Abstract
Panax notoginseng saponins (PNS) are components derived from Chinese herb panax notoginseng and play important roles in the cure of wounds. However, how PNS plays this function is still unclear. In this study, we used MTT assay, wound healing assay, western blot, quantitative real time PCR and enzyme-linked immunosorbent assay to detect the effects of PNS on the proliferation, migration and expression of collagen and fibronectin of anterior cruciate ligament (ACL) fibroblasts as well as the underlying mechanism. We found that PNS promoted the proliferation and migration of ACL fibroblasts and increased the expression levels of collagen and fibronectin. Further mechanism study indicates that PNS might play its function through the phosphorylation of PI3K, AKT and ERK. This study provides a possible mechanism for the function of PNS and lays foundation for further study on the function of panax notoginseng.
Keywords: Panax notoginseng saponins, proliferation, migration, collagen, PI3K/AKT, ERK
Introduction
Anterior cruciate ligament (ACL) injury is a commonly problem encountered in athletic individuals. After ACL damage, the proliferation and migration of ACL fibroblasts towards injury part is very important for the healing of ligament. Large amounts of inflammatory factors are secreted in the injured portion and affect synthesis of extracellular matrix impeding repair of injured tissues [1]. These inflammatory factors also inhibit migration of ACL fibroblasts thus affecting self-repairing of ACL injury. Current opinion for the treatment of ACL injury ranges from non-operative managements to multiple surgical options. As the poor self-healing capacity of ACL, outcomes of these treatments of ACL injury are often unsatisfactory and this may limit career of athletes [2,3].
Traditional Chinese medicinal herb panax notoginseng has been used as a hemostatic medicine promoting blood clotting, relieving swelling and alleviating pain for thousands of years [4,5]. Panax notoginseng saponins (PNS) are important components derived from panax notoginseng. PNS plays important roles in damage repairing. PNS can inhibit the aggregation of platelets and promote flow of blood [6]. PNS also promotes angiogenesis, helps the injured vessel to repair [7,8]. PNS promotes proliferation and osteogenic differentiation of bone marrow stromal cells [9], helps the injured bones to repair. PNS can decrease the levels of serum lactate dehydrogenase, creatine kinase and normalize the activities of superoxide dismutase, glutathione peroxidase and catalase [10], thus playing a protective role in cardiovascular system. Moreover, PNS inhibits cell apoptosis of cardiomyocytes [11] and attenuates damage induced by oxidative stress [12]. PNS is a promising drug for the treatment of heart diseases. PNS has extensive anti-cancer activities, it can promote apoptosis of cancer cells and arrest the cell cycle [13-15]. In addition, PNS shows various activities including anti-inflammatory effect [16], neuroprotective effect [17], immunologic adjuvant [16] and prevention of diabetes [18].
PNS plays an important role in the cure of wounds. Whereas, how PNS plays its function is not yet clear. In this study, we explored the effects of PNS on the proliferation, migration and expression of collagen and fibronectin in ACL fibroblasts as well as the underlying mechanism. We found that PNS promoted the proliferation and migration of ACL and enhanced the expression of collagen and fibronectin through the phosphorylation of PI3K, AKT and ERK. These results provide a possible mechanism for the function of panax notoginseng and also suggest that PNS could be a promising drug for injury treatment.
Materials and methods
Isolation of ACL fibroblasts and cell culture
Ten weeks-old New Zealand white rabbits were purchased from China Medical University (Shenyang, China). The ACLs were harvested under sterile condition and cut into small pieces after removal of adipose tissues and connective tissues. Tissue pieces were digested with 0.1% (w/v) collagenase I and 0.25% (w/v) trypsin and cultured in Dulbecco’s modified Eagle’s medium (DMEM, Gibco, Grand Island, NY, USA) with 10% (v/v) fetal bovine serum (FBS, HyClone, Logan, UT, USA) and maintained at 37°C and 5% (v/v) CO2 in a saturated humidity. Cells were washed with phosphate-buffered saline (PBS) to remove cells non-attaching. The isolated ACL fibroblasts were cultured in DMEM with 10% FBS and maintained in 37°C incubator with 5% CO2. All of animal experiments were approved by Animal Care and Use Committee of China Medical University.
Immunohistochemistry
After isolation, cells were fixed with 4% paraformaldehyde and permeabilized with 0.5% Triton X-100. Endogenous peroxidase was quenched with 3% H2O2 and unspecific sites were blocked by goat serum. Then cells were incubated with primary antibody against vimentin (1:200, Wanleibio, Shenyang, China) at 4°C overnight, followed by incubation with Biotin conjugated secondary antibody and HRP conjugated streptavidin. Then cells were incubated with 3,3’-diaminobenzidine (DAB) development reagent, stained with hematoxylin and observed in an inverted microscopy.
MTT assay
Cell viability was detected using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, Sigma, St. Louis, USA). ACL fibroblasts were harvested and seeded in 96-well plates at density of 2 × 103 cells/well. After attaching to the plates, cells were treated with 0, 0.05, 0.1, 0.2 and 0.4 mg/ml of PNS (Melonepharma, Dalian, China). After incubation for 24 h, MTT at a final concentration of 0.2 mg/ml was added to each well and incubated for additional 4 h. The supernatant was removed subsequently and 200 μl dimethyl sulfoxide (DMSO, Sigma) was added to each well. The absorbance at 490 nm was measured with a microplate reader.
Wound healing assay
Cells were harvested and seeded in 6-well plates at density of 1 × 105 cells/well. Scratches were made on the cell surface with pipette tips. Cells were subsequently washed with serum-free medium and cultured in serum-free medium. Images of each sample were captured at 0 h, 8 h, 24 h, 48 h after treatment with different concentration of PNS and relative migration ratios were calculated according to the following formula: relative migration ratio = (1-distance between edges of migrated scratch/distance between edges of initial scratch) × 100%.
Quantitative real time PCR (qPCR)
After different treatment, cells of each group were collected by centrifugation. Total RNA was extracted from each sample using Total RNA Extraction Kit (Tiangen, Beijing, China) according to the manufacturer’s protocol. Total RNA was then reverse transcribed to cDNA using M-MLV reverse transcriptase (BioTeke, Beijing, China). The mRNA expression levels of tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), interleukin-1β (IL-1β), collagen I, collagen III and fibronectin were detected by SYBR Green qPCR method. The mRNA expression levels were normalized to β-actin and the relative mRNA expression levels were calculated using the 2-∆∆Ct method [19]. qPCR program was as follows: 95°C 10 min; 95°C 10 s, 60°C 20 s, 72°C 30 s, 40 cycles; 4°C 5 min. SYBR Green reagent was purchased from Solarbio (Beijing, China) and 2 × Power Taq PCR MasterMix was purchased from BioTeke. The primers used were shown in Table 1.
Table 1.
Primers for quantitative real time PCR
| Gene names | Forward Primer (5’-3’) | Reverse Primer (5’-3’) |
|---|---|---|
| TNF-α | TCTTCTGCCTGCTGCACTTC | CTTGCGGGTTTGCTACTACG |
| IL-6 | AAGAAGCCACCCTCAAGCC | AGCAAGGACACCCGCACTC |
| IL-1β | GTCTTGTCAGTCGTTGTGGCTCT | GTAGTCATCCCAGGTGTTGCA |
| Collagen I | CCGGCTCCTGCTCCTCTTA | TCTGCACGCATGTGACTGG |
| Collagen III | CTCTGCTTCATCCCACTGTTATT | TTGGCACGGTTCGGGTTTC |
| Fibronectin | CACAGAAGGGCGACAGGAT | GCATAAGGCACCATTGGAATT |
| β-actin | AAGTGCTTCTAGGCGGACTGT | ATGCTCGCTCCAACGACTGCT |
Western blot
Cells were harvested and lysed with NP-40 lysis buffer (Beyotime, Shanghai, China) on ice. The protein concentration was detected using Enhanced BCA Protein Assay Kit (Beyotime). Equal amount of protein was subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). The separated proteins were then transferred to polyvinylidene difluoride (PVDF) membranes. After blocking with 5% skim milk or 5% BSA, the PVDF membranes were incubated with primary antibody against MMP-2, MMP-9, collagen I, collagen III, PI3K, p-PI3K, AKT, p-AKT, ERK, and p-ERK (1:1000, Wanleibio) at 4°C overnight, followed by incubating with horseradish peroxidase (HRP)-conjugated secondary antibody (Beyotime). Proteins were detected with the ECL detection system and analyzed with Gel-Pro-Analyzer software using β-actin as reference.
Enzyme-linked immunosorbent assay (ELISA)
Levels of TNF-α, IL-6, IL-1β in the media were detected by ELISA. The cell media was collected and levels of TNF-α, IL-6, IL-1β were measured with Rabbit TNF-α ELISA Kit, Rabbit IL-6 ELISA Kit and Rabbit IL-1β ELISA Kit (USCN, Wuhan, China) according to the manufacturer’s instruction. Briefly, test samples and standard specimens were added into individual wells, and the biotin-labeled secondary antibodies and HRP- labeled avidin were added and incubated at 37°C for 60 min. Later, the color development reagent was added and incubated for 10 min at 37°C. Then the stop solution was added to stop the reaction. The absorbance was measured at 450 nm. Concentrations of TNF-α, IL-6, IL-1β were analyzed with curve expert 1.3 software.
Statistical analysis
All experiments were performed three times. The experimental results were presented as mean ± standard deviation (SD). Differences between two groups were analyzed using one-way analysis of variance (ANOVA) and Bonferroni’s multiple comparison. P < 0.05 was considered to be significant.
Results
PNS relieves inflammatory response in ACL fibroblasts
The isolated ACL fibroblasts were identified by Immunohistochemistry (Figure 1A). TNF-α, IL-6 and IL-1β are important inflammatory cytokines induced during ACL injury. Here qPCR was used to measure changes in the mRNA expression levels of TNF-α, IL-6 and IL-1β. Results of qPCR showed that after treatment with different concentration of PNS, the mRNA levels of TNF-α, IL-6 and IL-1β were decreased in a dose-dependent manner (Figure 1B). Concentrations of TNF-α, IL-6 and IL-1β in media supernatant were also detected by ELISA. Consistent with qPCR, the results of ELISA showed that the concentrations of TNF-α, IL-6 and IL-1β were decreased in a dose-dependent manner after treatment with different concentration of PNS (Figure 1C). These results indicate that PNS can relieve inflammatory response in ACL fibroblasts.
Figure 1.
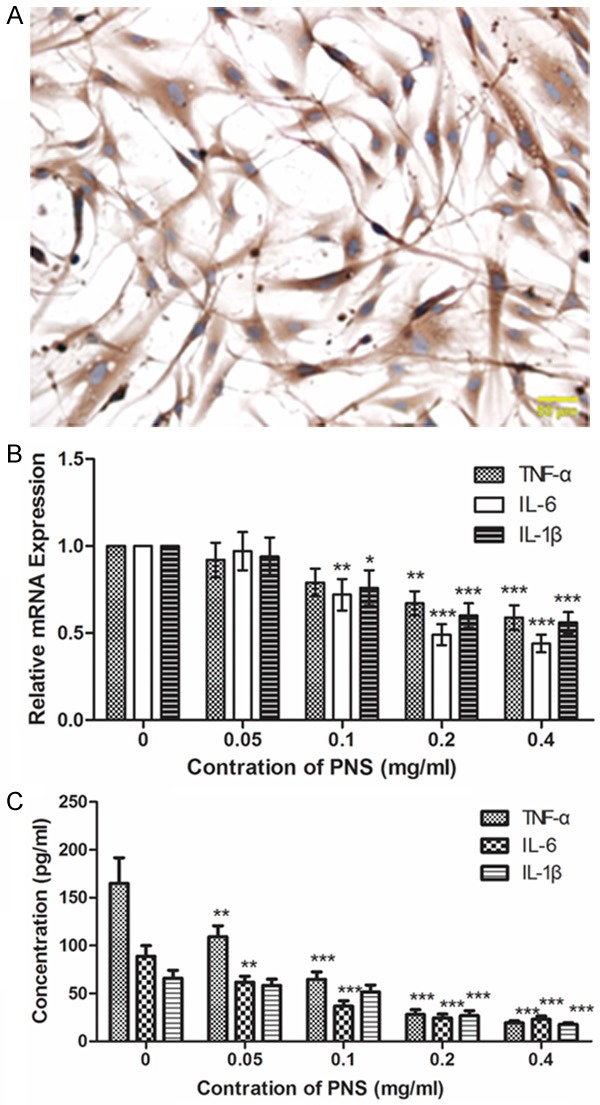
PNS relieves inflammatory response in ACL fibroblasts. A. Isolated ACL fibroblasts were identified by Immunohistochemistry. Cells were stained with antibody against Vimentin. Scale bar = 50 μm. B. The mRNA expression levels of TNF-α, IL-6 and IL-1β were detected by qPCR after treatment with 0, 0.05, 0.1, 0.2 and 0.4 mg/ml of PNS. The relative mRNA expression levels were calculated using the 2-∆∆Ct method and β-actin was used as a reference. C. Concentrations of TNF-α, IL-6 and IL-1β in the media were measured by ELISA after treatment with different concentration of PNS. Each experiment was repeated three times. Results were presented as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001.
PNS promotes the proliferation and migration of ACL fibroblasts
Proliferation and migration of ACL fibroblasts are important for the healing of injured ligament. Wound healing assay was performed to explore the function of PNS on migration of ACL fibroblasts. Results of wound healing assay showed that, after different treatment with PNS, the relative migration ratio of ACL fibroblasts was increased markedly (Figure 2A and 2B, P < 0.05). MTT assay was used to detect the effect of PNS on proliferation of ACL fibroblasts. After treatment with different concentration of PNS, cell viability of ACL fibroblasts was increased significantly (Figure 2C, P < 0.05). These results demonstrate that PNS promotes the proliferation and migration of ACL fibroblasts.
Figure 2.
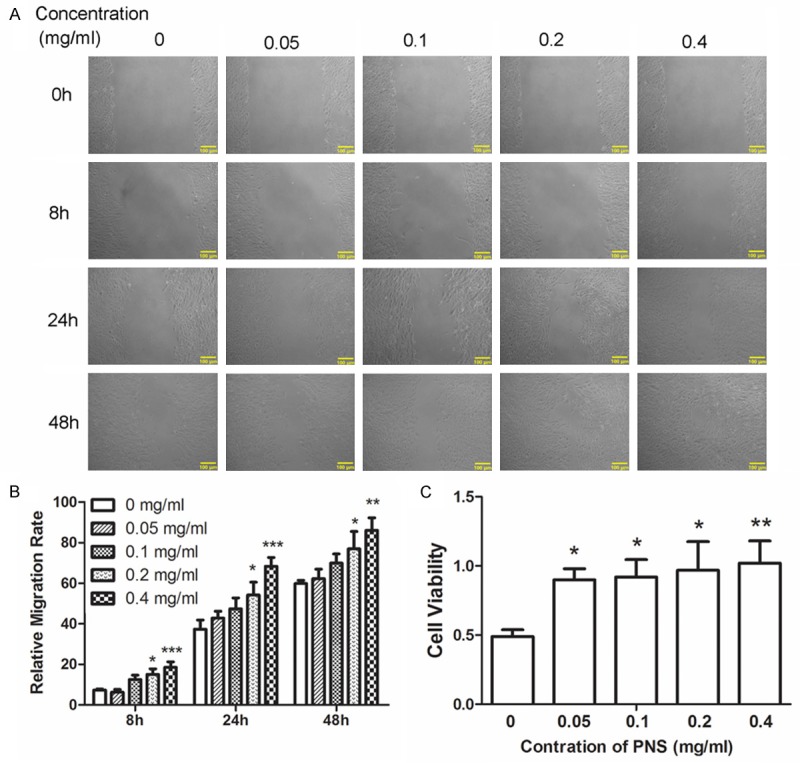
PNS promotes the proliferation and migration of ACL fibroblasts. A, B. The migration capability of cells was detected by wound healing assay after treatment with 0, 0.05, 0.1, 0.2 and 0.4 mg/ml of PNS. C. The cell viability was detected by MTT assay after treatment with different concentration of PNS. All experiments were repeated three times and the results were presented as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001.
PNS promotes the expression of collagen and fibronectin
Western blot was utilized to detect the expression levels of MMP-2 and MMP-9. Results of western blot showed that expression levels of MMP-2 and MMP-9 were decreased in a dose-dependent manner after treatment with different concentration of PNS (Figure 3A and 3B). Collagen is important for injury healing. Changes in levels of collagen I, collagen III were detected by qPCR and western blot. Results of qPCR showed that the mRNA levels of collagen I and collagen III were increased in a dose-dependent manner after treatment with different concentration of PNS (Figure 3C). Consistent with the results of qPCR, western blot also showed a promontory in the expression levels of collagen I and collagen III (Figure 3D and 3E). Fibronectin is another kind of extracellular matrix that is important for the healing of damage. The expression of fibronectin was measured by qPCR and western blot. Results of qPCR showed that the mRNA level of fibronectin was increased in a dose-dependent manner after different treatment (Figure 3F). There was a similar swell in the protein level of fibronectin detected by western blot (Figure 3G and 3H). Results of qPCR and western blot illustrate that PNS promotes the expression of collagen and fibronectin.
Figure 3.
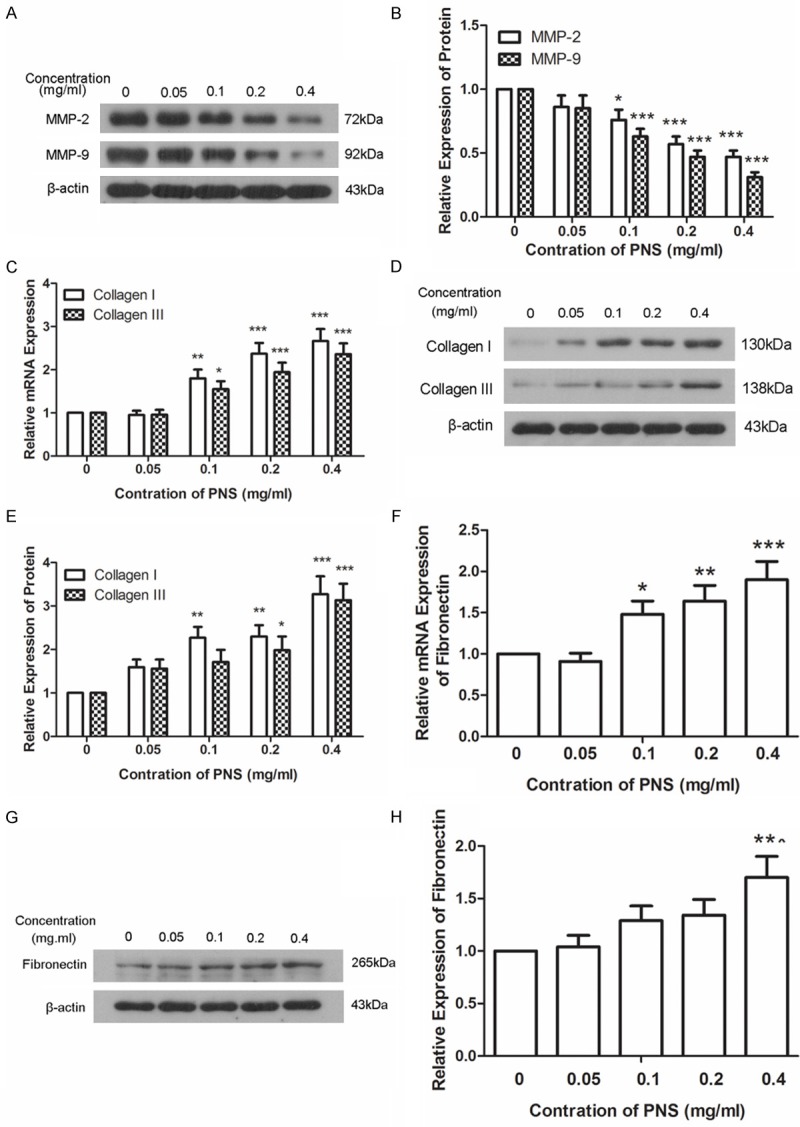
PNS promotes the expression of collagen and fibronectin. A, B. Protein levels of MMP-2 and MMP-9 were measured by western blot after treatment with 0, 0.05, 0.1, 0.2 and 0.4 mg/ml of PNS. The relative expression levels of MMP-2 and MMP-9 were calculated using β-actin as reference. C. The mRNA levels of collagen I and collagen III were measured by qPCR after treatment with different concentration of PNS. The relative mRNA expression levels were calculated using the 2-∆∆Ct method with β-actin as reference. D, E. The protein levels of collagen I and collagen III were detected by western blot after treatment with different concentration of PNS. β-actin was used as reference when the relative expression levels of protein were calculated. F. The mRNA level of fibronectin was detected by qPCR using β-actin as reference. The relative mRNA expression levels were calculated using the 2-∆∆Ct method. G, H. The protein level of fibronectin was detected by western blot using β-actin as reference. All experiments were repeated three times and the results were presented as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001.
PNS promotes the phosphorylation of PI3K, AKT and ERK
To further study how PNS promotes the proliferation and migration of ACL fibroblasts, the levels of PI3K, phosphorylated PI3K (p-PI3K), AKT, phosphorylated AKT (p-AKT), ERK and phosphorylated ERK (p-ERK) were detected by western blot. After treatment with different concentration of PNS, there were no changes in the protein level of PI3K. However, the level of p-PI3K showed a dose-dependent increase after treatment with PNS (Figure 4A and 4B). Similar results were also discovered in the levels of AKT/p-AKT and ERK/p-ERK (Figure 4C-F). These results demonstrate that PNS promotes the phosphorylation of PI3K, AKT and ERK.
Figure 4.
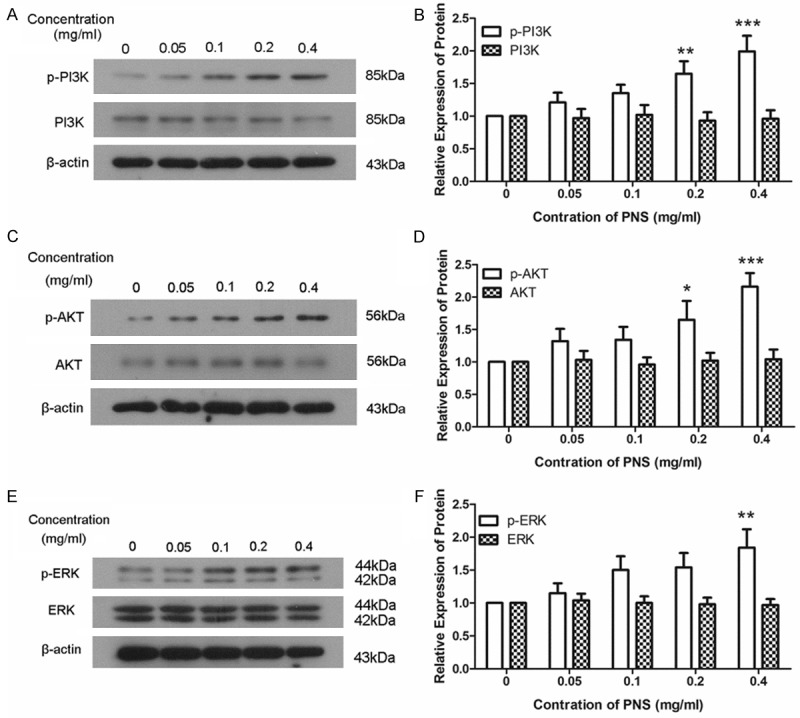
PNS promotes the phosphorylation of PI3K, AKT and ERK. A, B. The protein levels of PI3K and p-PI3K were detected by western blot after treatment with 0, 0.05, 0.1, 0.2 and 0.4 mg/ml of PNS. The relative protein levels were calculated using β-actin as reference. C, D. The protein levels of AKT and p-AKT were measured by western blot after treatment with different concentration of PNS. β-actin was used as reference when the relative protein levels were calculated. E, F. Western blot was used to detect the protein levels of ERK and p-ERK using β-actin as reference. Each experiment was repeated three times and the results were presented as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001.
Discussion
PNS was the main component of panax notoginseng and plays important roles in the healing of ACL injury. In this study, we explored the mechanism how PNS played its functions. We found that PNS promoted the proliferation and migration of ACL fibroblast as well as the expression of collagen and fibronectin through the phosphorylation of PI3K, AKT and ERK.
ACL injury usually causes inflammatory responses in the damaged part, and is usually accompanied with a large amount of inflammatory cytokines such as TNF-α, IL-6, IL-1β [20]. In our study, we found that PNS attenuated inflammatory response, and decreased the levels of TNF-α, IL-6 and IL-1β. These results demonstrate that PNS has a protective effect on ACL injury. Similar results are found in the report of He et al. PNS inhibits NF-κB activity and inhibits cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) induced by TNF-α [15]. PNS is also found to protect hemorrhagic shock by increasing SOD activity, decreasing levels of MDA and MPO and reducing the expression levels of TNF-α and IL-6 [16]. Also PNS protects lung injury induced by lipopolysaccharide (LPS) and decreases the inflammatory cytokines, such as TNF-α, IL-6 and IL-10 [21].
In our study, we found that PNS promoted the proliferation and migration of ACL fibroblasts. Similar to our study, PNS promotes the proliferation and differentiation of neural stem cells and mesenchymal stem cells as well as NIH3T3 [17,22]. In addition, PNS also attenuates H2O2 induced cell death in primary rat cortical astrocytes by reduction of reactive oxygen species (ROS) [23]. However, in cancer cells, PNS plays opposite effects. PNS has been reported to inhibit the metastasis of breast cancer [14]. Wang et al also reported that 25-OCH (3)-PPD, which is a newly identified component of PNS, inhibits the migration of breast cancer cells and reduces the expression of epithelial-to-mesenchymal transition (EMT) markers [24]. Ft1, which is an ingredient of PNS, inhibits the proliferation of human neuroblastoma cells, arrests the cell cycle, promotes cell apoptosis, actives caspase-3, p53 and p21, regulates the expression of proteins related to cell cycle and apoptosis [13]. Other ingredients of PNS, such as GF4 and GRg6, inhibit the proliferation of human lymphoma and induce cell apoptosis [25,26].
ACL injury was usually accompanied with an increase of matrix metalloproteinase (MMP) which was considered to be a main reason of the poor healing ability after ACL [27,28]. A decrease in the protein levels of MMP-2 and MMP-9 was discovered after treatment with PNS. This result indicates that PNS may relieve the cause of the poor healing ability of ACL. Similar to our results, Jang et al reported that PNS can decrease MMP-2 induced by LPS [29]. Collagen and fibronectin are extracellular matrixes regulated by MMP and are also very important for the healing of wounded tissue. In our study, increase in the mRNA levels and protein levels of collagen I, collagen III and fibronectin was discovered, which is consistent with the decrease of MMP. Along with the above results, it is suggested that PNS promotes repair of the injured ACL.
In the mechanism study, we found that the phosphorylation levels of PI3K, AKT and ERK were increased by PNS. This suggests that PNS promotes the activation of PI3K, AKT and ERK and PNS might promote the healing of injured ACL through PI3K/AKT and ERK signaling pathway. PNS is shown to promote the proliferation and migration of human umbilical vein endothelial cells (HUVECs) by regulating the PI3K/AKT and Raf/MEK/ERK signaling pathways [8]. And in human neuroblastoma SHSY5Y cells, Ft1, an ingredient of PNS, enhances the phosphorylation of ERK1/2, JNK and p38 MAPK [13]. However, in the report of Sun et al, the expression levels of PI3K, AKT and ERK are increased by PNS in different blood corpuscles [30], which is a little bit different from our results. This may be because of the different contexts in different types of cells. Through the PI3K/Akt signaling pathway, PNS also attenuates oxygen-glucose deprivation injury in PC12 cells [31].
In our study, we explored function of PNS as well as the underlying mechanism. PNS was found to promote the proliferation and migration of ACL fibroblast as well as the expression of collagen I, collagen III and fibronectin to attenuate ACL injury. And PNS might perform this function through the phosphorylation of PI3K, AKT and ERK. This study provides a possible mechanism for the function of PNS and suggests that PNS may become a promising drug for injury treatment, and this study also lays foundation for further study of panax notoginseng.
Disclosure of conflict of interest
None.
References
- 1.Elsaid KA, Fleming BC, Oksendahl HL, Machan JT, Fadale PD, Hulstyn MJ, Shalvoy R, Jay GD. Decreased lubricin concentrations and markers of joint inflammation in the synovial fluid of patients with anterior cruciate ligament injury. Arthritis Rheum. 2008;58:1707–1715. doi: 10.1002/art.23495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shafizadeh S, Schneider MM, Bouillon B. Injuries of the anterior cruciate ligament in athletes. Chirurg. 2014;85:888–894. doi: 10.1007/s00104-014-2773-3. [DOI] [PubMed] [Google Scholar]
- 3.Witonski D. [Natural history of anterior cruciate and medial collateral ligament healing--literature review] . Chir Narzadow Ruchu Ortop Pol. 2002;67:93–97. [PubMed] [Google Scholar]
- 4.Dong TT, Cui XM, Song ZH, Zhao KJ, Ji ZN, Lo CK, Tsim KW. Chemical assessment of roots of Panax notoginseng in China: regional and seasonal variations in its active constituents. J Agric Food Chem. 2003;51:4617–4623. doi: 10.1021/jf034229k. [DOI] [PubMed] [Google Scholar]
- 5.Lee MR, Yun BS, Sung CK. Comparative Study of White and Steamed Black Panax ginseng, P. quinquefolium, and P. notoginseng on Cholinesterase Inhibitory and Antioxidative Activity. J Ginseng Res. 2012;36:93–101. doi: 10.5142/jgr.2012.36.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang X, Xiong X, Wang H, Wang J. Protective effects of panax notoginseng saponins on cardiovascular diseases: a comprehensive overview of experimental studies. Evid Based Complement Alternat Med. 2014;2014:204840. doi: 10.1155/2014/204840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng H, Liu C, Ou Y, Zhang Y, Fu X. Total saponins of Panax notoginseng enhance VEGF and relative receptors signals and promote angiogenesis derived from rat bone marrow mesenchymal stem cells. J Ethnopharmacol. 2013;147:595–602. doi: 10.1016/j.jep.2013.03.043. [DOI] [PubMed] [Google Scholar]
- 8.Shen K, Ji L, Gong C, Ma Y, Yang L, Fan Y, Hou M, Wang Z. Notoginsenoside Ft1 promotes angiogenesis via HIF-1alpha mediated VEGF secretion and the regulation of PI3K/AKT and Raf/MEK/ERK signaling pathways. Biochem Pharmacol. 2012;84:784–792. doi: 10.1016/j.bcp.2012.05.024. [DOI] [PubMed] [Google Scholar]
- 9.Li XD, Wang JS, Chang B, Chen B, Guo C, Hou GQ, Huang DY, Du SX. Panax notoginseng saponins promotes proliferation and osteogenic differentiation of rat bone marrow stromal cells. J Ethnopharmacol. 2011;134:268–274. doi: 10.1016/j.jep.2010.11.075. [DOI] [PubMed] [Google Scholar]
- 10.Shi R, Liu L, Huo Y, Cheng YY. [Study on protective effects of Panax notoginseng saponins on doxorubicin-induced myocardial damage] . Zhongguo Zhong Yao Za Zhi. 2007;32:2632–2635. [PubMed] [Google Scholar]
- 11.Chen SC, Cheng JJ, Hsieh MH, Chu YL, Kao PF, Cheng TH, Chan P. Molecular mechanism of the inhibitory effect of trilinolein on endothelin-1-induced hypertrophy of cultured neonatal rat cardiomyocytes. Planta Med. 2005;71:525–529. doi: 10.1055/s-2005-864153. [DOI] [PubMed] [Google Scholar]
- 12.Qiang H, Zhang C, Shi ZB, Yang HQ, Wang KZ. Protective effects and mechanism of Panax Notoginseng saponins on oxidative stress-induced damage and apoptosis of rabbit bone marrow stromal cells. Chin J Integr Med. 2010;16:525–530. doi: 10.1007/s11655-010-0566-1. [DOI] [PubMed] [Google Scholar]
- 13.Gao B, Shi HL, Li X, Qiu SP, Wu H, Zhang BB, Wu XJ, Wang ZT. p38 MAPK and ERK1/2 pathways are involved in the pro-apoptotic effect of notoginsenoside Ft1 on human neuroblastoma SH-SY5Y cells. Life Sci. 2014;108:63–70. doi: 10.1016/j.lfs.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 14.Wang P, Cui J, Du X, Yang Q, Jia C, Xiong M, Yu X, Li L, Wang W, Chen Y, Zhang T. Panax notoginseng saponins (PNS) inhibits breast cancer metastasis. J Ethnopharmacol. 2014;154:663–671. doi: 10.1016/j.jep.2014.04.037. [DOI] [PubMed] [Google Scholar]
- 15.He F, Ding Y, Liang C, Song SB, Dou DQ, Song GY, Kim YH. Antitumor effects of dammaranetype saponins from steamed Notoginseng. Pharmacogn Mag. 2014;10:314–317. doi: 10.4103/0973-1296.137372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu HZ, Liu ZL, Zhao SP, Sun CZ, Yang MS. Protective Mechanism of Panax notoginseng Saponins on Rat Hemorrhagic Shock Model in Recovery Stage. Cell Biochem Biophys. 2014;70:1719–1724. doi: 10.1007/s12013-014-0119-x. [DOI] [PubMed] [Google Scholar]
- 17.Si YC, Li Q, Xie CE, Niu X, Xia XH, Yu CY. Chinese herbs and their active ingredients for activating xue (blood) promote the proliferation and differentiation of neural stem cells and mesenchymal stem cells. Chin Med. 2014;9:13. doi: 10.1186/1749-8546-9-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uzayisenga R, Ayeka PA, Wang Y. Anti-diabetic potential of Panax notoginseng saponins (PNS): a review. Phytother Res. 2014;28:510–516. doi: 10.1002/ptr.5026. [DOI] [PubMed] [Google Scholar]
- 19.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2 (-Delta Delta C (T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 20.Naraoka T, Ishibashi Y, Tsuda E, Yamamoto Y, Kusumi T, Kakizaki I, Toh S. Time-dependent gene expression and immunohistochemical analysis of the injured anterior cruciate ligament. Bone Joint Res. 2012;1:238–244. doi: 10.1302/2046-3758.110.2000118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen YQ, Rong L, Qiao JO. Antiinflammatory effects of Panax notoginseng saponins ameliorate acute lung injury induced by oleic acid and lipopolysaccharide in rats. Mol Med Rep. 2014;10:1400–1408. doi: 10.3892/mmr.2014.2328. [DOI] [PubMed] [Google Scholar]
- 22.Yin LM, Wang X, Qian XD, Lin XJ, Chen XH, Gao RL. Effects of Panax notoginseng saponins on proliferation and differentiation in NIH3T3 cells. Chin J Integr Med. 2012;18:616–620. doi: 10.1007/s11655-012-1179-7. [DOI] [PubMed] [Google Scholar]
- 23.Zhou N, Tang Y, Keep RF, Ma X, Xiang J. Antioxidative effects of Panax notoginseng saponins in brain cells. Phytomedicine. 2014;21:1189–1195. doi: 10.1016/j.phymed.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang W, Zhang X, Qin JJ, Voruganti S, Nag SA, Wang MH, Wang H, Zhang R. Natural product ginsenoside 25-OCH3-PPD inhibits breast cancer growth and metastasis through down-regulating MDM2. PLoS One. 2012;7:e41586. doi: 10.1371/journal.pone.0041586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen B, Shen YP, Zhang DF, Cheng J, Jia XB. The apoptosis-inducing effect of ginsenoside F4 from steamed notoginseng on human lymphocytoma JK cells. Nat Prod Res. 2013;27:2351–2354. doi: 10.1080/14786419.2013.828290. [DOI] [PubMed] [Google Scholar]
- 26.Chen B, Jia XB. Apoptosis-inducing effect of ginsenoside Rg6 on human lymphocytoma JK cells. Molecules. 2013;18:8109–8119. doi: 10.3390/molecules18078109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Y, Tang Z, Xue R, Singh GK, Shi K, Lv Y, Yang L. Combined effects of TNF-alpha, IL-1beta, and HIF-1alpha on MMP-2 production in ACL fibroblasts under mechanical stretch: an in vitro study. J Orthop Res. 2011;29:1008–1014. doi: 10.1002/jor.21349. [DOI] [PubMed] [Google Scholar]
- 28.Xue R, Yang L, Tang Z, Zhang J, Wang Y, Tang X, Jiang J, Wu Y, Yang R, Chen P, Sung KL. The profile of MMP and TIMP in injured rat ACL. Mol Cell Biomech. 2010;7:115–124. [PubMed] [Google Scholar]
- 29.Jang YJ, Kim ME, Ko SY. n-Butanol extracts of Panax notoginseng suppress LPS-induced MMP-2 expression in periodontal ligament fibroblasts and inhibit osteoclastogenesis by suppressing MAPK in LPS-activated RAW264.7 cells. Arch Oral Biol. 2011;56:1319–1327. doi: 10.1016/j.archoralbio.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 30.Sun X, Gao RL, Lin XJ, Xu WH, Chen XH. Panax notoginseng saponins induced up-regulation, phosphorylation and binding activity of MEK, ERK, AKT, PI-3K protein kinases and GATA transcription factors in hematopoietic cells. Chin J Integr Med. 2013;19:112–118. doi: 10.1007/s11655-012-1306-4. [DOI] [PubMed] [Google Scholar]
- 31.Huang Y, Yu J, Wan F, Zhang W, Yang H, Wang L, Qi H, Wu C. Panaxatriol saponins attenuated oxygen-glucose deprivation injury in PC12 cells via activation of PI3K/Akt and Nrf2 signaling pathway. Oxid Med Cell Longev. 2014;2014:978034. doi: 10.1155/2014/978034. [DOI] [PMC free article] [PubMed] [Google Scholar]


