in hippocampus and leptomeningeal vessels of case 1 and in neocortex, entorhinal cortex, hippocampus, cingulate gyrus and amygdale of case 3. Vessel infarcts were observed in cases 1, which corresponded to neuroimaging vascular foci. Cortical laminar III necrosis in case 1 supported the evidence of hypoxia due to the ischemic damage. According to previous volumetric MRI study, atrophy of the thalamus was characteristic in PSP, while the enlargement of the ventricular system can be found in both PSP and MSA-P [21]. In our examination, mild thalamus atrophy with neuron loss and gliosis, and ventricular dilation were detected in cases 1 and 3, which confirmed the findings.
Neuropsychiatric symptoms and cognitive decline are not rare in PSP patients; therefore, limbic systems were carefully examined. No hippocampal sclerosis (HS) was found in the three cases. Actually, HS may be concurrent with TDP-43 pathology, which is not common in coexist with tauopathy in PSP [22]. AGs and Alzheimer’s changes were found in some cases, because all the three cases are very old age and coexisted changes are not rare, which might contribute to the symptoms. Furthermore, tufed astrocytes and Aβ amyloid depositions were seen in amygdala and cingulate gyrus. In previous study, midcingulate cortex (MCC) involvement was proven in PSP, and the anterior MCC is associated with cognitive domain [23].
Speech disturbance was noticed in all the three PSP cases, which can be attributed to neocortex pathology, especially in posterior frontal, anterior temporal, perisylvian or parietal cortex [24]. Actually, speech problem is not indispensable in the diagnosis of PSP. On the contrary, it is more common seen in frontotemporal lobe degeneration (FTLD). Therefore, FTLD should be a possibility in differential diagnosis of PSP. Concerning gaze palsy, the impairment of vertical eye movement was reported in all the three case. And pathological changes in oculomotor nucleus can partially explain the symptom. Of Note, other regions were also closely associated with saccadic eye movement include the supplementary motor area (Brodmann area 6), the frontal eye fields (Brodmann area 8), the posterior parietal cortex (Brodmann area 39), and the dorsolateral prefrontal cortex (Brodmann area 46). Apraxia in case 2 and asymmetric atrophy in the left cortex of case 3 were found, therefore, CBD was necessarily required to be excluded in the pathology. Interestingly, some rare cases, e.g. dual pathology of CBD and PD with clinical features of PSP, have ever been reported [25]. Symptoms of lower cranial nerve palsy occurred in all the three cases, which suggest the value of these symptoms in the clinical diagnosis of PSP.
Pathology in cerebellum is characteristic in PSP, although clinical cerebellar signs are rarely seen. There were PSP cases with predominant cerebellar ataxia [26]. However, the clinicopathological relationships are still unknown in comparison to the cases without ataxia. Typical pathological findings in cerebellum were observed in all the three cases, such as tau-positive globose NFTs and oligdendrocytes with the tau-positive coiled-body and threads in dentate nucleus and white matter pathology [27,28]. Cervical spinal cords in cases 2 & 3 were obtained with tau-positive globose NFTs, tufted astrocytes and neuropil threads were respectively found in the neurons of anterior horn and surrounding white matters. Therefore, cervical spinal cord should be routinely examined in the PSP autopsy, considering its frequency of involvement [29]. In conclusion, PSP does not simply present as a clinical extrapyramidal disease, or a pathological entity in basal ganglia and hindbrain. The clinical and pathological heterogeneity makes PSP as a great challenge for physicians in clinical diagnosis and for scientists in basic scientific research.
Disclosure of conflict of interest
None.
References
- 1.Schrag A, Ben-Shlomo Y, Quinn NP. Prevalence of progressive supranuclear palsy and multiple system atrophy: a cross-sectional study. Lancet. 1999;354:1771–1775. doi: 10.1016/s0140-6736(99)04137-9. [DOI] [PubMed] [Google Scholar]
- 2.Steele JC, Richardson JC, Olszewski J. Progressive Supranuclear Palsy. A Heterogeneous Degeneration Involving the Brain Stem, Basal ganglia and cerebellum with vertical gaze and pseudobulbar palsy, nuchal dystonia and dementia. Arch Neurol. 1964;10:333–359. doi: 10.1001/archneur.1964.00460160003001. [DOI] [PubMed] [Google Scholar]
- 3.Williams DR, Holton JL, Strand C, Pittman A, de Silva R, Lees AJ, Revesz T. Pathological tau burden and distribution distinguishes progressive supranuclear palsy-parkinsonism from Richardson’s syndrome. Brain. 2007;130:1566–1576. doi: 10.1093/brain/awm104. [DOI] [PubMed] [Google Scholar]
- 4.Josephs KA, Boeve BF, Duffy JR, Smith GE, Knopman DS, Parisi JE, Petersen RC, Dickson DW. Atypical progressive supranuclear palsy underlying progressive apraxia of speech and nonfluent aphasia. Neurocase. 2005;11:283–296. doi: 10.1080/13554790590963004. [DOI] [PubMed] [Google Scholar]
- 5.Gerstenecker A, Duff K, Mast B, Litvan I ENGENE-PSP Study Group. Behavioral abnormalities in progressive supranuclear palsy. Psychiatry Res. 2013;210:1205–1210. doi: 10.1016/j.psychres.2013.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Litvan I, Agid Y, Caline D, Campbell G, Dubois B, Duvoisin RC, Goetz CG, Golbe LI, Grafman J, Growdon JH, Hallett M, Jankovic J, Quinn NP, Tolosa E, Zee DS. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology. 1996;47:1–9. doi: 10.1212/wnl.47.1.1. [DOI] [PubMed] [Google Scholar]
- 7.Collins SJ, Ahlskog JE, Parisi JE, Maraganore DM. Progressive supranuclear palsy: neuropathologically based diagnostic clinical criteria. J Neurol Neurosurg Psychiatry. 1995;58:167–173. doi: 10.1136/jnnp.58.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dickson DW, Rademakers R, Hutton ML. Progressive supranuclear palsy: pathology and genetics. Brain Pathol. 2007;17:74–82. doi: 10.1111/j.1750-3639.2007.00054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiu WZ, Papma JM, de Koning I, Donker Kaat L, Seelaar H, Reijs AE, Valkema R, Hasan D, Boon AJ, van Swieten JC. Midcingulate involvement in progressive supranuclear palsy and tau positive frontotemporal dementia. J Neurol Neurosurg Psychiatry. 2012;83:910–5. doi: 10.1136/jnnp-2011-302035. [DOI] [PubMed] [Google Scholar]
- 10.Mazère J, Meissner WG, Mayo W, Sibon I, Lamare F, Guilloteau D, Tison F, Allard M. Progressive supranuclear palsy: in vivo SPECT imaging of presynaptic vesicular acetylcholine transporter with [123I] -iodobenzovesamicol. Radiology. 2012;265:537–543. doi: 10.1148/radiol.12112650. [DOI] [PubMed] [Google Scholar]
- 11.Matsusaka H, Ikeda K, Akiyama H, Arai T, Inoue M, Yaqishita S. Astrocytic pathology in progressive supranuclear palsy: significance for neuropathological diagnosis. Acta Neuropathol. 1998;96:248–252. doi: 10.1007/s004010050891. [DOI] [PubMed] [Google Scholar]
- 12.Tsuboi Y, Ahlskog JE, Apaydin H, Parisi JE, Dickson DW. Lewy bodies are not increased in progressive supranuclear palsy compared with normal controls. Neurology. 2001;57:1675–1678. doi: 10.1212/wnl.57.9.1675. [DOI] [PubMed] [Google Scholar]
- 13.Oshima K, Dickson DW. Cortical Alzheimer type pathology does not influence tau pathology in progressive supranuclear palsy. Int J Clin Exp Pathol. 2009;2:399–406. [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu MW, Liu J, Wang LN, Lu DH. Development of clinical neuropathology in china during the past half century: a publication survey. J Neuropathol Exp Neurol. 2013;72:892–894. doi: 10.1097/NEN.0b013e3182a330aa. [DOI] [PubMed] [Google Scholar]
- 15.Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 2006;112:389–404. doi: 10.1007/s00401-006-0127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thal DR, Rüb U, Orantes M, Braak H. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58:1791–1800. doi: 10.1212/wnl.58.12.1791. [DOI] [PubMed] [Google Scholar]
- 17.Ferrer I, Santpere G, van Leeuwen FW. Argyrophilic grain disease. Brain. 2008;131:1416–1432. doi: 10.1093/brain/awm305. [DOI] [PubMed] [Google Scholar]
- 18.Josephs KA, Katsuse O, Beccano-Kelly DA, Lin WL, Uitti RJ, Fujino Y, Boeve BF, Hutton ML, Baker MC, Dickson DW. Atypical progressive supranuclear palsy with corticospinal tract degeneration. J Neuropathol Exp Neurol. 2006;65:396–405. doi: 10.1097/01.jnen.0000218446.38158.61. [DOI] [PubMed] [Google Scholar]
- 19.Graber JJ, Staudinger R. Teaching Neuro-Images: “Penguin” or “hummingbird” sign and midbrain atrophy in progressive supranuclear palsy. Neurology. 2009;72:e81. doi: 10.1212/WNL.0b013e3181a2e815. [DOI] [PubMed] [Google Scholar]
- 20.Santpere G, Ferrer I. Delineation of early changes in cases with progressive supranuclear palsy-like pathology. Astrocytes in striatum are primary targets of tau phosphorylation and GFAP oxidation. Brain Pathol. 2009;19:177–187. doi: 10.1111/j.1750-3639.2008.00173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Messina D, Cerasa A, Condino F, Abrabia G, Novellino F, Nicoletti G, Salsone M, Morelli M, Lanza PL, Quattrone A. Patterns of brain atrophy in Parkinson’s disease, progressive supranuclear palsy and multiple system atrophy. Parkinsonism Relat Disord. 2011;17:172–176. doi: 10.1016/j.parkreldis.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 22.Yokota O, Davidson Y, Bigio EH, Ishizu H, Terada S, Arai T, Haseqawa M, Akiyama H, Sikkink S, Pickering-Brown S, Mann DM. Phosphorylated TDP-43 pathology and hippocampal sclerosis in progressive supranuclear palsy. Acta Neuropathol. 2010;120:55–66. doi: 10.1007/s00401-010-0702-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chiu WZ, Papma JM, de Koning I, Donker Kaat L, Seelaar H, Reijs AE, Valkema R, Hasan D, Boon AJ, van Swieten JC. Midcingulate involvement in progressive supranuclear palsy and tau positive frontotemporal dementia. J Neurol Neurosurg Psychiatry. 2012;83:910–5. doi: 10.1136/jnnp-2011-302035. [DOI] [PubMed] [Google Scholar]
- 24.Gorno-Tempini ML, Hillis AE, Weintraub S. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–1014. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mooney T, Tampiyappa A, Robertson T, Grimley R, Burke C, Nq K, Patrikios P. Dual pathology of corticobasal degeneration and Parkinson’s disease in a patient with clinical features of progressive supranuclear palsy. Neurol India. 2011;59:887–890. doi: 10.4103/0028-3886.91371. [DOI] [PubMed] [Google Scholar]
- 26.Kanazawa M, Tada M, Onodera O, Takahashi H, Nishizawa M, Shimohata T. Early clinical features of patients with progressive supranuclear palsy with predominant cerebellar ataxia. Parkinsonism Relat Disord. 2013;19:1149–1151. doi: 10.1016/j.parkreldis.2013.07.019. [DOI] [PubMed] [Google Scholar]
- 27.Mizusawa H, Yen SH, Hirano A, Llena JF. Pathology of the dentate nucleus in progressive supranuclear palsy: a histological, immunohistochemical and ultrastructural study. Acta Neuropathol. 1989;78:419–428. doi: 10.1007/BF00688179. [DOI] [PubMed] [Google Scholar]
- 28.Armstrong RA. White matter pathology in progressive supranuclear palsy (PSP): a quantitative study of 8 cases. Clin Neuropathol. 2013;32:399–405. doi: 10.5414/NP300608. [DOI] [PubMed] [Google Scholar]
- 29.Iwasaki Y, Yoshida M, Hashizume Y, Hattori M, Aiba I, Sobue G. Widespread spinal cord involvement in progressive supranuclear palsy. Neuropathology. 2007;27:331–340. doi: 10.1111/j.1440-1789.2007.00787.x. [DOI] [PubMed] [Google Scholar]