Abstract
Background: Accumulating evidence demonstrated a link of increased expression of Angiopoietin-2 (Ang-2) with invasive and metastatic phenotypes of various types of human cancers. However, until now, the serum level and its diagnostic and prognostic potential in breast cancer have not been investigated. Methods: Enzyme-linked immunosorbent assays were used to measure the levels of Ang-2. Sensitivity, specificity and area under curve (AUC) for serum Ang-2 levels were determined using receiver operator characteristic (ROC) analysis. Survival curves were plotted using the Kaplan-Meier method and differences in survival rates were analyzed using the log-rank test. Prognostic relevance of each variable to overall survival (OS) and disease-free survival (DFS) were analyzed using the Cox regression model. Results: Serum expression of Ang-2 in patients with breast cancer was significantly higher than healthy control group (3171 ± 1024 vs. 1800 ± 874 pg/ml, P < 0.0001). ROC curve analysis showed that at the optimal cut-off (2558.5 pg/ml), serum level of Ang-2 had a sensitivity of 78.3% and a specificity of 77.0% for distinguishing breast cancer patients from healthy controls with an area under the curve (AUC) of 0.836 (P < 0.001, 95% confidence interval: 0.787-0.885). The 5-year OS of high Ang-2 expression group was significantly shorter than that of low Ang-2 expression group (55.9% vs. 80.3%; P = 0.018). Moreover, the 5-year DFS of high Ang-2 expression group was also significantly shorter than that of low Ang-2 expression group (46.0% vs. 68.7%; P = 0.029). Conclusions: Our data indicate that serum Ang-2 level has potential value for early detection of breast cancer. Furthermore, Ang-2 has prognostic value in patients with breast cancer.
Keywords: Angiopoietin-2, breast cancer, diagnosis, prognosis
Introduction
Breast cancer is the most common cancer and the second leading cause of cancer-related deaths in females worldwide. It has been a leading cause of death among women in China, with 1.1 million new cases [1]. The major cause of breast cancer death is not due to local tumors but distant tumor metastases that are often undiscovered during initial diagnosis and are resistant to conventional therapies. The angiopoietins (Angs) are novel endothelial growth factors, found to be ligands for the endothelium-specific tyrosine receptor Tie-2 [2-5]. Angiopoietin 2 (Ang-2) was initially identified as an antagonist for angiopoietin 1 (Ang-1) activation of Tie2 that modulates vessel stability [6-8]. Accumulating evidence demonstrated a link of increased expression of Ang-2 with invasive and metastatic phenotypes of various types of human cancers including breast cancers [8,9]. Previously, Sfiligoi C et al found that increased expression of Ang-2 in breast cancer tissues correlated with lymph node invasion and short survival [10]. However, until now, the serum level and its diagnostic and prognostic potential in breast cancer have not been investigated.
In the present study, we aimed to investigate the association between serum expression level of Ang-2 and clinicopathological and survival data from breast cancer patients, and to determine whether circulating Ang-2 was a useful diagnostic biomarker for breast cancer.
Materials and methods
Patients and sample collection
The study was approved by the ethics committee of the third Xiangya Hospital and all the patients provided written informed consent. We prospectively collected serum samples from patients undergoing surgery for breast cancer. Sample collection was performed between June 2008 and May 2013 at the Department of Breast Surgery, the third Xiangya Hospital. 143 patients with histologically confirmed breast cancer and 100 healthy controls with no previous history of any cancer were included in this analysis. The median follow-up time was 47 months. Detailed clinicopathological parameters of patients are summarized in Table 1.
Table 1.
serum Ang-2 expression level in relation to clinicopathological features in patients with breast cancer
| Clinicopathological data | Case number (%) | Ang-2, pg/ml (mean ± SD) | P value |
|---|---|---|---|
| Age (years) | |||
| ≤ 55 | 79 (55.2%) | 3029 ± 998 | |
| > 55 | 64 (44.8%) | 3273 ± 1011 | 0.76 |
| Tumor size (cm) | |||
| ≤ 2.0 | 69 (48.3%) | 2834 ± 879 | |
| > 2.0 | 74 (51.7%) | 3566 ± 1023 | 0.09 |
| Histological grade | |||
| I/II | 86 (60.1%) | 2577 ± 1123 | |
| III | 57 (39.9%) | 3908 ± 791 | 0.03 |
| Lymph node metastasis | |||
| Absent | 91 (63.6%) | 2671 ± 997 | |
| Present | 52 (32.4%) | 3761 ± 1019 | 0.002 |
| Clinical stage | |||
| I/II | 82 (57.3%) | 2512 ± 1023 | |
| III | 61 (42.7%) | 3973 ± 921 | < 0.001 |
| Histological type | |||
| Ductal | 89 (62.2%) | 2999 ± 1013 | |
| Lobular | 54 (37.8%) | 3409 ± 1314 | 0.08 |
| ER status | |||
| Negative | 81 (56.6%) | 3387 ± 1290 | |
| Positive | 62 (43.4%) | 2971 ± 797 | 0.11 |
| PR status | |||
| Negative | 79 (55.2%) | 3346 ± 1091 | |
| Positive | 64 (44.8%) | 3031 ± 1001 | 0.79 |
| HER-2 status | |||
| Negative | 83 (56.5%) | 3276 ± 1213 | |
| Positive | 60 (43.5%) | 2923 ± 792 | 0.43 |
ER: estrogen receptor; PR: progesterone receptor; HER-2: C-erbB-2; SD: standard deviation.
Serum Ang-2 measurements
Blood samples were obtained prior to surgery. Serum was separated after centrifugation (2,500 rpm, 10 min) and stored at -80°C. Enzyme-linked immunosorbent assays were used to measure the levels of Ang-2 according to manufacturer’s instructions (Quantikine; R&D Systems, Minneapolis, Minn). All samples were examined in duplicate, and the mean values were used for statistical analysis. Measurements were done in blinded manner.
Statistical methods
Statistical analyses were performed using SPSS 18.0 statistical software package (SPSS Inc., Chicago, IL, USA). The relationship between clinicopathological parameters and serum Ang-2 levels was examined using the Mann-Whitney U or Kruskal-Wallis test, as appropriate. Sensitivity, specificity and area under curve (AUC) for serum Ang-2 levels were determined using receiver operator characteristic (ROC) analysis. Survival curves were plotted using the Kaplan-Meier method and differences in survival rates were analyzed using the log-rank test. Prognostic relevance of each variable to overall survival (OS) and disease-free survival (DFS) were analyzed using the Cox regression model. P-values of < 0.05 were considered to represent statistical significance.
Results
Increased serum expression levels of Ang-2 in patients with breast cancer
Enzyme-linked immunosorbent assay was performed to detect the serum expression level of Ang-2 in 143 patients with breast cancer and 100 healthy controls. Serum expression level of Ang-2 in patients with breast cancer was significantly higher than healthy control group (3171 ± 1024 vs. 1800 ± 874 pg/ml, P < 0.0001, shown in Figure 1). The median of Ang-2 expression levels in all 143 patients with breast cancer was 3353 pg/ml. We divided the patients into two groups according to their expression levels of Ang-2 using its median as a cutoff: high Ang-2 expression group (n = 72) and low Ang-2 expression group (n = 71).
Figure 1.
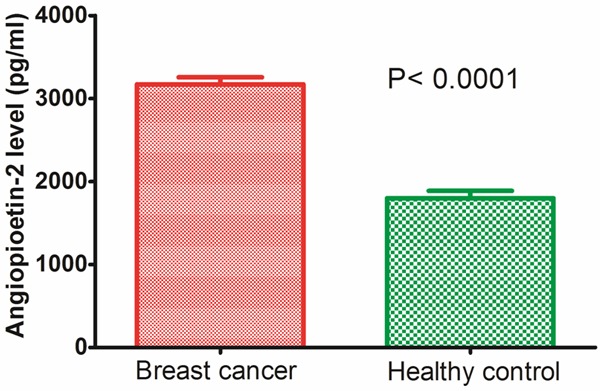
Serum Ang-2 expression level in 143 patients with breast cancer and 100 healthy controls. Serum expression level of Ang-2 in patients with breast cancer was significantly higher than healthy control group (3171 ± 1024 vs. 1800 ± 874 pg/ml, P < 0.0001).
Associations between serum expression levels of Ang-2 and clinical characteristics of breast cancer
We investigated the associations between the serum expression levels of Ang-2 and the clinicopathological characteristics of patients with breast cancer. Finally, we found that increased Ang-2 expression was strongly associated with histological grade (P = 0.03), lymph node metastasis (P = 0.002), and clinical stage (P < 0.001, shown in Table 1). However, no significant correlation was observed between serum Ang-2 level and other clinicopathologic parameters tested, including age (P = 0.77), tumor size (P = 0.91), histological type (P = 0.08), ER status (P = 0.11), PR status (P = 0.79), and HER-2 status (P = 0.43).
Circulating Ang-2 as a diagnostic biomarker in breast cancer
We plotted expression data for Ang-2 using ROC curves to identify a cut-off value that could distinguish breast cancer patients from healthy controls. ROC curve analysis showed that at the optimal cut-off (2558.5 pg/ml), serum level of Ang-2 had a sensitivity of 78.3% and a specificity of 77.0% for distinguishing breast cancer patients from healthy controls with an area under the curve (AUC) of 0.836 (P < 0.001, 95% confidence interval: 0.787-0.885, Figure 2).
Figure 2.
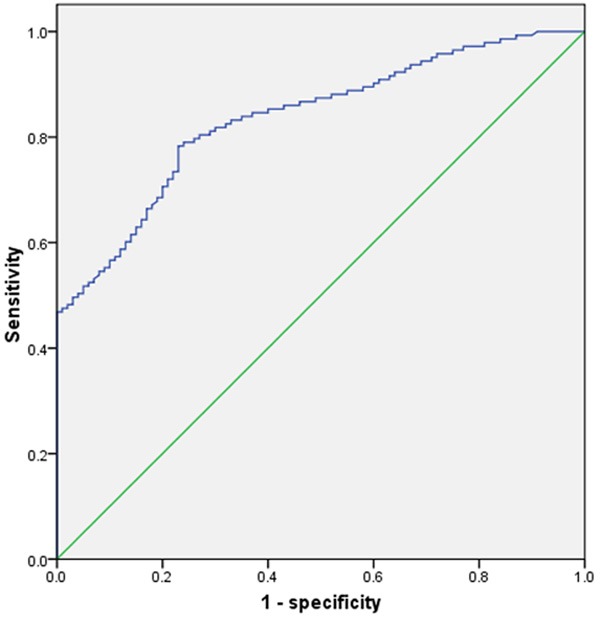
Receiver operator characteristic (ROC) analysis showed that at the optimal cut-off (2558.5 pg/ml), serum level of Ang-2 had a sensitivity of 78.3% and a specificity of 77.0% for distinguishing breast cancer patients from healthy controls with an area under the curve (AUC) of 0.836 (P < 0.001, 95% confidence interval: 0.787-0.885).
Increased expression of Ang-2 as a prognostic biomarker in breast cancer
To further investigate the correlation of serum Ang-2 expression level with survival of patients with breast cancer, Kaplan-Meier analyses were performed. As shown in Figure 3A, the 5-year OS of high Ang-2 expression group was significantly shorter than that of low Ang-2 expression group (55.9% vs. 80.3%; P = 0.018). Moreover, the 5-year DFS of high Ang-2 expression group was also significantly shorter than that of low Ang-2 expression group (46.0% vs. 68.7%; P = 0.029, shown in Figure 3B).
Figure 3.
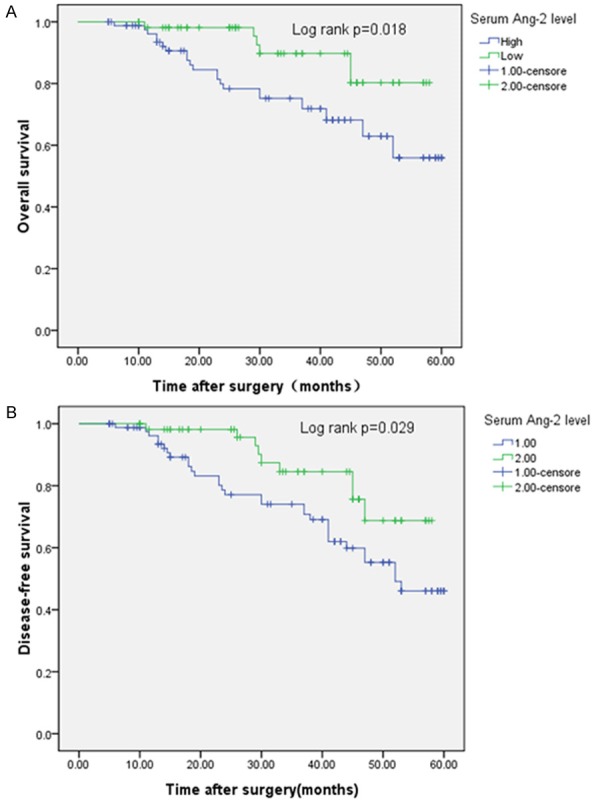
Kaplan-Meier curves of patients with breast cancer. Elevated Ang-2 level was associated with shorter overall survival (A) and disease-free survival (B).
Discussion
Ang-2 has been found to be highly expressed in some cancer tissues, including breast cancer [8,9,11-16]. Previously, Sfiligoi C et al found that increased expression of Ang-2 in breast cancer tissues correlated with lymph node invasion and short survival [10]. In their study, matching data with available clinicopathologic and biochemical data revealed a significant association between Ang-2 expression and lymph node invasion. Univariate and multivariate survival analysis, by means of Kaplan-Meier method and Cox’s proportional hazards model, showed significant and independent association between Ang-2 mRNA level and both disease-free and overall survival. However, they haven’t detected the serum expression level of Ang-2. Until now, it’s still unclear whether the serum expression level of Ang-2 is increased. Also, the diagnostic and prognostic potential of serum Ang-2 expression in breast cancer hasn’t been investigated. Previous studies have investigated the serum Ang-2 expression level and its diagnostic and prognostic potential in several other cancers. For example, Sallinen H et al found that Ang-2 levels were significantly elevated in serum samples of patients with ovarian carcinoma compared with healthy controls. In addition, Ang-2 levels were significantly higher in patients with ovarian carcinoma compared with patients with benign or borderline ovarian tumors. In ROC analysis, the area under the curve for serum Ang-2 (0.77) was lower than CA125 (0.95) to differentiate ovarian cancer from healthy control. High serum levels of Ang-2 were associated with primary residual tumor more than 1 cm after debulking surgery, and high Ang-2 levels correlated positively with an advanced tumor stage. Elevated Ang-2 level (> 2.7 ng/mL) was a significant predictor of poor overall and recurrence-free survival when assessing Kaplan-Meier curves by a log-rank test. These results suggested that Ang-2 may serve as an angiogenic marker of decreased patient survival in ovarian cancer [17]. Similar to their results, Engin H et al found that the plasma concentrations of Ang-2 were significantly higher in patients with colon cancer compared with healthy controls. Furthermore, plasma concentrations of Ang-2 were found to be significantly higher in stage III patients compared to stage II patients. Therefore, plasma concentrations Ang-2 may be a valuable, additional, tumor marker in colon cancer [18]. Furthermore, Fawzy A et al concluded that serum Ang-2 was a useful marker for the diagnosis of non-small cell lung cancer (NSCLC) by ELIZA technique [19].
In the present study, our results showed that the levels of serum Ang-2 were significantly increased in patients with breast cancer compared with healthy controls. Further more, we found that serum Ang-2 level expression levels were significantly positively correlated with histological grade, lymph node involvement, and clinical stage, suggesting that Ang-2 might be involved in the carcinogenesis and metastasis of breast cancer. More importantly, ROC curve analysis showed that serum level of Ang-2 had a sensitivity of 78.3% and a specificity of 77.0% for distinguishing breast cancer patients from healthy controls with an AUC of 0.836, and we proved that patients with a high expression of Ang-2 tended to have shorter DFS and OS than patients with lower levels, indicating that high Ang-2 level is a promising non-invasive biomarker for diagnosis and prognosis of patients with breast cancer.
In summary, we found a significant elevation of serum Ang-2 levels in patients with breast cancer compared with those of healthy controls. Our data indicate that serum Ang-2 level has potential value for early detection of breast cancer. Furthermore, Ang-2 has prognostic value in patients with breast cancer.
Disclosure of conflict of interest
None.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, Daly TJ, Davis S, Sato TN, Yancopoulos GD. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Sci. 1997;277:55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- 3.Conteduca V, Kopf B, Burgio SL, Bianchi E, Amadori D, De Giorgi U. The emerging role of anti-angiogenic therapy in ovarian cancer (Review) Int J Oncol. 2014;44:1417–1424. doi: 10.3892/ijo.2014.2334. [DOI] [PubMed] [Google Scholar]
- 4.Katoh M. Therapeutics targeting angiogenesis: genetics and epigenetics, extracellular miRNAs and signaling networks (Review) Int J Mol Med. 2013;32:763–767. doi: 10.3892/ijmm.2013.1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eroglu Z, Stein CA, Pal SK. Targeting angiopoietin-2 signaling in cancer therapy. Expert Opin Investig Drugs. 2013;22:813–825. doi: 10.1517/13543784.2013.793306. [DOI] [PubMed] [Google Scholar]
- 6.Thomas M, Augustin HG. The role of the Angiopoietins in vascular morphogenesis. Angiogenesis. 2009;12:125–137. doi: 10.1007/s10456-009-9147-3. [DOI] [PubMed] [Google Scholar]
- 7.Gerald D, Chintharlapalli S, Augustin HG, Benjamin LE. Angiopoietin-2: an attractive target for improved antiangiogenic tumor therapy. Cancer Res. 2013;73:1649–1657. doi: 10.1158/0008-5472.CAN-12-4697. [DOI] [PubMed] [Google Scholar]
- 8.Hu B, Cheng SY. Angiopoietin-2: development of inhibitors for cancer therapy. Current Oncology Reports. 2009;11:111–116. doi: 10.1007/s11912-009-0017-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Helfrich I, Edler L, Sucker A, Thomas M, Christian S, Schadendorf D, Augustin HG. Angiopoietin-2 levels are associated with disease progression in metastatic malignant melanoma. Clin Cancer Res. 2009;15:1384–92. doi: 10.1158/1078-0432.CCR-08-1615. [DOI] [PubMed] [Google Scholar]
- 10.Sfiligoi C, de Luca A, Cascone I, Sorbello V, Fuso L, Ponzone R, Biglia N, Audero E, Arisio R, Bussolino F, Sismondi P, De Bortoli M. Angiopoietin-2 expression in breast cancer correlates with lymph node invasion and short survival. Int J Cancer. 2003;103:466–74. doi: 10.1002/ijc.10851. [DOI] [PubMed] [Google Scholar]
- 11.Coelho AL, Araujo A, Gomes M, Catarino R, Marques A, Medeiros R. Circulating Ang-2 Mrna Expression Levels: Looking ahead to a New Prognostic Factor for NSCLC. PLoS One. 2014;9:e90009. doi: 10.1371/journal.pone.0090009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen HH, Weng BQ, Cheng KJ, Liu HY, Wang SQ, Lu YY. Effect of the vascular endothelial growth factor expression level on angiopoietin-2-mediated nasopharyngeal carcinoma growth. Vasc Cell. 2014;6:4. doi: 10.1186/2045-824X-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li C, Fan J, Song X, Chen Y, Li C, Mi K, Ma H, Song Y, Tao X, Li G. Expression of angiopoietin-2 and vascular endothelial growth factor receptor-3 correlates with lymphangiogenesis and angiogenesis and affects survival of oral squamous cell carcinoma. PLoS One. 2013;8:e75388. doi: 10.1371/journal.pone.0075388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pappa CA, Tsirakis G, Samiotakis P, Tsigaridaki M, Alegakis A, Goulidaki N, Alexandrakis MG. Serum levels of angiopoietin-2 are associated with the growth of multiple myeloma. Cancer Invest. 2013;31:385–389. doi: 10.3109/07357907.2013.800093. [DOI] [PubMed] [Google Scholar]
- 15.Li C, Sun CJ, Fan JC. Angiopoietin-2 expression is correlated with angiogenesis and overall survival in oral squamous cell carcinoma. Med Oncol. 2013;30:571. doi: 10.1007/s12032-013-0571-2. [DOI] [PubMed] [Google Scholar]
- 16.Diaz-Sanchez A, Matilla A, Nunez O. Serum angiopoietin-2 level as a predictor of tumor invasiveness in patients with hepatocellular carcinoma. Scand J Gastroenterol. 2013;48:334–343. doi: 10.3109/00365521.2012.746391. [DOI] [PubMed] [Google Scholar]
- 17.Sallinen H, Heikura T, Laidinen S, Kosma VM, Heinonen S, Ylä-Herttuala S, Anttila M. Preoperative angiopoietin-2 serum levels: a marker of malignant potential in ovarian neoplasms and poor prognosis in epithelial ovarian cancer. Int J Gynecol Cancer. 2010;20:1498–1505. doi: 10.1111/IGC.0b013e3181f936e3. [DOI] [PubMed] [Google Scholar]
- 18.Engin H, Ustundag Y, Tekin IO, Gökmen A, Ertop Ş, İlikhan SU. Plasma concentrations of angiopoietin-1, angiopoietin-2 and Tie-2 in colon cancer. Eur Cytokine Netw. 2012;23:68–71. doi: 10.1684/ecn.2012.0308. [DOI] [PubMed] [Google Scholar]
- 19.Fawzy A, Gaafar R, Kasem F, Ali SS, Elshafei M, Eldeib M. Importance of serum levels of angiopoietin-2 and survivin biomarkers in non-small cell lung cancer. J Egypt Natl Canc Inst. 2012;24:41–45. doi: 10.1016/j.jnci.2011.12.006. [DOI] [PubMed] [Google Scholar]


