Abstract
The development of novel antitumor drugs for the treatment of non-small cell lung carcinoma NSCLC is imperative in order to improve the efficacy of lung cancer therapy and prognosis. In the current study, we demonstrated the antitumor activity of isorhamnetin and its combinations with cisplatin and carboplatin against A-549 lung cancer cells. In order to assess the anticancer enhancing effect of isorhamnetin on cisplatin and carboplatin, A-549 cells were treated with isorhamnetin, cisplatin, carboplatin and their combinations and cell viability, cell apoptosis, cell cycle arrest as well as loss of mitochondrial membrane potential were evaluated by MTT assay, flow cytometry, confocal microscopy and fluorescence microscopy. The effect of the drugs on cancer cell migration, microtubule depolymerization as well activation of caspases was also studied. The results revealed that, as compared to single drug treatment, the combination of isorhamnetin with cisplatin and carboplatin resulted in greater effect in inhibiting cancer cell growth and inducing apoptosis. Combination of isorhamnetin with cisplatin and carboplatin resulted in more potent apoptosis induction as revealed by fluorescence microscopy using AO/PI double staining. Isorhamnetin and its combinations also triggered microtubule distortion and depolymerization. The combination of isorhamnetin with cisplatin and carboplatin increased the number of cells in G2/M phase dramatically as compared to single drug treatment. Moreover, isorhamnetin and its combinations with known anticancer drugs induced disruption of the mitochondrial membrane potential as well as activation of caspases 3, 9 and poly-(ADP-ribose) polymerase in A-549 cells. Isorhamnetin as well as its combinations with cisplatin and carboplatin resulted in inhibition of cancer cell migration significantly. Results of the current study suggest that isorhamnetin combinations with cisplatin and carboplatin might be a potential clinical chemotherapeutic approach for NSCLC.
Keywords: Non-small cell lung carcinoma, isorhamnetin, apoptosis, cell migration, tubulin, chemotherapy
Introduction
Lung cancer, also known as pulmonary carcinoma, is a malignant lung tumor characterized by uncontrolled cell growth in tissues of the lung. Lung cancer is initiated by activation of oncogenes or inactivation of tumor suppressor genes. Carcinogens cause mutations in these genes which induce the development of cancer. Mutations in the K-ras proto-oncogene are responsible for 10-30% of lung adenocarcinomas. About 4% of non-small-cell lung carcinomas involve an EML4-ALK tyrosine kinase fusion gene. Epigenetic changes such as alteration of DNA methylation, histone tail modification, or microRNA regulation may lead to inactivation of tumor suppressor genes. The epidermal growth factor receptor (EGFR) regulates cell proliferation, apoptosis, angiogenesis, and tumor invasion. Mutations and amplification of EGFR are common in non-small-cell lung carcinoma and provide the basis for treatment with EGFR-inhibitors [1-5].
Lung cancer is the most frequently diagnosed cancer responsible for almost 1.38 million deaths every year worldwide [6,7]. In China, lung cancer has replaced liver cancer as the leading cause of death among people with malignant tumors in 2008. According to the statistics from the National office on tumor cure and prevention, about 600000 people die of lung cancer each year in China. For therapeutic purposes, clinically two broad classes of lung cancer have been distinguished, non-small-cell lung carcinoma (NSCLC) and small-cell lung carcinoma (SCLC). NSCLC is more prevalent, accounting for almost 80% of lung cancers. Non-small cell lung cancer is composed of several subtypes, including lung adenocarcinoma, which is the most common lung cancer in the western world. NSCLC also includes squamous-cell carcinoma and large-cell carcinoma. Squamous-cell carcinoma accounts for about 30% of lung cancers. About 9% of lung cancers are large-cell carcinoma [8-11].
Among the conventional anticancer drugs, platinum complexes such as cisplatin (cis-diamminedichloroplatinum II) and carboplatin (cis-diammine [1,1-cyclobutane-dicarboxylato] platinum) have been investigated comprehensively [12-15]. The chemotherapy regimen for lung cancer depends on the tumor type. Small-cell lung carcinoma (SCLC) is treated primarily with chemotherapy and radiotherapy [16]. In SCLC, cisplatin and etoposide are most commonly used. Combinations with carboplatin, gemcitabine, paclitaxel, vinorelbine, topotecan, and irinotecan are also used [17,18]. In advanced non-small cell lung carcinoma (NSCLC), chemotherapy improves survival and is used as first-line treatment. The most frequently used therapies for NSCLC are chemotherapy, radiotherapy and surgical treatments. Generally NSCLC is treated by surgical operation followed by chemotherapy to avoid recurrence. Unfortunately, 2/3 of the patients are inoperable when diagnosed. Among the patients who undergo surgical operation with overall 5-year survival rates of about 40%, nearly 40% belong to stage IIIA and a few to stage IIIB with a 5-year survival rate of about 20%. About 50% of the NSCLS patients receive chemotherapy with paclitaxel and carboplatin. However, these chemotherapeutic drugs produce serious side-effects such as leukopenia, hepatic dysfunction, disorder in renal function, nausea and vomiting. These side-effects are most commonly attributed to tissue necrosis, apoptosis and inflammation caused by the chemotherapy treatment [19-22]. The toxic side-effects of these anticancer drugs can be minimized by using non-toxic potentiating agent or synergistic compound in combination with anticancer drugs which may possibly potentiate their effectiveness while minimizing their toxicity by reducing the dose. Such non-toxic potentiating agents which can possibly solve many problems of the clinical cancer treatment include flavonoids from plants [23,24].
Therefore, a main attention of pre-clinical and clinical research in recent years has been to design innovative treatment strategies capable of selectively inhibiting crucial pathways that control cancer cell proliferation and apoptosis [18-20 Silibinin]. Combination therapy/prevention, in this regard, is gaining increased attention as an effective alternative to increase efficacy and minimize systemic toxicity of chemotherapeutic agents [25,26]. The present study is an effort to analyze the effects of a combination of isorhamnetin and platinum compounds (cisplatin and carboplatin) on growth inhibition, cell cycle regulation and apoptosis in non-small cell lung carcinoma cells, and to define molecular mechanisms involved in the observed increased efficacy. This study constitutes first such report on isorhamnetin combination treatment of NSCLC with cisplatin and carboplatin.
Materials and methods
Cell lines and chemicals
A-549 non-small-cell lung cancer cell lines were purchased from China Center for Type Culture Collection (Wuhan, China). Cells were cultured in RPMI 1640 with 10% fetal bovine serum (Hyclone Laboratories, Logan, UK) and 100 units/ml penicillin -100 µg/ml streptomycin, and were grown at 37°C in a humidified 95% air and 5% CO2 atmosphere. RPMI 1640 and other culture materials were from GIBCO/BRL Life Technologies (Grand Island, NY).Isorhamnetin flavonoid (> 95% purity by HPLC), cisplatin and carboplatin were from Sigma Chemical Co. (St. Louis, MO.), and for all the studies, isorhamnetin and cisplatin were dissolved in dimethyl sulfoxide (DMSO) and carboplatinin distilled water as stock solutions and diluted as per requirement. Unless specified otherwise, the final concentration of DMSO in the culture medium during different treatments did not exceed 0.2% (v/v), and therefore the same concentration of DMSO was present in control dishes.
MTT assay for evaluating the cytotoxic effect
The cytotoxic effect of isorhamnetin, cisplatin and carboplatin and their combination on the proliferation of A-549 cells was measured by MTT assay. Briefly, A-549 cells were plated at a density of 1×105 cells per well in 96 well plates overnight and then treated with 0, 2.5, 5, 25, 50, 75 and 100 μM isorhamnetin; 0.5 μM cisplatin and carboplatin each or their combination (25 μM isorhamnetin plus 0.5 μM cisplatin or 0.5 μM carboplatin) for 24 h. Ten microliters of MTT solution (1 mg/ml in PBS) were added to each well and the cells were cultured for another 4 h at 37°C. The medium was completely removed and 200 μl DMSO was added to solubilize MTT formazan crystals. The plates were then agitated and the optical density was determined at 570 nm (OD570) using an ELISA plate reader (Model 550; Bio-Rad, Hercules, CA, USA). At least three independent experiments were performed.
Immunofluorescence assay of microtubule
The A-549 cells were cultured on the coverslips and treated with 0.030% DMSO, 25 μM isorhamnetin, 0.5 µM cisplatin and carboplatin each (or their combination viz., isorhamnetin+cisplatin and isorhamnetin+carboplatin) for 48 h. The cells were fixed with 2.5% formaldehyde for 20 min and were then permeabilized with 0.5% Triton ×-100 in PBS for 10 min. After blocking with 5% BSA for 20 min, the cells were incubated with anti-β-tubulin monoclonal antibody (1:50 in the blocking solution) for 3 h at 37°C followed by incubation with FITC-conjugated goat anti-mouse IgG (1:1000) for 1 h at 37°C. Images of the microtubules were obtained using a confocal fluorescence microscope. Mitotic index was calculated by the ratio of the number of cells in mitosis (including the cell with condensed chromosomes, aligned chromosomes and segregated chromosomes) to the total number of cells. The experiment was repeated thrice.
Acridine orange/propidium iodide staining for the detection of apoptosis
For the detection of apoptosis in the treated cells, a propidium iodide (PI) and acridine orange (AO) double staining assay were done using a fluorescent microscope (Leica attached with Q-Floro software) according to a standard procedure. A-549 cells (1×106 cells/mL in a 50 mL culture flask) were plated, treated with isorhamnetin (IR, 50 µM), cisplatin (CP, 0.5 µM), carboplatin (CB, 0.5 µM) and their combinations (IR+CP) and (IR+CB), and incubated for 48 h. After harvesting the cells, they were stained with fluorescent dyes and observed under a UV-fluorescent microscope (Olympus B ×51).
Immunoblotting assay for evaluating activation of caspases and PARP induced by isorhamnetin, cisplatin, carboplatin and their combinations
After treatment of A-549 cells with isorhamnetin (25 μM), cisplatin (25 μM), carboplatin (25 μM) and their combinations (IR+CP) and (IR+CB)or DMSO for 48 h, the cells were collected by trypsinization, washed twice with cold PBS, and then lysed with ice-cold lysis buffer consisting of 30 mMTris-HCl (pH 7.4), 0.2% SDS, 100 mM NaCl, 1 mMEDTA (Merck), 1% Triton X-100 and a protease inhibitor cocktail (Tianjin Resent Chemicals CO., Ltd.) of 0.002% (w/v) aprotinin, 0.002% (w/v) leupeptin, 0.00025% (w/v) pepstain A, and 0.085% (w/v) PMSF. Cell lysates were kept on ice for 30 min after gentle vortexing, and then centrifuged at 15,000 g for 10 min at 4°C. The supernatants were immediately analyzed by western blotting or stored at -80°C until use. The protein concentration was measured by a Bio-Rad assay. For western blot analysis, equal amounts of proteins (30~60 μg) from each treatment were loaded on 12% SDS-PAGE gels. SDS-PAGE and electrophoretic transfer of proteins onto PVDF membranes (Millipore, Billerica, MA, USA) were performed with Hoefer and Bio-Rad apparati, respectively. Then, PVDF membranes were incubated with antibodies against caspase 8 (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA), caspase 9, caspase 3, PARP (Cell Signaling), and β-actin for 1 h or overnight. Membranes were then incubated with the appropriate secondary antibody conjugated to horseradish peroxidase for 1 h. After the membranes were exposed to reagents for ECL immunodetection, the labeled proteins were detected by autoradiographic film.
Cell cycle evaluation by flow cytometry
The cell cycle analysis was performed by flow cytometry with DNA propidium iodide (PI) staining. The A549 cells were treated with isorhamnetin (25 μM), cisplatin (0.5 μM), carboplatin (0.5 μM) and their combinations (IR 25 µM+CP 0.5 µM) and (IR 25 µM+CB 0.5 µM) or 0.020% DMSO for 48 hand then harvested by trypsinization. The cells were fixed with 2 ml of 75% cold ethanol overnight and then stained with staining buffer containing 0.02% PI, 0.2% sodium citrate, 0.5% Triton ×-100 and 0.02% RNase A for 1 h in the dark. The DNA content and cell cycle phase distribution were examined using a BD FACS Calibur flow cytometer and Cell Quest software 3.0 (Becton-Dickinson). The experiment was repeated three times.
Cell apoptosis evaluation and its quantification
Apoptosis detection was performed using the Annexin V-FITC and PI apoptosis kit (Bestbio, Shanghai, China). A-549 cells were plated at a density of 2×106 cells/well into 12-well plates and incubated overnight. Apoptosis was triggered by exposing cells with isorhamnetin (25 µM), cisplatin (0.5 µM), carboplatin (0.5 µM) or their combinations (IR 25 µM+CP 0.5 µM) and (IR 25 µM+CB 0.5 µM) for 48 h. Cells grown in media containing an equivalent amount of DMSO without any drug served as control. After 48 h of incubation, the cells with different treatments were harvested and the final samples were measured on a BD FACS Calibur flow cytometer and Cell Quest software 3.0 (Becton-Dickinson). The experiment was repeated three times.
Mitochondrial membrane potential (mmp) assay
The mitochondrial membrane potential in A-549 cells was assessed by using the JC-1 mitochondria staining kit for flow cytometry as per the recommendations of the manufacturer. Briefly, cells were treated with isorhamnetin (25 µM), cisplatin (0.5 µM), carboplatin (0.5 µM) or their combinations (IR 25 µM+CP 0.5 µM) and (IR 25 µM+CB 0.5 µM)for the indicated time then incubated in medium containing JC-1 probe (3-5 μGu/mL) for 20 min at 37°C The cells were then incubated with 5 mM of JC-1 for 30 min at 350°C. After washing with ice-cold JC-1 binding buffer twice, mitochondrial membrane potential (MMP) was measured immediately using flow cytometry. Two excitation wavelengths, 527 nm (green) and 590 nm (red) were used to detect the JC-1 monomer form and the JC-1 aggregate form, respectively. The red fluorescence was primarily detected in healthy viable cells with high MMP, while red fluorescence was decreased in damaged mitochondria. The cells incubated with JC-1 were also detected under laser scanning confocal microscope.
Cell migration assay
Cell monolayer (90% confluent) was allowed to become quiescent in medium with 0.1% dialyzed fetal bovine serum for 24 h. Then cells were scraped to make a straight line wound and treated with isorhamnetin (25 µM), cisplatin (0.5 µM), carboplatin (0.5 µM) or their combinations (IR 25 µM+CP 0.5 µM) and (IR 25 µM+CB 0.5 µM) for 48 h. Photographs were taken at 48 h and lengths of wound were determined by Image J (version 1.46) software.
Statistical analyses
Data analysis. All results are presented as means ± SD of n observations, unless otherwise noted. Statistical significance was determined at the 95% confidence level using one-way ANOVA with Scheffe’s test.
Results
Antiproliferative activity of isorhamnetin, cisplatin, carboplatin and their combinations against A-549 cancer cells
The antiproliferative activity of isorhamnetin (IR), cisplatin (CP), carboplatin (CB) and their combinations are shown in Figures 1 and 2. Cells were initially treated with varying concentrations of isorhamnetin (2.5, 5, 25, 50, 75 and 100 µM) for 24 h. Isorhamnetin showed a dose-dependent growth inhibition of A-549 cells. For combination treatment, 25 µM of isorhamnetin was used with 0.5 µM each of cisplatin and carboplatin. The results of the combination treatment showed much more potent growth inhibition of the cell growth. As compared to the isorhamnetin, cisplatin or carboplatin growth inhibitory effects, their combination was much more effective in curbing the growth of the A-549 cancer cells. These results indicate that these potential combinations are encouraging for cancer chemotherapy, since single drug therapy is less effective now because of multidrug resistance exhibited by cancer cells. DMSO served as vehicle control.
Figure 1.
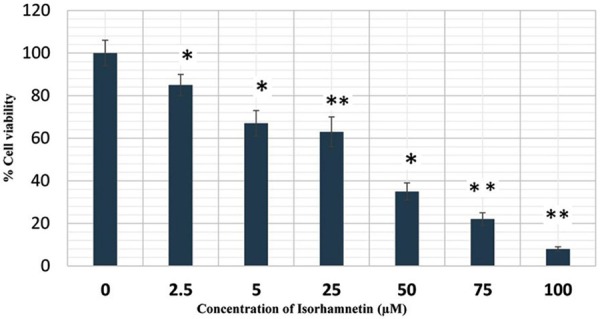
Effect of isorhamnetin on A-549 cancer cell proliferation. These cells were treated with DMSO as vehicle control and with isorhamnetin at different concentrations from (0, 2.5, 5, 25, 50 and 100 μM) for 24 h and cell viability was determined by MTT assay. *P = 0.05, **P = 0.01 vs. vehicle control.
Figure 2.
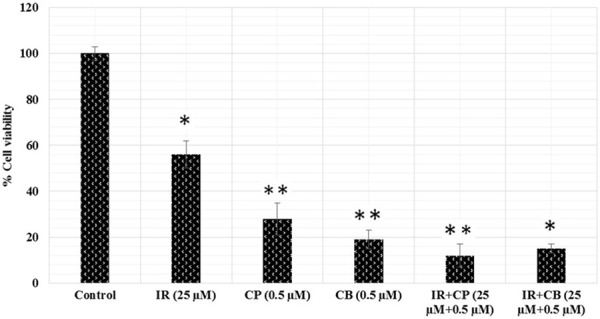
Effect of isorhamnetin, cisplatin, carboplatin and their combinations on A-549 cancer cell proliferation. The cells were treated with DMSO as vehicle control and with isorhamnetin (IR, 25 μM), cisplatin (CP, 0.5 μM), carboplatin (CB, 0.5 μM) and their combinations (IR+CP and IR+CB) for 24 h and cell viability was determined by MTT assay. *P = 0.05, **P = 0.01 vs. vehicle control.
Quantification of apoptosis using ao/pi double staining and phase-contrast microscopy
Morphological changes in A-549 cells treated with isorhamnetin, cisplatin, carboplatin or their combinations were observed under a fluorescent microscope for 48 h. The cells were counted under a fluorescent microscope to analyze viable cells, early apoptosis, and late apoptosis. Early apoptosis, demarcated as intervening AO within the damaged DNA, was detected under bright green fluorescence. Simultaneously, control cells (no drug treatment) were visualized with a green intact nuclear structure (Figure 3). Control (A, untreated) A-549 cells after 48 h showed normal structure without noticeable apoptosis. (B-D) Isorhamnetin, cisplatin and carboplatin-treated cells respectively showed early apoptosis features including blebbing and chromatin condensation after 48 h treatment. (E and F) isorhamnetin+cisplatin and isorhamnetin+carboplatin-treated cells respectively showed late apoptosis in addition to early apoptotic features.
Figure 3.
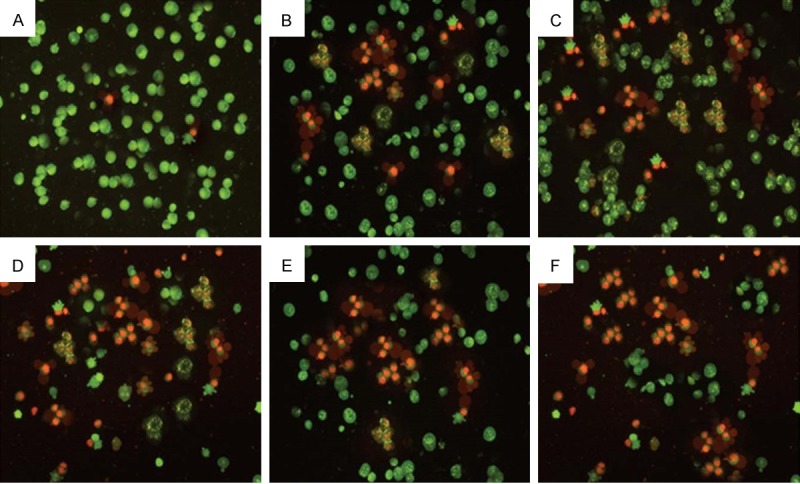
Effect of Isorhamnetin, cisplatin, carboplatin and their combinations on cell apoptosis in A-549 cancer cells. Control (Untreated) A-549 cells after 48 h showed normal structure without noticeable apoptosis. (B-D) Isorhamnetin, cisplatin and carboplatin-treated cells respectively showed early apoptosis features including blebbing and chromatin condensation after 48 h treatment. (E and F) isorhamnetin+cisplatin and isorhamnetin+carboplatin-treated cells respectively showed late apoptosis in addition to early apoptotic features. (magnification: 200×).
Effect of isorhamnetin, cisplatin, carboplatin and their combinations on microtubules in A549 cells
We also investigated the effect of isorhamnetin, cisplatin, carboplatin and their combinations on microtubules in A-549 cancer cells. Microtubules play a key role in the process of mitosis. The immunofluorescence staining of microtubules with anti-β-tubulin monoclonal antibody followed by FITC-conjugated goat anti-mouse IgG was performed. No aberrant microtubule disruption was detected in the control cells; however, aberrant microtubule disruption was seen in the cells treated with isorhamnetin, cisplatin, carboplatin and their combinations (Figure 4). The effect of the combination of isorhamnetin with cisplatin and carboplatin was much more pronounced as compared to single agents.
Figure 4.
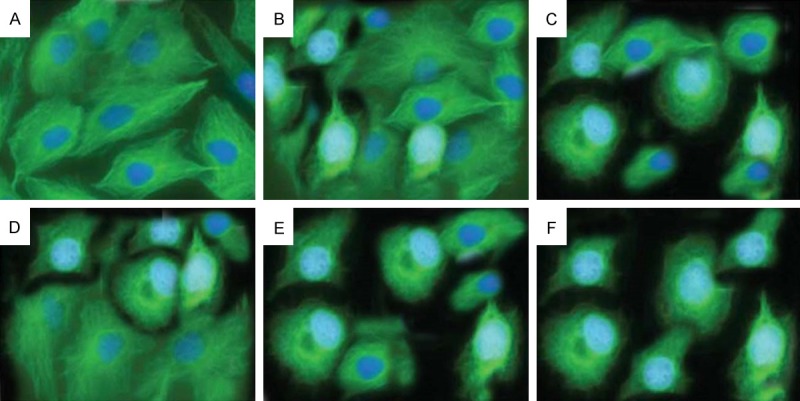
The effects of Isorhamnetin, cisplatin, carboplatin and their combinations on microtubules in A-549 cells. A549 cells were treated with 0.025% DMSO (A), 25 μM isorhamnetin (B), 0.5 μM cisplatin (C) and carboplatin (D) each or their combination (IR+CP) (E) and (IR+CB) (F) for 48 h. The images were visualized under a fluorescence microscope. The representative from three independent experiments is shown.
Evaluation and quantification of cell apoptosis in A-549 cells after isorhamnetin, cisplatin, carboplatin and their combination treatment
An early indicator of apoptosis is the rapid translocation and buildup of the membrane phospholipid phosphatidylserine from the cytoplasmic interface to the extracellular surface. This membrane asymmetry loss could be identified by utilizing the binding properties of Annexin V. To substantiate the synergistic effects of isorhamnetin with cis-platin and carboplatin on A-59 cells, the apoptotic effects of IR, CP, CB, IR+CP and IR+CB were tested using Annexin V-FITC and PI apoptosis kit. Annexin V staining can detect phosphatidyl serine and as such can be used for its analysis. The typical dot-plots illustrating apoptotic status are shown in Figure 5. The percentage of early apoptotic as well as late apoptotic cells increases dramatically after combination treatment. Cells were treated with either 25 µM isorhamnetin (IR, B), 0.5 µM cis-platin (CP, C), 0.5 µM carboplatin (CB, D) or their combinations (IR+CP, E) and (IR+CB, F) for 48 h, while DMSO-PBS served as control (A). The apoptotic cell percentage was 5.2±0.5% (control, A), 21.1±1.5% (isorhamnetin, B) (P < 0.05 vs. control), 54.0±2.1% (cisplatin, C) (P < 0.05 vs. control), 58.1±3.4% (carboplatin, D) (P < 0.01 vs. control), 78.1±8.6% (IR+CP, E) and 84.2±3.2% (IR+CB, F) (P < 0.01 vs. control).
Figure 5.
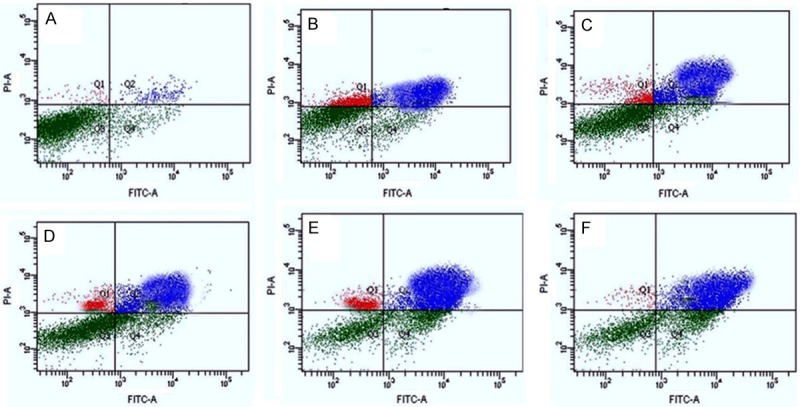
Isorhamnetin, cis-platin, carboplatin-induced apoptosis of A-549 cells analyzed by FACS, stained with annexin V-FITC/PI. Cells were treated with either 25 μM isorhamnetin (IR, B), 0.5 μM cisplatin (CP, C), 0.5 μM carboplatin (CB, D) or their combinations (IR+CP, E) and (IR+CB, F) for 48 h, while DMSO-PBS served as control (A). Data summary and analysis of the proportion of A-549 cells in different periods was according to the results of flow cytometric analysis.
Effect of isorhamnetin, cis-platin, carboplatin and their combinations on cell cycle phase distribution in A-549 cells
The effect of the isorhamnetin (25 µM), cis-platin (0.5 µM), carboplatin (0.5 µM) and their combinations is shown in Figure 6. Both the single drug treatment as well as the combination treatment of isorhamnetin with cis-platin and carboplatin induced disturbances in the cell cycle phase distribution in A-549 cancer cells. Untreated cells showed that 58.1% cells were in the cell growth (G1) phase, 27.6% in DNA synthesis (S) phase and 9.1% in division (G2/M) phase. Treatment with isorhamnetin at a concentration of 25 µM increased the amount of cells in G2/M to 32.5% (Figure 6B). Treatment with cis-platin at 0.5 µM increased the percentage further to 36.7% (Figure 6C) and increased significantly on treatment with carboplatin at 0.5 µM to 49.2% (Figure 6D), reaching a maximum of 78.9% (Figure 6E), and 84.1% (Figure 6F), respectively for isorhamnetin+cisplatin and isorhamnetin+carboplatin combinations. Therefore, the ability of these drugs to induce mitotic block was shown to increase in the order control < isorhamnetin < cisplatin < carboplatin < isorhamnetin+cisplatin < isorhamnetin+carboplatin.
Figure 6.
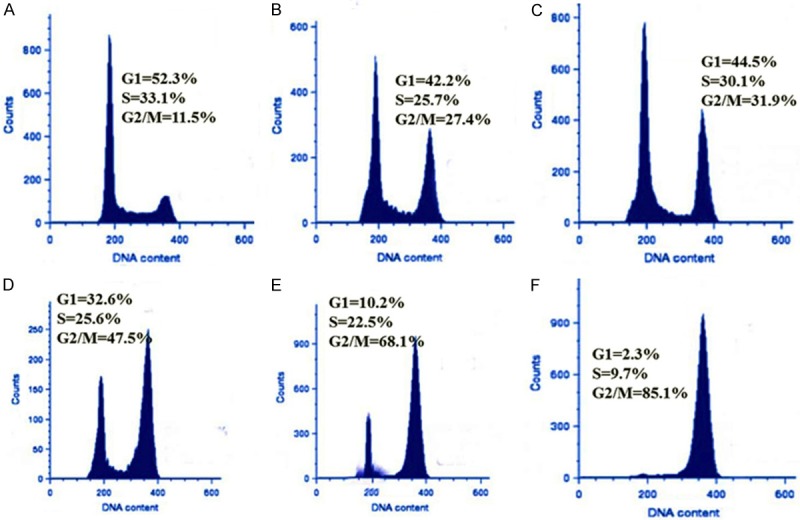
Effect of isorhamnetin, cis-platin, carboplatin and their combinations on cell cycle phase distribution in A-549 cells using FACS analyzer. A-549 cells (lung cancer) cells were stained with propidium iodide after 48 h exposure to control (A), 25 μM Isorhamnetin (IR, B), 0.5 μM cisplatin (CP, C), 0.5 μM carboplatin (CB, D), IR+CP (E) and IR+CB (F). Data is representative of three independent experiments.
Effect of isorhamnetin, cis-platin, carboplatin and their combinations on Mitochondrial Membrane Potential in A-549 cells
The effect of the compounds and their combinations is shown in Figure 7. Mitochondrial membrane potential (MMP, ΔΨm) is an indicator of mitochondrial function which is intimately related with mitochondrial membrane permeability. In this study, we determined whether the apoptosis induced in A-549 cells by isorhamnetin, cis-platin, carboplatin and their combinations was accompanied with loss of mitochondrial membrane potential and hence the mitochondrial dysfunction. The MMP of the A-549 cells treated with isorhamnetin, cis-platin, carboplatin and their combinations was measured by flow cytometry using JC-1 probe. The results revealed that the mean fluorescence intensity ratio of red fluorescence and green fluorescence (FL2-H/FL1-H) which pointed towards the fact that MMP levels declined considerably after isorhamnetin, cis-platin, carboplatin and their combinations (Figure 7). Thus, this assay gave some evidence that the apoptosis in A-549 cells occurred via mitochondrial pathway.
Figure 7.
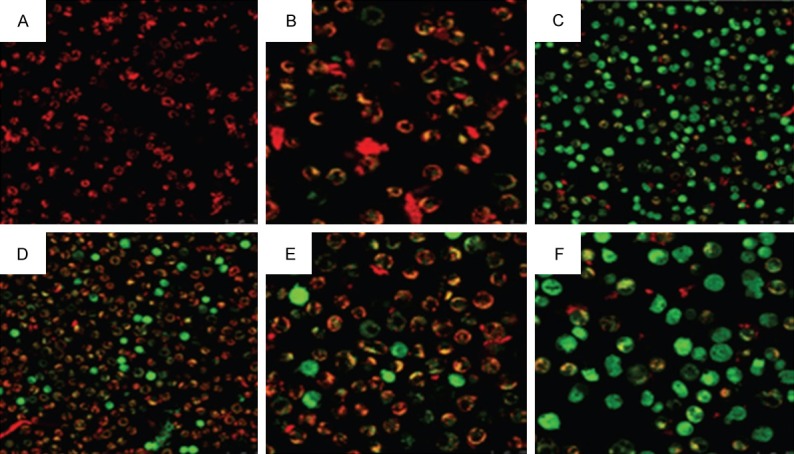
Evaluation of mitochondrial membrane potential of A-549 cells analyzed by laser confocal microscopy and flow cytometry after treatment with 25 μM Isorhamnetin (IR, B), 0.5 μM cisplatin (CP, C), 0.5 μM carboplatin (CB, D), IR+CP (E) and IR+CB (F). The ratio of red and green mean fluorescence intensity obtained under FACS shows mitochondrial membrane potential loss induced by isorhamnetin, cis-platin, carboplatin and their combinations.
Isorhamnetin, cis-platin, carboplatin and their combinations induce activation of caspases and PARP in A-549 cancer cells
Since caspase family members (caspase 3, 8 and 9) as well as Poly (ADP-ribose) polymerase (PARP), are the key mediators of the apoptotic process, we evaluated in this study whether induction of apoptosis in A-549 cells by isorhamnetin, cis-platin, carboplatin and their combinations was accompanied with the activation of caspases and PARP. The results are presented in Figure 8, which shows that after A-549 cells were treated with isorhamnetin, cis-platin, carboplatin and their combinations, these triggered the activation of caspase 9 in the apoptotic intrinsic pathway leading to slight alteration of cleaved caspase 8 in the apoptotic extrinsic pathway compared with that in the control. Further, the results also showed that PARP had undergone cleavage while as the expression of procaspase 3 got diminished. The expression of these proteins after combination treatment decreased remarkably. These results point out to the fact that isorhamnetin, cis-platin, carboplatin and their combination-induced apoptosis in A-549 cells is connected with caspase 3 and 9.
Figure 8.
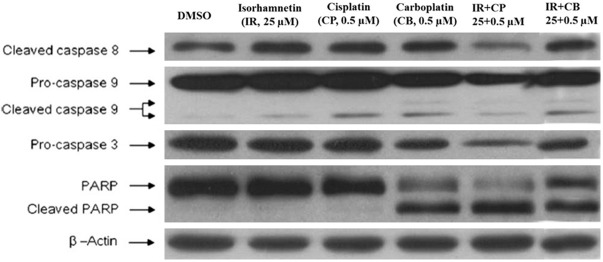
Apoptosis of A-549 cells is mediated through the activation of caspases. The effect of isorhamnetin (IR), cisplatin (CP), carboplatin (CB) and their combinations on the activation of caspases and PARP was analyzed by immunoblotting. Equivalent amounts of each sample were loaded on SDS-PAGE gels. The expression of caspases 3, 8 and 9, PARP, and β-actin were detected using specific antibodies. The results are symbolic of at least three independent experiments.
Effect of isorhamnetin, cis-platin, carboplatin and their combinations on cell migration of A-549 monolayers: Inhibition of cell migration
Enhanced migratory capacity is one of the hall marks of highly invasive tumor cells. Inhibition of cancer cell migration could be an effective means of preventing cancer metastasis, thereby enabling confinement of primary tumors in manageable form, making surgical options more viable [27]. Wound closure experiments were executed to confirm the inhibitory effect of isorhamnetin and its combinations with cis-platin and carboplatin in A-549 cell migration. As shown in Figure 9, the wound got almost healed in untreated and slightly so by is orhamnetin. However, after combination treatment, invasiveness of A-549 cells showed potent inhibition in cell migration. Thus combination treatment results in significant inhibition of A-549 cell invasion compared to negative control (0 µM). Figure 10 shows percentage of migrated cells which were quantified by migration protocol.
Figure 9.
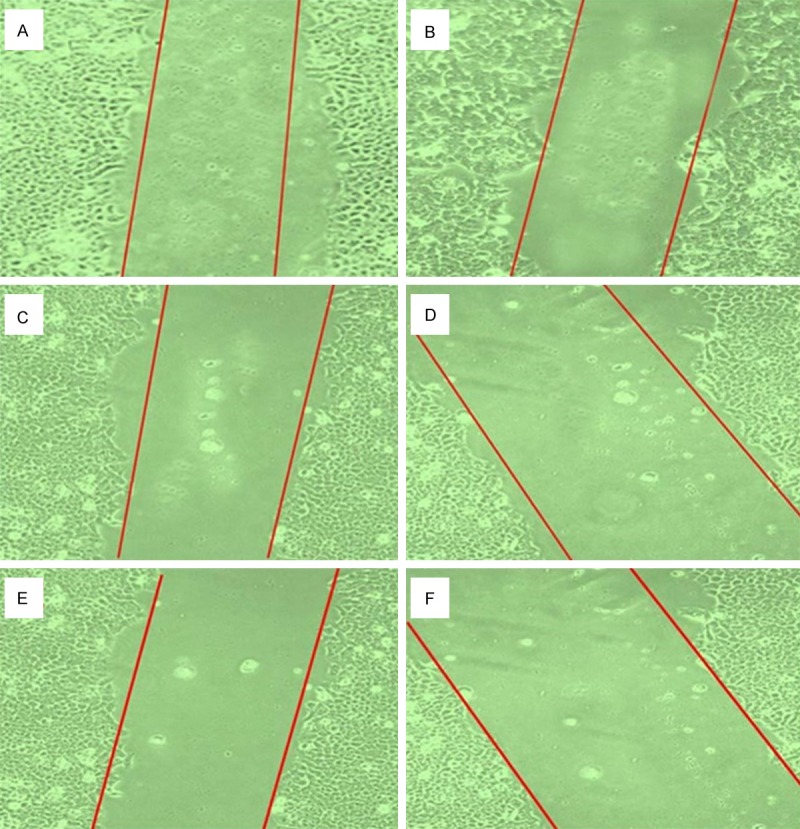
Effect of Isorhamnetin, cisplatin, carboplatin and their combinations on cell migration of A-549 monolayers. Cells were exposed to 25 μM isorhamnetin (B), 0.5 μM cisplatin (C), 0.5 μM carboplatin (D), Isorhamnetin+cisplatin (E) and Isorhamnetin+carboplatin (F) for 48 h. (A) untreated control cells.
Figure 10.
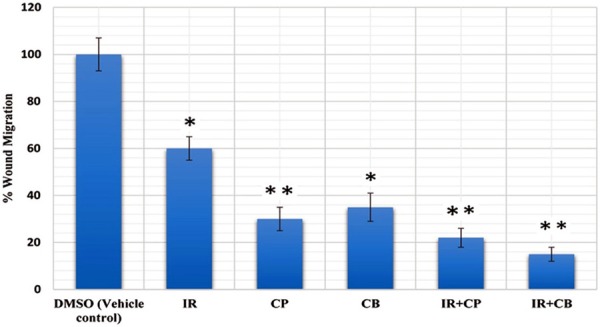
Inhibition of cell migration by isorhamnetin, cisplatin, carboplatin and their combinations in A-549 cells. The areas of migrated cells were quantified and represented as bar graph. *P = 0.05, **P = 0.01 vs. vehicle control.
Discussion
The biological mechanisms of antitumor drugs include the inhibition of proliferation, growth arrest in the cell cycle, enhanced apoptosis and the modulation of signal transduction pathways. In the introductory investigation, we found that isorhamnetin along with its combinations with cis-platin and carboplatin treatment markedly reduced the proliferation and viability of A-549 cells (Figures 1 and 2), thus raising the possibility that capsaicin might be a potential chemopreventive or therapeutic agent. Numerous natural phytochemicals have been reported to inhibit the growth of cancer cells through disruption of cell cycle progression [28,29]. Escaping cell cycle arrest is the most commonly observed phenomenon in tumor development. The cell cycle comprises of a number of checkpoints that act as surveillance mechanisms. Upon cellular stress or DNA damage, these mechanisms induce cells to undergo either cell cycle arrest, activation of repair systems, or apoptotic induction. Checkpoints at G1/S and G2/M transitions are essential regulatory gates during cell cycle progression [30,31]. In this study, the treatment with isorhamnetin or its combinations with cisplatin and carboplatin increased the population of G2/M cells. Thus these compounds induced mitotic block in A-549 cells thus triggering G2/M cell cycle phase arrest (Figure 6).
Apoptosis induction in cancer cells is also seen as a potent mechanism through which a wide variety of natural phytochemicals suppress cancer growth. Our results revealed that isorhamnetin along with its combinations with cis-platin and carboplatin induced significant apoptosis in A-549 cells (Figures 3 and 5). Control (untreated) A-549 cells after 48 h showed normal structure without noticeable apoptosis. However (B-D) Isorhamnetin, cis-platin and carboplatin-treated cells respectively showed early apoptosis features including blebbing and chromatin condensation after 48 h treatment. (E and F) isorhamnetin+cisplatin and isorhamnetin+carboplatin-treated cells respectively showed late apoptosis in addition to early apoptotic features.
Highly invasive tumors are characterized by an enhanced migratory capacity to normal tissues. Invasion of cancer cells into neighboring tissue and the vasculature is a preliminary step in tumor metastasis. This involves chemotactic migration of cancer cells, directed by bulging activity of the cell membrane and its attachment to the extracellular matrix. Inhibition of cancer cell migration could be an effective means of preventing cancer metastasis, thereby enabling confinement of primary tumors in manageable form, making surgical options more viable. During inaugural phase of the metastatic process, cancer cells penetrate into surrounding tissues and blood vessels and it has been shown to be the rate limiting step [32,33]. Therefore, inhibition of the cell migration represents a prospective therapeutic approach for the treatment of tumor metastasis. In this study, we evaluated the inhibitory effects of isorhamnetin, cis-platin, carboplatin and their combinations in A-549 cell migration. Percentage of wound closure was determined by the difference in area covered by migrated cells in control versus treated with isorhamnetin, cis-platin, carboplatin and their combinations for 24 h. The results show that the combinations of isorhamnetin with cis-platin and carboplatin were much more effective in inhibiting A-549 cell migration as compared to isorhamnetin, cis-platin or carboplatin alone.
In conclusion, our study indicates that exposure of human A-549 cancer cells to isorhamnetin and its combinations with cis-platin and carboplatin reduces cell viability significantly, induces cell cycle arrest at G2/M phase, inhibits cancer cell migration and induces apoptosis that involves mitochondria and caspase members. Isorhamnetin and its combinations induced early and late apoptosis respectively.
The apoptosis of A-549 cells treated with isorhamnetin, cis-platin, carboplatin and their combinations is associated with the induction of caspase 3 and 9, as well as loss of the mitochondrial membrane potential. In summary, these findings suggest that isorhamnetin and its combination with cis-platin and carboplatin possesses significant anti-cancer activity and may be potential candidates in cancer chemotherapy.
Disclosure of conflict of interest
None.
References
- 1.Larsen JE, Minna D. Molecular biology of lung cancer: clinical implications. Clin Chest Med. 2011;32:703–740. doi: 10.1016/j.ccm.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cooper WA, Lam DC, O’Toole SA, Minna JD. Molecular biology of lung cancer. J Thorac Dis. 2013;5:S479–90. doi: 10.3978/j.issn.2072-1439.2013.08.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tobias J, Hochhauser D. Cancer and its Management. Chapter 12. 6th edition. Wiley-Blackwell; 2010. p. 200. [Google Scholar]
- 4.Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med. 2008;359:1367–1380. doi: 10.1056/NEJMra0802714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aviel-Ronen S, Blackhall FH, Shepherd FA, Tsao MS. K-ras mutations in non-small-cell lung carcinoma: a review. Clin Lung Cancer. 2006;8:30–38. doi: 10.3816/CLC.2006.n.030. [DOI] [PubMed] [Google Scholar]
- 6.Alberg AJ, Brock MV, Stuart JM. Epidemiology of lung cancer: Looking to the future. J. Clin. Oncol. 2005;23:3175–3185. doi: 10.1200/JCO.2005.10.462. [DOI] [PubMed] [Google Scholar]
- 7.American Cancer Society. Cancer Facts & Figures 2014. Atlanta, Ga: American Cancer Society; 2014. [Epub ahead of print] [Google Scholar]
- 8.Posther KE, Harpole DH Jr. The surgical management of lung cancer. Cancer Invest. 2006;24:56–67. doi: 10.1080/07357900500449611. [DOI] [PubMed] [Google Scholar]
- 9.Quoix E, Ramlau R, Westeel V, Papai Z, Madroszyk A, Riviere A, Koralewski P, Breton JL, Stoelben E, Braun D, Debieuvre D, Lena H, Buyse M, Chenard MP, Acres B, Lacoste G, Bastien B, Tavernaro A, Bizouarne N, Bonnefoy JY, Limacher JM. Therapeutic vaccination with TG4010 and first-line chemotherapy in advanced non-small-cell lung cancer: A controlled phase 2B trial. Lancet Oncol. 2011;12:1125–1133. doi: 10.1016/S1470-2045(11)70259-5. [DOI] [PubMed] [Google Scholar]
- 10.Naruke T, Goya T, Tsuchiya R, Suemasu K. The importance of surgery to non-small cell carcinoma of lung with mediastinal lymph node metastasis. Ann Thorac Surg. 1988;46:603–10. doi: 10.1016/s0003-4975(10)64717-0. [DOI] [PubMed] [Google Scholar]
- 11.Li Y, Li H, Hu Y. Clinical study of lymph node metastasis in lung cancer. Chinese J Thorac Cardiovasc Surg. 2000;16:10–2. [Google Scholar]
- 12.Raghavan D, Koczwara B, Javle M. Evolving strategies of cytotoxic chemotherapy for advanced prostate cancer. Eur J Cancer. 1997;33:566–74. doi: 10.1016/s0959-8049(96)00510-2. [DOI] [PubMed] [Google Scholar]
- 13.Yagoda A, Watson RC, Natale RB, Barzell W, Sogani P, Grabstald H, Whitmore WF. A critical analysis of response criteria in patients with prostatic cancer treated with cis-diamminedichloride platinum II. Cancer. 1979;44:1553–62. doi: 10.1002/1097-0142(197911)44:5<1553::aid-cncr2820440502>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 14.Drelichman A, Oldford J, Al-Sarraf M. Evaluation of cyclophosphamide, adriamycin, and cis-platinum (CAP) in patients with disseminated prostatic carcinoma: a phase II study. Am J Clin Oncol. 1985;8:255–9. doi: 10.1097/00000421-198506000-00011. [DOI] [PubMed] [Google Scholar]
- 15.Trump DL, Marsh JC, Kvols LK, Citrin D, Davis TE, Hahn RG, Vogl SE. A phase II trial of carboplatin (NSC 241240) in advanced prostate cancer, refractory to hormonal therapy: an Eastern Cooperative Group Study. Invest New Drugs. 1990;8:S91–4. doi: 10.1007/BF00171992. [DOI] [PubMed] [Google Scholar]
- 16.Hann CL, Rudin CM. Management of small-cell lung cancer: incremental changes but hope for the future. Oncology (Williston Park) 2008;22:1486–92. [PMC free article] [PubMed] [Google Scholar]
- 17.Fairchild A, Harris K, Barnes E, Wong R, Lutz S, Bezjak A, Cheung P, Chow E. Palliative thoracic radiotherapy for lung cancer: a systematic review. J. Clin. Oncol. 2008;26:4001–4011. doi: 10.1200/JCO.2007.15.3312. [DOI] [PubMed] [Google Scholar]
- 18.Murray N, Turrisi AT 3rd. A review of first-line treatment for small-cell lung cancer. J Thorac Oncol. 2006;1:270–278. doi: 10.1016/s1556-0864(15)31579-3. [DOI] [PubMed] [Google Scholar]
- 19.Ukena D. Chemotherapy in stage I+II non-small cell lung cancer. Lung Cancer. 2001;33:S25–8. doi: 10.1016/s0169-5002(01)00299-9. [DOI] [PubMed] [Google Scholar]
- 20.Waller DA. Neoadjuvant chemotherapy in non-small cell lung cancer-the UK experience. Lung Cancer. 2001;34:S31–3. doi: 10.1016/s0169-5002(01)00379-8. [DOI] [PubMed] [Google Scholar]
- 21.Azim HA, Ganti AK. Treatment options for relapsed small-cell lung cancer. Anticancer Drugs. 2007;18:255–261. doi: 10.1097/CAD.0b013e328011a547. [DOI] [PubMed] [Google Scholar]
- 22.MacCallum C, Gillenwater HH. Second-line treatment of small-cell lung cancer. Curr Oncol Rep. 2006;8:258–264. doi: 10.1007/s11912-006-0030-8. [DOI] [PubMed] [Google Scholar]
- 23.Eid SY, El-Readi MZ, Wink M. Carotenoids reverse multidrug resistance in cancer cells by interfering with ABC-transporters. Phytomedicine. 2012;19:977–987. doi: 10.1016/j.phymed.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 24.Gómez-Contreras PC, Hernández-Flores G, Ortiz-Lazareno PC, Del Toro-Arreola S, Delgado-Rizo V, Lerma-Díaz JM, Barba-Barajas M, Domínguez-Rodríguez JR, Bravo Cuellar A. In vitro induction of apoptosis in U937 cells by perillyl alcohol with sensitization by pentoxifylline: Increased Bcl-2 and Bax protein expression. Chemotherapy. 2006;52:308–315. doi: 10.1159/000096003. [DOI] [PubMed] [Google Scholar]
- 25.Moffat KA, Johannes WU, Miller GJ. 1, 25-dihydroxyvitamin D3 and platinum drugs act synergistically to inhibit the growth of prostate cancer cell lines. Clin Cancer Res. 1999;5:695–703. [PubMed] [Google Scholar]
- 26.Huan SD, Stewart DJ, Aitken SE, Segal R, Yau JC. Combination of epirubicin and cisplatin in hormone-refractory metastatic prostate cancer. Am J Clin Oncol. 1999;22:471–4. doi: 10.1097/00000421-199910000-00010. [DOI] [PubMed] [Google Scholar]
- 27.Hedley BD, Winquist E, Chambers AF. Therapeutic targets for antimetastatic therapy. Expert Opin Ther Targets. 2004;8:527–536. doi: 10.1517/14728222.8.6.527. [DOI] [PubMed] [Google Scholar]
- 28.Chen C, Kong AN. Dietary cancer-chemopreventive compounds: from signaling and gene expression to pharmacological effects. Trends Pharmacol Sci. 2005;26:318–326. doi: 10.1016/j.tips.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 29.Aggarwal BB, Takada Y, Oommen OV. From chemoprevention to chemotherapy: common targets and common goals. Expert Opin Investig Drugs. 2004;13:1327–1338. doi: 10.1517/13543784.13.10.1327. [DOI] [PubMed] [Google Scholar]
- 30.Hartwell LH, Kastan MB. Cell cycle control and cancer. Science. 1994;266:1821–1828. doi: 10.1126/science.7997877. [DOI] [PubMed] [Google Scholar]
- 31.Kastan MB, Bartek J. Cell-cycle checkpoints and cancer. Nature. 2004;432:316–323. doi: 10.1038/nature03097. [DOI] [PubMed] [Google Scholar]
- 32.Maggioni D, Nicolini G, Rigolio R, Biffi L, Pignataro L, Gaini R, Garavello W. Myricetin and Naringenin Inhibit Human Squamous Cell Carcinoma Proliferation and Migration In Vitro. Nutr Cancer. 2014;66:1257–67. doi: 10.1080/01635581.2014.951732. [DOI] [PubMed] [Google Scholar]
- 33.Peng C, Zhou K, An S, Yang J. The effect of CCL19/CCR7 on the proliferation and migration of cell in prostate cancer. Tumour Biol. 2014 doi: 10.1007/s13277-014-2642-1. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]


