Abstract
The cancer stem cell (CSC) paradigm is one possible way to understand the genesis of cancer, and cervical cancer in particular. We quantified and enriched ALDH1+ cells within cervical cancer cell lines and subsequently characterized their phenotypical and functional properties like invasion capacity and epithelial-mesenchymal transition (EMT). ALDH1 expression in spheroid-derived cells (SDC) and the parental monolayer-derived cell (MDC) line was compared by flow-cytometry. Invasion capability was evaluated by Matrigel assay and expression of EMT-related genes Twist 1, Twist 2, Snail 1, Snail 2, Vimentin and E-cadherin by real-time PCR. ALDH1 expression was significantly higher in SDC. ALDH1+ cells showed increased colony-formation. SDC expressed lower levels of E-cadherin and elevated levels of Twist 1, Twist 2, Snail 1, Snail 2 and Vimentin compared to MDC. Cervical cancer cell lines harbor potential CSC, characterized by ALDH1 expression as well as properties like invasiveness, colony-forming ability, and EMT. CSC can be enriched by anchorage-independent culture techniques, which may be important for the investigation of their contribution to therapy resistance, tumor recurrence and metastasis.
Keywords: Cervical cancer, cancer stem cell, ALDH1, spheroid derived cell, EMT
Introduction
Cervical cancer remains high morbidity and a major cause of mortality amongst women worldwide especially in developing countries, despite many steps that have been dedicated to reducing the disease burden in the past decades [1]. The cancer stem cell (CSC) paradigm might be one possible way to understand cervical carcinogenesis. The CSCs, as postulated in the CSC theory, are held responsible for the persistence or recurrence of the tumor after conventional therapy [2]. This rare subpopulation of tumor cells exhibits stem cell properties like self-renewal capacity, multilineage differentiation and maintenance of a heterogeneous bulk tumor mass, increased motility, invasiveness, and heightened resistance to apoptosis [3]. These characteristics are held responsible for tumor maintenance and metastasis and possibly also for resistance toward chemotherapy and radiation therapy.
Epithelial-to-mesenchymal transition (EMT), a trans-differentiation program that converts adherent epithelial cells into individual migratory cells, is a key event in metastasis [4]. The EMT process disrupts E-cadherin mediated cell-cell adhesion during embryonic development and changes the cell phenotype into a more loosely mesenchymal-like cell, leading to the invasion of extracellular matrix [5]. Intensive studies revealed that transcriptional factors, such as Snail, Slug, and Twist, regulate EMT process [6]. Accumulating evidences have shown that cells with an EMT phenotype induced by different factors are rich sources for CSCs, suggesting the biological similarities between CSCs and EMT-phenotypic cells [7]. Moreover, induction of EMT in tumor cells not only promotes tumor cell invasion and metastasis but also contributes to drug resistance, suggesting that the molecular characterization of these cells will allow the development of newer therapies for complete eradication of tumors, which will certainly improve the overall survival of patients diagnosed with cancers.
In the present paper, we investigated whether anchorage-independent cell culture techniques allow the generation of spheroid from 3 cervical cancer cell lines and whether these cultures were enriched for cells with CSCs-like or EMT function and phenotype.
Material and methods
Cell lines and cell culture
Three human cervical cancer cell lines SiHa, HeLa and CaSki, were purchased from the American Type Culture Collection (Manassas, VA). All cell lines were maintained in Eagle’s Minimum Essential Medium (EMEM) obtained from Sigma Chemical Co. (St. Louis, MO), supplemented with 10% fetal bovine serum (FBS; Biochrom, Berlin, Germany), and 1% Penicillin and Streptomycin (10,000 U/ml and 10,000 mg/ml, respectively; Biochrom) at 37°C in 5% CO2.
Spheroid cell formation assay
Adherent monolayer cells were cultured in normal 10 cm2 culture flasks (BD Sciences, Franklin Lakes, USA) in DMEM containing 10% heat inactivated FBS and 1% Penicillin/Streptomycin, until 70-75% density. Culture medium was discarded and cells were washed with PBS without Ca2+/Mg2+. PBS was aspired and 1 ml Trypsin/EDTA (Biochrom AG, Berlin, Germany) was added. Cells were shaken off by tapping the plate, resuspended in Quantum 263 medium (Biochrom AG, Berlin, Germany), supplemented with 10 ng/mL Epidermal Growth Factor (EGF) and 10 ng/mL base Fibroblast Growth Factor (bFGF) (Biochrom AG, Berlin, Germany). To generate spheroids, single cells were plated in Corning* Ultra-Low Attachment flasks (Corning, New York, USA) at a specific density of 2 × 104 cells/ml. Cells were kept in the incubator at 37°C in humidified atmosphere with 5% CO2. After 5-7 days, spheroids were collected by filtration through a 40-μm mesh for the following experiments. The cervical cancer cell line SiHa never formed any spheroids and was cultured by replacing medium instead of passaging.
Multicolor flow-cytometric analysis and sorting (FACS)
The identification of aldehyde dehydrogenase 1 (ALDH1) activity from spheroid-derived and monolayer-derived cells was conducted by using the ALDEFLUOR assay (StemCell Technologies, Durham, NC, USA). Spheroids were disaggregated into single cells by Trypsin/EDTA digestion. The single cell suspension was washed twice in PBS buffer then suspended in ALDEFLUOR assay buffer containing ALDH substrate (BAAA, 1 mol/L per 1 × 106 cells) and incubated for 40 min at 37°C. As a negative control, for each sample of cells an aliquot was treated with 50 mmol/L diethylaminobenzaldehyde (DEAB), a specific ALDH inhibitor.
For FACS, cells were resuspended in PBS buffer at 1 × 107 cells per mL and separated on an Aria cell sorter (BD Biosciences). The sorted cells could not be assessed for purity by reanalysis due to the low numbers of cells obtained. The sorting gates were established, using as negative controls the cells treated with DEAB.
Clone formation assay
Freshly sorted ALDH1 positive and ALDH1 negative cells were inoculated into Ultra-low attachment 96-well plates at a density of 1000 cells/mL in Quantum 263 medium supplemented with 10 ng/mL EGF and 10 ng/mL bFGF. Culture medium was replaced every three days. After 2 weeks, the colonies contained in each well were counted and photographed.
Invasion assay
Cells cultivated in a monolayer or in a spheroid-culture were separated into single cells by careful trypsin digestion and resuspended in 1% BSA-DMEM culture medium. Cells (5 × 104) were seeded into the upper compartments of BD BioCoat™ Matrigel Invasion Chambers (BD Bioscience), and DMEM supplemented with 10% FBS was added to the lower compartment according to manufacturer’s instructions. The invasion chamber was kept for 24 h at 37°C in a humidified atmosphere containing 5% CO2. After incubation, the non-invading cells were removed from the upper surface of the membrane by gentle scrubbing, and the cells on the lower surface of the membrane were stained with crystal violet. Cell counting was facilitated by photographing the membrane through the microscope and 3 fields per membrane of triplicate membranes were counted under 200 × magnification (Axiovert, Axiovision, Zeiss, Germany).
Immunofluorescence staining
For immunofluorescence staining, spheroids were fixed in 4% paraformaldehyde and blocked in medium containing 10% FCS and 0.5% Triton. Cells were stained over night at 4°C with the pluripotency markers Sox2 (monoclonal antibody, Epitomics, Abcam, USA), OCT4 (monoclonal antibody, Abcam) and Nanog (monoclonal antibody, Abcam). Alexa Fluor 488 (Invitrogen) antibodies were used as secondary antibodies. Excess antibodies were washed away by PBS and then cells were fixed again using 4% paraformaldehyde. Cells were finally mounted in VECTASHIELD® Mounting Medium with DAPI (Vector Laboratories). All staining were examined under a fluorescence microscope (Nikon, Japan).
Quantitative real-time PCR
Real-time RT-PCR analysis was also conducted to measure the expression level of EMT related genes: Twist 1, Twist 2, Snail 1, Snail 2, Vimentin and E-cadherin (Table 1). Briefly, 1 μg of total RNA from each sample was subjected to reverse transcription using the High-Capacity RNA-to-cDNA Kit (Applied Biosystems) according to the manufacturer’s protocol. Real-time PCRs were then carried out in a total of 25 μL reaction mixture (2 μL cDNA, 12.5 μL of 2 × SYBR Green PCR Master Mix from Applied Biosystems, 1.5 μL of each 5 μmol/L forward and reverse primers, and 7.5 μL distilled H2O) in SmartCycler II (Cepheid). The PCR program was initiated by 10 min at 95°C before 40 thermal cycles, each at 15 s at 95°C and 1 min at 60°C. Data were analyzed using the modified delta delta Ct method. Primer sequences are listed in Table 1.
Table 1.
Primer sequences used for RT-PCR in EMT-related genes expression analysis (26)
| Transcript name | Forward primer sequence | Reverse primer sequence |
|---|---|---|
| SNAIL 1 | GGCGCACCTGCTCGGGGAGTG | GCCGATTCGCGCAGCA |
| SNAIL 2 | GGGGAGAAGCCTTTTTCTTG | TCCTCATGTTTGTGCAGGAG |
| Twist 1 | GGAGTCCGCAGTCTTACGAG | TCTGGAGGACCTGGTAGAGG |
| Twist 2 | AGCGACGAGATGGACAATAAGATGACC | CGGTCCGGAGGTGGGTGGCG |
| Vimentin | ACAACCTGGCCGAGGACATC | AGAGACGCATTGTCAACATCCTG |
Statistical analysis
For statistical comparison, the SPSS software for Windows (version 15; SPSS, Chicago, IL, USA) was used. Student’s t test was used to analyze statistical significance of the data.
Results
Spheroid formation of cervical cancer cell lines
Spheroid culture is widely used as it provides an in vitro 3-dimensional (3D) model to study proliferation, cell death, differentiation, and metabolism of cells derived from tumors and the response of tumor cells to radiotherapy and chemotherapy. Furthermore, it is also easier to enrich CSC populations when compared to other methods. The ability of purified CSCs to grow anchorage independently and to form spheres in cell culture has been demonstrated for breast, prostate, colon, head and neck, pancreatic, and melanoma derived CSCs. Here, we selected spheroid culture as an essential step to enrich for CSC-like populations from our cervical cancer cell lines.
Cells from three cervical cancer cell lines were grown in suspension at low density in defined serum-free medium with bFGF and EGF for 7-14 days. The spheroid formation usually started at the first day after starting suspension culture and the size became progressively larger. At days 3-5 an initial spheroid formation could be observed. After 4-7 days the morphology of spheroids would not change in size, but the number of spheroids still continued to grow. CaSki formed spheroids which were highly compact (Figure 1A). HeLa formed only loose aggregates of cells (Figure 1B). SiHa never formed any spheroids (Figure 1C). When the spheroids were transferred back to a regular tissue culture flask coated for monolayer cell culture, the spheroids adhered to the flask and cells grew out from the spheroid and formed a confluent monolayer. The phenotype of these cells was identical to the parental cell lines (Figure 1D).
Figure 1.
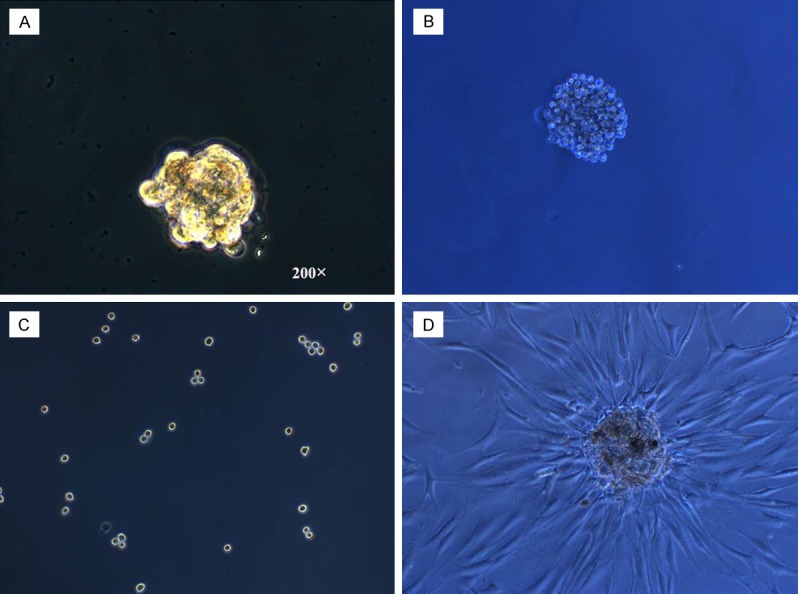
Spheroids generated from cervical cancer cell lines in serum-free medium have self-renewing capacity. Example of SDC formed by CaSki (A), HeLa (B), and MRIH215 (C) in suspension cultures in defined serum-free medium supplemented with bFGF and EGF after 7-10 days in culture(magnification × 200). (D) Spheroid adhering and growing to confluence after replating into flasks coated for tissue culture.
ALDH1 expression in cervical cancer cell lines
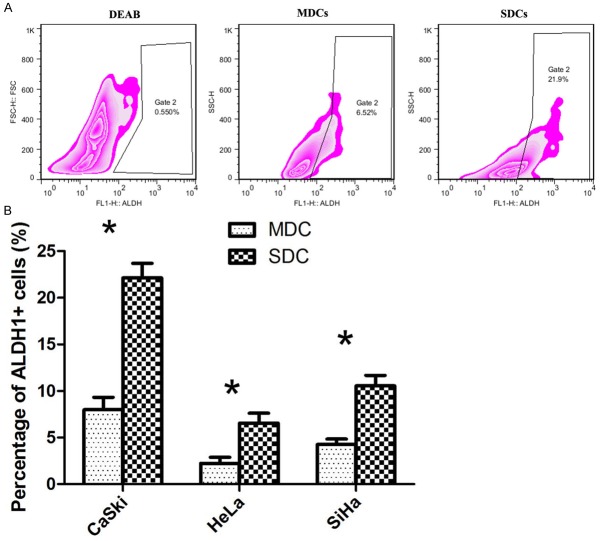
We measured ALDH1 enzymatic activity of the SDC (spheroid derived cell) of 3 cervical cancer cell lines and their matched MDC (monolayer derived cell) to verify the presence of a stem cell-like population. As control cells incubated with ALDEFLUOR substrate (BAAA) together with the specific ALDH inhibitor (DEAB) were used to establish the background fluorescence and to define the ALDH1 positive population (Figure 2A).
Figure 2.
Representative figures of flow cytometric analysis of the expression of ALDH1 in SDC compared to MDC, and control cells treated with DEAB, a specific inhibitor of ALDH1 (A). Mean percent of ALDH1+ cells in SDC and MDC of the 3 cell lines (B). Mean values ± SD of three determinations. Statistically significant differences are *P < 0.05 (Student’s t test).
As it is shown in Figure 2B, MDC and SDC of CaSki showed the highest expression of ALDH-positive cells (MDC: 6.70 ± 1.59%, SDC: 22.70 ± 3.57%) (P < 0.05) as compared to HeLa (MDC: 3.25 ± 0.97%, SDC: 7.56 ± 1.77 %) (P < 0.05) and SiHa (MDC: 5.11 ± 1.53%, SDC: 10.79 ± 3.27%) (P < 0.05). The data showed that SDC from all 3 cell lines had a significantly increased frequency of ALDH expressing cells as compared to parental MDC.
ALDH1+ cells in cervical cancer cell lines exhibit higher clonogenic ability than ALDH1- cells
In order to determine the clonogenic ability of ALDH1 sorted cells in vitro, a clone formation assay was conducted. After 14 days of culture, the colonies that formed were quantified macroscopically (Figure 3). The ALDH1+ subpopulation in CaSki and HeLa cell lines have a higher clone formation efficiency as compared to the ALDH1- subpopulation (**P < 0.05).
Figure 3.
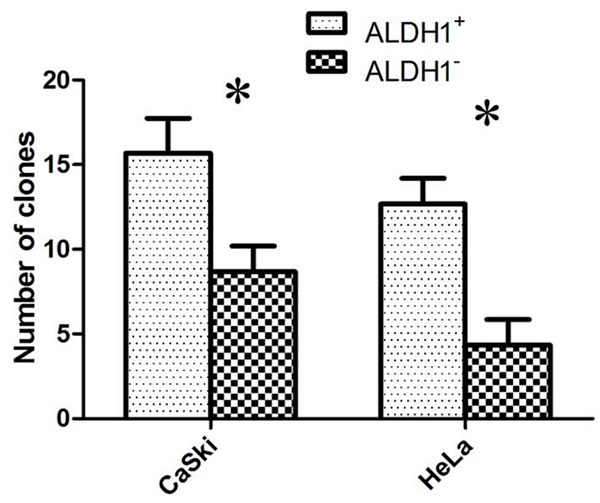
Clone formation assay with FACS-sorted and cloned cells. Mean values ± SD of three determinations. Statistically significant differences are *P < 0.05; (Student’s t test).
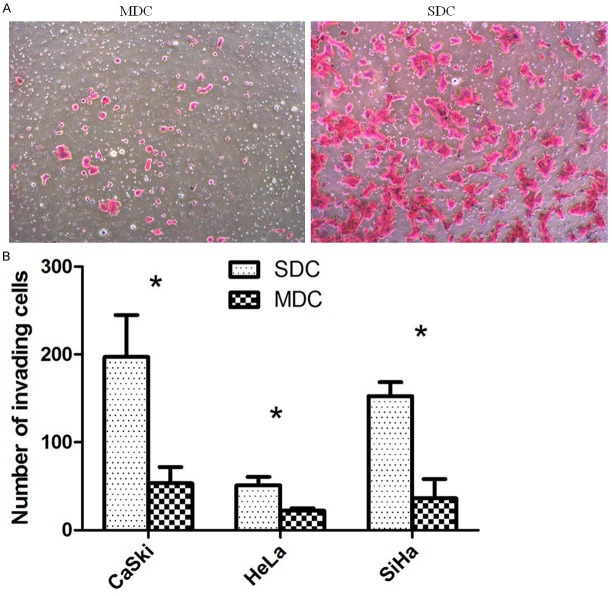
SDC exhibit increased invasion capacity over MDC in vitro
We compared the invading capacity of cells either raised in spheroid or monolayer culture by transwell invasion assay. SDC from all three tested cell lines showed a significantly increased invasion capacity of 2.3 to 7.4 fold over the parental control (Figure 4).
Figure 4.
ECM invasion assay. Representative figures of SDC and MDC in lower chamber in transwell invasion assay. Magnification in all figures: × 200 (A). Boxplot of the transwell invasion assay results. Mean values ± SD of three determinations (B). Statistically significant differences are *P < 0.05; (Student’s t test).
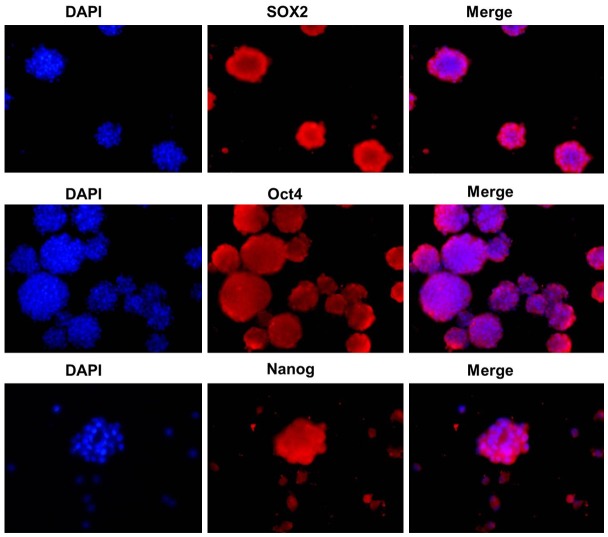
Intracellular localization of Sox2, Oct4 and Nanog in spheroid-forming cells
To examine the subcellular localization of embryonal proteins Sox2, Oct4 and Nanog in spheroid-forming cells, immunofluorescent staining of Sox2, Oct4 and Nanog was performed. Sox2, Oct4 and Nanog proteins were positively stained within the perinuclei and cytoplasm of the spheroid-forming cells (Figure 5).
Figure 5.
Analysis of the expression of SOX2, Nanog and Oct4 expression by immunofluorescence staining in MDC versus SDC.
Overexpression of EMT-associated genes in SDC
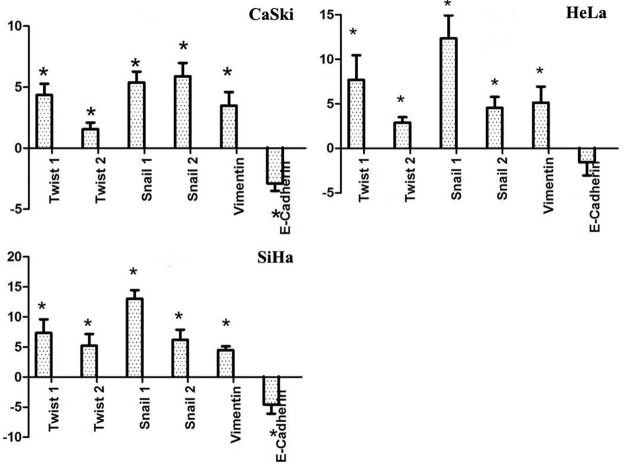
SDC from all 3 cervical cancer cell lines displayed characteristics of EMT by displaying lower expression levels of E-cadherin and increased levels of Twist 1, Twist 2, Snail 1, Snail 2 and Vimentin compared to MDC (Figure 6). These results further support the link between EMT and the acquisition of stem cell properties.
Figure 6.
Quantitative PCR analysis of mRNA expression of EMT-related genes. Mean values ± SD of three determinations. Statistically significant differences are *P < 0.05; (Student’s t test).
Discussion
CSCs are thought to be responsible for tumor maintenance, progression, and relapse of the disease due to, in part, an exhibition of multiple resistance mechanisms to chemotherapy and radiotherapy [8]. To date, the existence of CSCs has been documented in a number of human cancers, such as leukemia, breast cancer, prostate cancer, bladder cancer, lung cancer, head and neck cancer, liver cancer, ovarian cancer, colon carcinoma, malignant melanoma, cervical cancer, pancreatic cancer and Ewing sarcoma [9-20].
The spheroid formation assay is widely used to define CSC subpopulations. The stem cell-like characteristics of these cells were analyzed by comparing surface antigen expression and the expression of embryonal transcription factor that are markers of stemness. ALDH1 has been considered to be a marker for CSCs. As exemplified in breast cancer, for example, Ginestier et al. [21] reported that cells with high ALDH activity contain the tumorigenic cell fraction, are able to self-renew and to recapitulate the heterogeneity of the parental tumor. In our study, we found that SDC from all three cervical cancer cell lines contained a significantly higher number of ALDH1 positive cells than their corresponding MDCs. Cancer and normal stem cells (SCs) share proliferative properties of self-renewal and expression of key transcription factors. Nanog, Oct4, and Sox2 are the core regulators of mouse embryonic stem cell pluripotency and they cooperatively maintain the regulatory network responsible for self-renewal and pluripotency [22]. In our study, Oct4, Sox2, and Nanog were highly expressed in SDC derived of the three cervical cancer cell lines, which demonstrates that spheroids subcultivated from cancer cell lines exhibit CSC characteristics and are therefore useful for CSC research.
The spheroid-forming ability was found to correspond to expression of established CSC markers. However, it is reported that the spheroid-forming ability was not always reflected in tumor-initiating properties in vivo. In our experiments, we found that the three cervical cancer cell lines showed varying ability to form spheroids. The cervical cancer cell line SiHa that never formed any spheroids, also showed, after anchorage independent growth under culture conditions for spheroids, along with the increase of ALDH1 positive population when compared with adherently grown MDCs.
EMT plays a role in the generation of high-grade invasive cells with cancer stem cell-like properties [23]. This correlation between EMT and CSCs may provide a direct link between the CSCs and the metastatic potential of cancer. Characteristic changes during EMT include down-regulation of epithelial markers such as E-cadherin and up-regulation of mesenchymal markers like Vimentin. The EMT process is initiated by suppression of E-cadherin expression by the major EMT regulators, such as Snail and Twist [24]. The zinc-finger transcriptional repressors Snail 1 and Snail 2 and the basic helix-loop-helix (bHLH) transcription factor-Twist 1 were shown to induce EMT through repression of E-cadherin expression [25]. In our study, a significant decrease of E-Cadherin expression could be demonstrated in SDC. In addition, the expression of Twist 1, Twist 2, Snail 1, Snail 2 and Vimentin were up-regulated in SDC in the three cervical cancer cell lines. These suggest that cervical cancer SDC display EMT characteristics which together with their capacity to migrate through the basal membrane (invasion assay), are necessary for metastasis. We believe that cells derived from spheroids, which had undergone a transition to a mesenchymal phenotype, maintained CSC-characteristics such as expressing a high level of the CSC marker ALDH1, increased invading capacity, and elevated protein levels of stem cell-related transcription factors such as Nanog, Sox2, and Oct3/4.
In summary, in this study we provide an anchorage independent culturing method of cervical cancer cell lines suitable for the enrichment of cells with cancer stem cell properties and of cells undergoing EMT. Functionally, SDC from all three cervical cancer cell lines showed significantly higher invasion capability than their corresponding monolayer cells. FACS sorted ALDH1+ cells displayed higher colony forming efficiency than ALDH1- cells. Our findings may prove it useful to uncover the characteristics of EMT in vitro and thus support in vivo studies that investigating the role of CSC and EMT in the spread of cervical cancer.
Acknowledgements
This research was supported in part by grants 81070470 (XSL), 81370695 (XSL) and 81401183 (DD) from the National Science Foundation of China, and grant 11ZR1404100 (DD) from the Shanghai Science and Technology Commission, Shanghai Key Laboratory of Female Reproductive Endocrine-Related Diseases.
Disclosure of conflict of interest
None.
References
- 1.Arrossi S, Sankaranarayanan R, Parkin DM. Incidence and mortality of cervical cancer in Latin America. Salud pública Méx. 2003;45(Suppl 3):S306–14. doi: 10.1590/s0036-36342003000900004. [DOI] [PubMed] [Google Scholar]
- 2.Clevers H. The cancer stem cell: premises, promises and challenges. Nat Med. 2011;17:313–319. doi: 10.1038/nm.2304. [DOI] [PubMed] [Google Scholar]
- 3.Girouard SD, Murphy GF. Melanoma stem cells: not rare, but well done. Lab Invest. 2011;91:647–664. doi: 10.1038/labinvest.2011.50. [DOI] [PubMed] [Google Scholar]
- 4.Marquardt J, Factor V, Thorgeirsson S. Epigenetic regulation of cancer stem cells in liver cancer: current concepts and clinical implications. J Hepatol. 2010;53:568–577. doi: 10.1016/j.jhep.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiou SH, Wang ML, Chou YT, Chen CJ, Hong CF, Hsieh WJ, Chang HT, Chen YS, Lin TW, Hsu HS, Wu CW. Coexpression of Oct4 and Nanog Enhances Malignancy in Lung Adenocarcinoma by Inducing Cancer Stem Cell-Like Properties and Epithelial–Mesenchymal Transdifferentiation. Cancer Res. 2010;70:10433–44. doi: 10.1158/0008-5472.CAN-10-2638. [DOI] [PubMed] [Google Scholar]
- 6.Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9:265–73. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- 7.Kong D, Li Y, Wang Z, Sarkar FH. Cancer stem cells and epithelial-to-mesenchymal transition (EMT)-phenotypic cells: are they cousins or twins? Cancers. 2011;3:716–729. doi: 10.3390/cancers30100716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Santisteban M. ABC transporters as molecular effectors of pancreatic oncogenic pathways: the Hedgehog-GLI model. J Gastrointest Cancer. 2010;41:153–8. doi: 10.1007/s12029-010-9144-1. [DOI] [PubMed] [Google Scholar]
- 9.Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005;65:10946–10951. doi: 10.1158/0008-5472.CAN-05-2018. [DOI] [PubMed] [Google Scholar]
- 10.Schatton T, Murphy GF, Frank NY, Murphy GF, Frank NY, Yamaura K, Waaga-Gasser AM, Gasser M, Zhan Q, Jordan S, Duncan LM, Weishaupt C, Fuhlbrigge RC, Kupper TS, Sayegh MH, Frank MH. Identification of cells initiating human melanomas. Nature. 2008;451:345–349. doi: 10.1038/nature06489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 12.Chan KS, Espinosa I, Chao M, Wong D, Ailles L, Diehn M, Gill H, Presti J Jr, Chang HY, van de Rijn M, Shortliffe L, Weissman IL. Identification, molecular characterization, clinical prognosis, and therapeutic targeting of human bladder tumor-initiating cells. Proc Natl Acad Sci U S A. 2009;106:14016–14021. doi: 10.1073/pnas.0906549106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dalerba P, Dylla SJ, Park IK, Liu R, Wang X, Cho RW, Hoey T, Gurney A, Huang EH, Simeone DM, Shelton AA, Parmiani G, Castelli C, Clarke MF. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci U S A. 2007;104:10158–10163. doi: 10.1073/pnas.0703478104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prince ME, Sivanandan R, Kaczorowski A, Wolf GT, Kaplan MJ, Dalerba P, Weissman IL, Clarke MF, Ailles LE. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci U S A. 2007;104:973–978. doi: 10.1073/pnas.0610117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang S, Balch C, Chan MW, Lai HC, Matei D, Schilder JM, Yan PS, Huang TH, Nephew KP. Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Res. 2008;68:4311–4320. doi: 10.1158/0008-5472.CAN-08-0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, Bruns CJ, Heeschen C. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1:313–323. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 18.Yang ZF, Ho DW, Ng MN, Lau CK, Yu WC, Ngai P, Chu PW, Lam CT, Poon RT, Fan ST. Significance of CD90+ cancer stem cells in human liver cancer. Cancer Cell. 2008;13:153–166. doi: 10.1016/j.ccr.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 19.Suva ML, Riggi N, Stehle JC, Baumer K, Tercier S, Joseph JM, Suvà D, Clément V, Provero P, Cironi L, Osterheld MC, Guillou L, Stamenkovic I. Identification of cancer stem cells in Ewing’s sarcoma. Cancer Res. 2009;69:1776–1781. doi: 10.1158/0008-5472.CAN-08-2242. [DOI] [PubMed] [Google Scholar]
- 20.Jordan CT, Guzman ML, Noble M. Cancer stem cells. N Engl J Med. 2006;355:1253–1261. doi: 10.1056/NEJMra061808. [DOI] [PubMed] [Google Scholar]
- 21.Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG, Liu S, Schott A, Hayes D, Birnbaum D, Wicha MS, Dontu G. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Z, Oron E, Nelson B, Razis S, Ivanova N. Distinct lineage specification roles for NANOG, OCT4, and SOX2 in human embryonic stem cells. Cell Stem Cell. 2012;10:440–454. doi: 10.1016/j.stem.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 23.Rosen JM, Jordan CT. The increasing complexity of the cancer stem cell paradigm. Science. 2009;324:1670–1673. doi: 10.1126/science.1171837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang MH, Chen CL, Chau GY, Chiou SH, Su CW, Chou TY, Peng WL, Wu JC. Comprehensive analysis of the independent effect of twist and snail in promoting metastasis of hepatocellular carcinoma. Hepatology. 2009;50:1464–1474. doi: 10.1002/hep.23221. [DOI] [PubMed] [Google Scholar]
- 25.Tsai JH, Yang J. Epithelial-mesenchymal plasticity in carcinoma metastasis. Genes Dev. 2013;27:2192–2206. doi: 10.1101/gad.225334.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gasparotto D, Polesel J, Marzotto A, Colladel R, Piccinin S, Modena P, Grizzo A, Sulfaro S, Serraino D, Barzan L, Doglioni C, Maestro R. Overexpression of TWIST2 correlates with poor prognosis in head and neck squamous cell carcinomas. Oncotarget. 2011;2:1165. doi: 10.18632/oncotarget.390. [DOI] [PMC free article] [PubMed] [Google Scholar]