Abstract
Background: Recent findings suggest decreasing TFPI-2 expression plays a significant role in inhibiting cell migration and tumor invasion. The clinicopathological significance of the expression of TFPI-2 and its possible correlation with the expression of CD133 in cholangiocarcinoma remains to be solved. Methods: We investigated if TFPI-2 was involved in the clinicopathological significance of cholangiocarcinoma. An immunohistochemical method was used to detect 218 cases of cholangiocarcinoma, 30 para-neoplastic and 20 normal bile ducts for their expression status of TFPI-2 and CD133, and then the results were analyzed with the patient’s age, sex, tumor site and the histological grade, clinical stage as well as overall mean survival time. Results: Compared with the para-neoplastic and normal cholangiocytes, the expression of TFPI-2 was obviously decreased while the expression of CD133 in carcinoma cells was increased. Carcinomas with low expression of TFPI-2 were significantly corresponding to the tumor site (P = 0.006), size (P = 0.005), histological grade (P = 0.0001) and clinical stage (P = 0.0001), but not to the age (P = 0.066) and sex (P = 0.411), respectively. By Kaplan-Meier survival analysis, the low expression of TFPI-2 was significantly correlative with the overall survival time (P = 0.0001). Further, the expression of TFPI-2 was found inversely correlative with the expression of CD133 (g = -0.3876, P < 0.0001). Conclusions: Our finding suggests that the decreased expression of TFPI-2 may play an important role in the carcinogenesis and progression, and may become a new adjunct marker in the diagnosis and prognosis in cholangiocarcinoma. The expression of TFPI-2 may be inversely correlative with the expression of CD133.
Keywords: Cholangiocarcinoma, TFPI-2, CD133, immunohistochemistry, prognosis
Introduction
Cholangiocarcinoma is one of the most aggressive malignancies, with an extremely poor prognosis. It is because that by the time of becoming clinically evident, most cancers of the biliary tract will have grown beyond the limits of curative resection. Complete resection looks the only potentially curative therapy for all types of biliary tract neoplasms. In prognosis, although 5-year survival rates for intrahepatic and distal cholangiocarcinomas are generally better than those for perihilar carcinomas (30-40%, 20-30%, and 9-18%, respectively) [1,2], stage-related 5-year survival rates seem more comparable. The morbility of bile duct cancer is as if different in the different country, the different area, and the different races [3]. In China, the number of this kind of rare carcinoma in Western country obviously increases with the tumor-free 5-year survival rate of 13.3%. Among the variously different causes for incidence, the hepatitis virus infection, especially hepatitis C virus (HCV), the chronic biliary infects of intestinal source, the primary desmoplastic cholangitis, the bile duct lithiasis, the infection of the Clonorchis sinensis, and the worse environmental carcinogens are possibly the pathogenesis factors for Chinese cholangiocarcinoma patients. Advances in biological research, especially those in mutation-independent activation of the Hedgehog pathway [4], have provided interesting information on the carcinogenesis of this rare tumor. Furthermore, these results bring us the opportunity of development of future targeted therapies in biliary tract cancer. However, early pathologic diagnosis is still difficult in these highly desmoplastic, submucosal, infiltrating cancers, and sensitivity for the diagnosis of cholangiocarcinoma is only 30% for cytology to 40 to 70% for combined brush cytology and biopsy, making a negative result virtually useless [1,2]. This reality has raised therapeutic problem, and new early diagnostic tools and therapeutic techniques in this disease are urgently needed.
Tissue factor pathway inhibitor-2 (TFPI-2), a 32-kDa broad-spectrum Kunitz-type serine proteinase inhibitor, abundantly produced by a variety of human tissues and directionally secreted into their extracellular matrix (ECM) [5-7]. Proteolytic degradation of the extracellular matrix (ECM) is considered to be an essential step in tumor growth and metastasis.TFPI-2 is thought to negatively regulate the enzymatic activity of ECM-associated trypsin, plasmin, and VIIa-tissue factor complexly to protect the ECM stability [8].
In humans, TFPI-2 gene is located on chromosome 7q22, and consists of three Kunitz-type serine proteinase inhibitory domains similar to the classical tissue factor pathway inhibitor (TFPI-1). While the first Kunitz-type domain of TFPI-2 appears to contain the main inhibitory activity towards a number of serine proteinases [9]. The degradation of ECM involves a variety of proteases, particularly metalloproteinases (MMPs). MMPs take part in virtually all events of ECM remodeling. It is reported that upregulation the expression of MMPs strongly associated with the progression of several malignancies, including cervical cancer [10]. TFPI-2 has also been reported to effectively regulate MMPs activity by inhibiting activation of proMMPs by trypsin-like serine proteinases [11]. TFPI-2 gene promote contains a complete CpG island region of at least 220-bp. It is observed that the TFPI-2 expression, decreasing or even diminishing, attributed to promoting hypermethylation in nasopharyngeal carcinoma [12]. Recent findings suggest decreasing TFPI-2 expression plays a significant role in inhibiting cell migration and tumor invasion by a mechanism that involves its inhibitory activity [13,14]. Recently it has been reported that TFPI-2 inhibits the growth and invasion of hepatocellular carcinoma cells and is inactivated in human hepatocellular carcinoma; and furthermore, adenovirus-mediated gene transfer of tissue factor pathway inhibitor-2 inhibits gallbladder carcinoma growth in vitro and in vivo. However, the expression of TFPI-2 has not yet been studied in cholangiocarcinoma. We wonder if TFPI-2 is a putative tumor suppressor in cholangiocarcinoma and thus the significance of expression of TFPI-2 in cholangiocarcinoma needs further explored.
CD133 is one of the most important cancer-initiating (stem) cell markers [15-17] and was confirmed to be expressed in solid cancers such as colon cancer [18] and brain tumors [19,20]. There has been a group of research on CD133 in cholangiocarcinoma from Japan [21], Thailand [22] and China [23], indicating that CD133 expression tends to be related to higher incidences of metastasis; and CD133 is independently related to worse prognosis in cholangiocarcinoma. However, there is no investigation in the relationship between TFPI-2 and CD133 in cholangiocarcinoma to date. It is interesting that if there is any association between TFPI-2 and CD133. We performed an immunohistochemical study to investigate the possible role of the TFPI-2 in clinicopathology and prognosis in two hundred-eighteen cases of cholangiocarcinoma, and then to analyze the possible relationship between the expression of TFPI-2 and CD133. It is hoped that the study will give information on the pathogenesis of this disease.
Materials and methods
Patients and specimens
Two Hundred and eighteen cases of cholangiocarcinoma tissue samples derived from a cohort of patients who had undergone surgery for cholangiocarcinoma, 30 cases of the corresponding para-neoplastic bile duct tissue and 20 cases of normal bile ducts with inflammation were retrieved from the archival file of the Department of Pathology, Chinese People’s Liberation Army (PLA) General Hospital. Each tissue specimen was histologically evaluated by at least two experienced pathologists. The carcinoma patients included 130 men and 88 women with the age from 17~73 (mean = 53.6; median = 56.0) years old. In tumor location, 53 were intrahepatic, 103 perihilar, and 62 distal cholangiocarcinomas. Tumor grading and staging was performed by applying WHO and UICC criteria, and in grading, 69 was at grade 1, 91 at grade 2 and 58 at grade 3; in staging, 7 was at stage I, 95 at stage II, 88 at stage III and 28 at stage IV. Ethical approval for the study was not required by our institution as the experiments carried out did not relate to patient’s privacy, impairment and treatment.
Immunohistochemical analysis
Paraffin tissue sections, 4 μm in thickness, were cut, dewaxed in xylene and rehydrated in a graded ethanol series. Then sections were immersed in 3% hydrogen peroxide in methanol for 10 minutes to block endogenous peroxidase activity and rinsed in running water. They were then immersed in boiling 0.01 M citrate buffer (pH 6.0) in a pressure cooker. The pressure cooker was then sealed and brought to full pressure. The heating time was 2 minutes. After that, the pressure cooker was de-pressured and cooled under running water. The lid was then removed, and the hot buffer was flushed out with cold water from a running tap. The cooled sections were washed twice in phosphate buffered saline (PBS) before immunohistochemical staining. The primary antibodies were then added to the sections and then the sections were incubated at 4°C overnight in a humidified chamber with monoclonal mouse antibody against human TFPI-2 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and polyclonal rabbit antibody against human CD133 (Abcam Inc., Cambridge, MA, USA) diluted 1:100 in blocking solution. After exposure to primary antibody, the sections were allowed to react with the poly peroxidase-anti-mouse/rabbit IgG for 20 minutes by the standard non-biotin PV-6000 Polymer Detection System (Zymed Laboratories Inc., South San Francisco, CA). The sections were then washed in water, counter-stained with Mayer’s haematoxylin for one minute at room temperature, dehydrated, cleaned and finally mounted. Paraffin blocks of human breast ductal carcinoma tissues were used as the positive controls. Negative controls were sections treated the same as above but with omission of the primary antibody and replaced by 0.01 M PBS. For immunohistochemical evaluation of TFPI-2 and CD133, membranous and cytoplasmic labeling of tumor cells was classified as either negative. In scoring expression of TFPI-2 and CD133 protein, both the extent and intensity of immunopositivity were considered, according to Hao et al. [24]. The intensity of positivity was scored as follows: 0, negative; 1, weak; 2, moderate; 3, strong. The extent of positivity was scored as follows: 0, < 5%; 1, > 5-25%; 2, > 25-50%; 3, > 50-75%; 4, > 75% of the cells in the respective lesions. The final score was determined by multiplying the intensity of positivity and the extent of positivity scores, yielding a range from 0 to 12. Scores ≥ 4 were defined as high expression pattern. Scores < 4 were recorded as low expression pattern.
Statistical analysis
Fisher’s exact test, Pearson Chi square’s test, Spearman’s correlation coefficient test for trends in proportions and Kaplan-Meier method with Log rank test or Cox regression method for univariate or multivariate overall survival analysis were used to assess the associations between TFPI-2 or CD133 expression and pathological indices. A P < 0.05 was considered statistically significant.
Results
TFPI-2 expression in normal, para-neoplastic bile duct tissue and cholangiocelluar carcinoma
TFPI-2 protein was expressed high or positive diffusely in the membrane and cytoplasm of cholangiocytes in all thirty para-neoplastic and twenty normal bile ducts. In carcinoma, TFPI-2 was expressed diffusely in the membrane and cytoplasm of cancer cells in one hundred and four out of two hundred-eighteen cases (47.7%). The expression of TFPI-2 was low and negative in 114 (52.3%) cases of carcinoma (Figure 1). Most poorly-differentiated cancer cells were negative for TFPI-2 protein. There was a statistical difference between cholangiocarcinomas and para-neoplastic or normal bile ducts (P < 0.0001).
Figure 1.
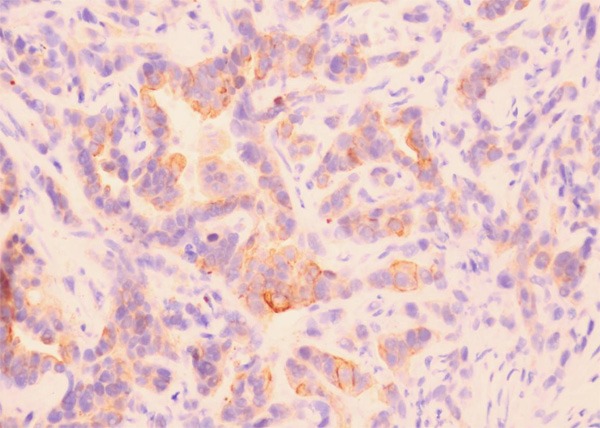
Expression of TFPI-2 in cholangiocarcinoma. TFPI-2 was lowly expressed positive in the membrane and cytoplasm of cancer cells in cholangiocarcinoma. (TFPI-2 ×400).
Relationship between TFPI-2 expression and age, sex, site, size, histological grade, clinical stage and prognosis
In this group of 218 cholangiocarcinomas, TFPI-2 low expression was correlative with tumor site (P = 0.006) and tumor size (P = 0.005) but not with age (P = 0.066) and sex (P = 0.411) (Table 1). The percentage of carcinomas with low expression of TFPI-2 increased from 26.1% (18 of 69) in well-differentiated cancers (grade 1) to 58.2% (53 of 91) in moderately differentiated cancers (grade 2) and to 74.1% (43 of 58) in poorly differentiated cancers (grade 3), thus this association of increased histological grade of tumors with low expression of TFPI-2 was statistically significant (P = 0.0001, Table 1). In this research, the carcinomas with TFPI-2 low expression was 36.3% (37 of 102) at stage I to II, but 66.4% (77 of 116) at stage III to IV. Statistically, the low expression of TFPI-2 was significantly associated with more advanced clinical stage of the tumors (P = 0.0001, Table 1). Follow-up data showed that there was a significant difference in overall mean survival time between the carcinomas with TFPI-2 low expression (16.8 months) and those with high expression (31.1 months) (Log rank = 45.149; P = 0.0001) (Figure 2). It was suggested that the TFPI-2 low expression was significantly related to the cancers with shorter mean survival time (P = 0.0001). In the result of multivariate analysis by Cox Regression, TFPI-2 expression was an independent prognostic factor (P = 0.0001).
Table 1.
The relationship between expression of TFPI-2 or CD133 and clinicopathological features
| Clinicopathological features | parameter | TFPI-2 | P | CD133 | P | ||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| low | high | low | high | ||||
| Age | ≥ 60 | 47 | 30 | 0.066 | 33 | 44 | 0.395 |
| < 60 | 67 | 74 | 70 | 71 | |||
| Sex | Man | 71 | 59 | 0.411 | 58 | 72 | 0.407 |
| Woman | 43 | 45 | 45 | 43 | |||
| Tumor site | Intrahepatic | 37 | 16 | 0.006 | 22 | 31 | 0.068 |
| Perihilar | 52 | 51 | 44 | 59 | |||
| Distal | 25 | 37 | 37 | 25 | |||
| Size | ≤ 2 cm | 45 | 58 | 0.005 | 60 | 43 | 0.003 |
| > 2 ≤ 5 cm | 50 | 41 | 37 | 54 | |||
| > 5 cm | 19 | 5 | 6 | 18 | |||
| Grade | 1 | 18 | 51 | 0.0001 | 45 | 24 | 0.0001 |
| 2 | 53 | 38 | 42 | 49 | |||
| 3 | 43 | 15 | 16 | 42 | |||
| TNM | I | 3 | 4 | 0.0001* | 5 | 2 | 0.0001* |
| II | 34 | 61 | 57 | 38 | |||
| III | 56 | 32 | 35 | 53 22 | |||
| IV | 21 | 7 | 6 | 16.9 | |||
| Survivalz | Mean (months) | 16.8 | 31.1 | 0.0001 | 31.2 | 0.0001 | |
I~II vs III~IV.
Figure 2.
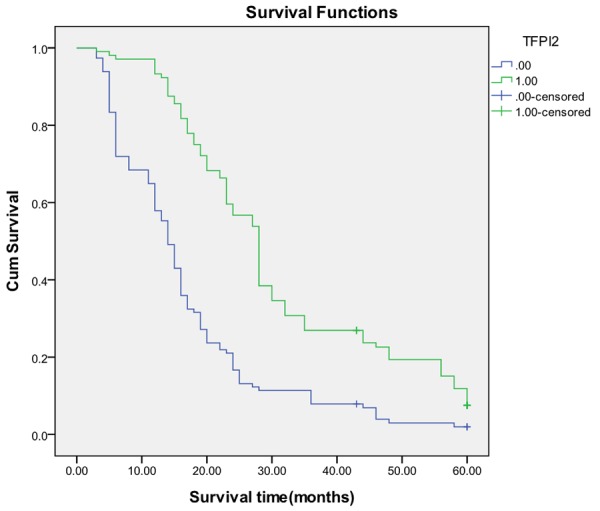
Kaplan-Meier survival analysis by TFPI-2 status (n = 218). The y-axis represents the percentage of patients; the x-axis, their survival in months. The green line represents TFPI-2- high expressive patients with a trend of better survival than the blue line representing TFPI-2- low expressive patients (Log rank = 45.149; P = 0.0001). Mean overall survival (OS) time was 31.1 months for the TFPI-2-high expressive group and 16.8 months for the TFPI-2- low expressive group.
CD133 expression in normal, para-neoplastic bile duct tissue and cholangiocelluar carcinoma
In carcinoma, CD133 expression was expressed high and observed diffusely and strongly in the membrane and cytoplasm of cancer cells in one hundred-fifteen out of two hundred-eighteen cases (52.8%). Most cancer cells were diffusely and strongly positive for CD133 protein in the membrane and cytoplasm of cholangiocarcinoma (Figure 3A). CD133 protein was expressed low or negative in all thirty para-neoplastic and twenty normal bile ducts. There was a statistical difference between cholangiocarcinomas and para-neoplastic or normal bile ducts (P < 0.0001).
Figure 3.
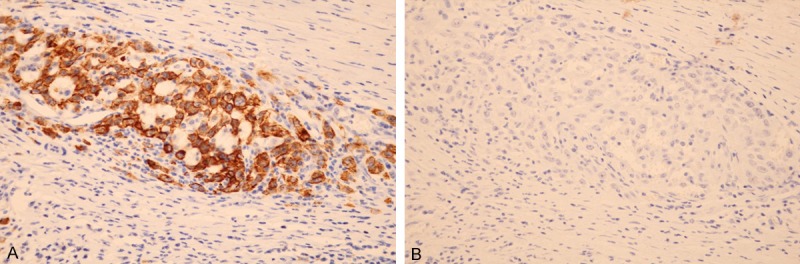
Expression of CD133 and TFPI-2 protein in cholangiocarcinoma. CD133 was highly expressed (A) but TFPI-2 was negatively expressed (B) in the same cancerous glands in moderately differentiated cholangiocarcinoma. (CD133, TFPI-2 ×400).
Relationship between CD133 expression and age, sex, site, size, histological grade, clinical stage and prognosis
In this group of 218 cholangiocarcinomas, CD133 high expression was correlative with tumor size (P = 0.003) but not with tumor site (P = 0.068), age (P = 0.395) and sex (P = 0.407) (Table 1). The percentage of carcinomas with high expression of CD133 increased from 34.8% (24 of 69) in well-differentiated cancers (grade 1) to 53.8% (49 of 91) in moderately differentiated cancers (grade 2) and to 72.4% (42 of 58) in poorly differentiated cancers (grade 3), thus this association of increased histological grade of tumors with expression of CD133 was statistically significant (P = 0.0001, Table 1). In this research, the carcinomas with CD133 high expression was 39.2% (40 of 102) at stage I to II, but 64.7% (75 of 116) at stage III to IV. Statistically, the high expression of CD133 was significantly associated with more advanced clinical stage of the tumors (P = 0.0001, Table 1). Follow-up data showed that there was a significant difference in overall mean survival time between the carcinomas with CD133 high expression (16.9 months) and those with low expression (31.2 months) (Log rank = 41.642; P = 0.0001) (Figure 4). It was suggested that the CD133 high expression was significantly related to the cancers with shorter mean survival time (P = 0.0001). In the result of multivariate analysis by Cox Regression, CD133 expression was also an independent prognostic factor (P = 0.0001).
Figure 4.
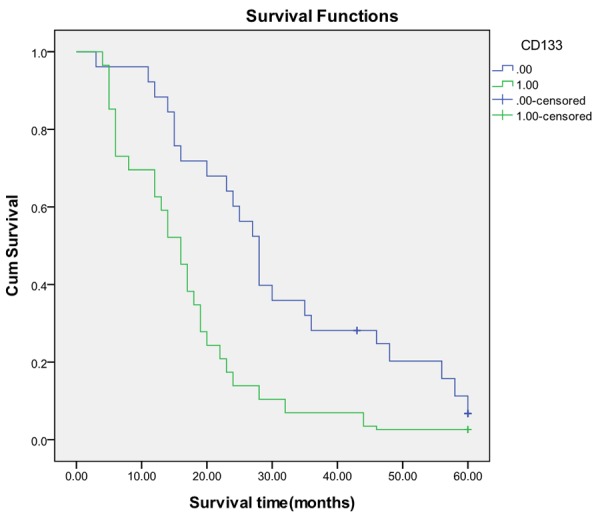
Kaplan-Meier survival analysis by CD133 status (n = 218). The y-axis represents the percentage of patients; the x-axis, their survival in months. The green line represents CD133-high expressive patients with a trend of worse survival than the blue line representing CD133- low expressive patients. Mean survival (OS) time was 16.9 months for the CD133- high expressive group and 31.2 months for the CD133- low expressive group (Log rank = 41.642; P = 0.0001).
Relationship between TFPI-2 and CD133 expression
Frequently, when TFPI-2 was negative or low expressive (Figure 3B), CD133 was found highly expressive in cancerous glands of cholangiocarcinoma (Figure 3A). Of the cholangiocarcinomas with high expression of CD133, TFPI-2 expression was found only in 35.7% (41/115), whereas of the cancers with low expression of CD133, TFPI-2 presented high up to 61.2% (63/103). A significantly inverse relationship was found between expressions of TFPI-2 and CD133 (r = -0.3876, P = 0.0001, Figure 5).
Figure 5.
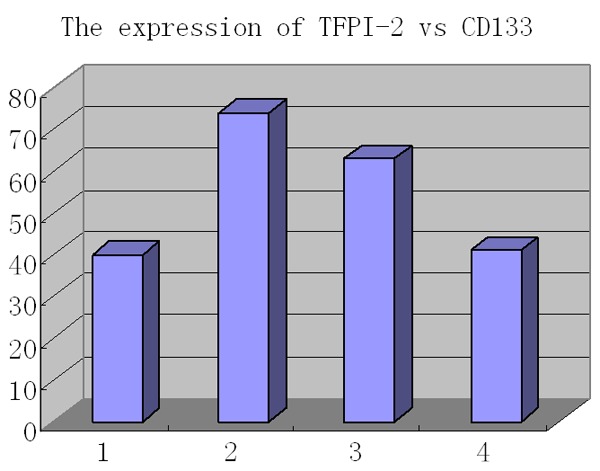
The inverse correlation between TFPI-2 and CD133. From left to right, expression of TFPI-2 compared with that of CD133 (n = 218) as low to low (1, n = 40), low to high (2, n = 74), high to low (3, n = 63) and high to high (4, n = 41) (r = -0.3876, P = 0.0001).
Discussion
Human TFPI-2, also known as placental protein (PP5) and matrix-associated serine protease inhibitor (MSPI), is an ECM-associated Kunitz-type serine proteinase inhibitor [25]. TFPI-2 plays an important role in normal ECM remodeling, and is also becoming increasingly recognized as a tumor suppressor gene. In several types of malignancies, such as choriocarcinoma [26], glioma [27], prostate cancer [28], pancreatic carcinoma [29] and lung cancer [30], TFPI-2 has significantly demonstrated tumor-suppressive functions during tumor cell invasion, metastasis, apoptosis, proliferation and angiogenesis. It was reported that, TFPI-2 showed high frequency of CpG islands aberrantly methylated in both cervical cancer specimens and cell lines [31,32]. But, to our knowledge, little is known on the role of TFPI-2 silencing in cholangiocarcinoma. To investigate the relationship between TFPI-2 and clinicopathological indices in patients with cholangial cancer, we analyzed the immunohistochemical expression levels of TFPI-2, with relationship to CD133 in cholangiocarcinoma tissues. In 218 cholangiocarcinoma with follow up data our results indicated that TFPI-2-low expression level was correlative with the histological degree (P = 0.0001) and the clinical stage (P = 0.0001). Furthermore, our data also showed that the low expression of the TFPI-2 protein was greatly associated with overall survival (P = 0.0001), suggesting TFPI-2 expression might be a potential prognostic factor in cholangiocarcinoma. Thus it is suggested that TFPI-2 might be a regulatory molecule as a tumor suppressor in cholangiocarcinoma.
CD133 is one of the hot markers in a variety of tumor stem cells [15-17]. In this research we found that CD133 is positive in 52.8% of cholangiocarcinoma patients in China. The present study investigated the expression of CD133 protein in 218 cholangiocarcinoma with follow up data and the results indicated that CD133 expression level was correlative with the histological degree (P = 0.0001) and the clinical stage (P = 0.0001). Furthermore, our data also showed that the expression of the CD133 protein was greatly associated with overall survival (P = 0.0001), suggesting CD133 expression might be a potential prognostic factor in cholangiocarcinoma. Our results are corresponding to previous reports in cholangiocarcinoma from Japan [21], Thailand [22] and another research group from Xi’an, China [23], in which the positive rate of CD133 was 48.3%, 67.6% and 74.0%, respectively and also CD133 expression was considered as a potential prognostic indicator. The expression of TFPI-2 can inhibit the growth of gall bladder cancer and hepatocellular carcinoma [33,34]. In this study, TFPI-2 expression was inversely associated with clinicopathological features as clinical stage, histological grade and poor prognosis in cholangiocarcinoma (Table 1). Our finding also suggests that TFPI-2 is inversely correlative with CD133 (P = 0.0001), implying loss of TFPI-2 function may exist in CD133-high expressive cancer cells. But the association between both genes is rather descriptive than causative to date and a clear functional relation needs to be explored further. Taken together, TFPI-2 is low expressed in cancer cells of cholangiocarcinoma. The combined detection of TFPI-2 and CD133 expression, to some extent, can reflect the biological behavior of cholangial cancer cells, thus guiding the choice of chemotherapy and molecular targeting therapy.
Acknowledgements
The authors thank all colleagues in the Department of Pathology, Chinese People’s Liberation Army General Hospital for their help and support with this study. All authors have contributed significantly, and all authors are in agreement with the content and writing of the paper.
Disclosure of conflict of interest
None.
References
- 1.Khan SA, Davidson BR, Goldin R, Pereira SP, Rosenberg WM, Taylor-Robinson SD, Thillainayagam AV, Thursz MR, Wasan H. Guidelines for the diagnosis and treatment of cholangiocarcinoma: consensus document. Gut. 2002;51(Suppl 6):VI1–VI9. doi: 10.1136/gut.51.suppl_6.vi1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gores GJ. A spotlight on cholangiocarcinoma. Gastroenterology. 2003;125:1536–1538. doi: 10.1016/j.gastro.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 3.Heron DE, Stein DE, Eschelman DJ, Topham AK, Waterman FM, Rosato EL, Alden M, Anne PR. Cholangiocarcinoma: the impact of tumor location and treatment strategy on outcome. Am J Clin Oncol. 2003;26:422–428. doi: 10.1097/01.COC.0000026833.73428.1F. [DOI] [PubMed] [Google Scholar]
- 4.Berman DM, Karhadkar SS, Maitra A, Montes-De-Oca R, Gerstenblith MR, Briggs K, Parker AR, Shimada Y, Eshleman JR, Watkins DN, Beachy PA. Widespread requirement for Hedgehog ligand stimulation in growth of digestive tract tumors. Nature. 2003;425:846–851. doi: 10.1038/nature01972. [DOI] [PubMed] [Google Scholar]
- 5.Udagawa K, Yasumitsu H, Esaki M, Sawada H, Nagashima Y, Aoki I, Jin M, Miyagi E, Nakazawa T, Hirahara F, Miyazaki K, Miyagi Y. Subcellular localization of PP5/TFPI-2 in human placenta: a possible role of PP5/TFPI-2 as an anti-coagulant on the surface of syncytiotrophoblasts. Placenta. 2002;23:145–153. doi: 10.1053/plac.2001.0774. [DOI] [PubMed] [Google Scholar]
- 6.Herman MP, Sukhova GK, Kisiel W, Foster D, Kehry MR, Libby P, Schönbeck U. Tissue factor pathway inhibitor-2 is a novel inhibitor of matrix metalloproteinases with implications for atherosclerosis. J Clin Invest. 2001;107:1117–1126. doi: 10.1172/JCI10403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sugiyama T, Ishii S, Yamamoto J, Irie R, Saito K, Otuki T, Wakamatsu A, Suzuki Y, Hio Y, Ota T, Nishikawa T, Sugano S, Masuho Y, Isogai T. cDNA macroarray analysis of gene expression in synoviocytes stimulated with TNF alpha. FEBS Lett. 2002;517:121–128. doi: 10.1016/s0014-5793(02)02588-7. [DOI] [PubMed] [Google Scholar]
- 8.Rao CN, Cook B, Liu Y Chilukuri K, Stack MS, Foster DC, Kisiel W, Woodley DT. HT-1080 fibrosarcoma cell matrix degradation and invasion are inhibited by the matrix-associated serine protease inhibitor TFPI-2/33 kDa MSPI. Int J Cancer. 1998;76:749–756. doi: 10.1002/(sici)1097-0215(19980529)76:5<749::aid-ijc21>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 9.Chand HS, Schmidt AE, Bajaj SP, Kisiel W. Structure function analysis of the reactive site in the first Kunitz type domain of human tissue factor pathway inhibitor-2. J Biol Chem. 2004;279:17500–17507. doi: 10.1074/jbc.M400802200. [DOI] [PubMed] [Google Scholar]
- 10.Libra M, Scalisi A, Vella N, Clementi S, Sorio R, Stivala F, Spandidos DA, Mazzarino C. Uterine cervical carcinoma: role of matrix metalloproteinases. Int J Oncol. 2009;34:897–903. doi: 10.3892/ijo_00000215. [DOI] [PubMed] [Google Scholar]
- 11.Hitendra Chand S, Donald Foster C, Walter Kisiel. Structure, function and biology of tissue factor pathway inhibitor-2. Thromb Haemost. 2005;94:1122–1130. doi: 10.1160/TH05-07-0509. [DOI] [PubMed] [Google Scholar]
- 12.Wang S, Xiao X, Zhou X, Huang T, Du C, Yu N, Mo Y, Lin L, Zhang J, Ma N, Murata M, Huang G, Zhang Z. TFPI-2 is a putative tumor suppressor gene frequently inactivated by promoter hypermethylation in nasopharyngeal carcinoma. BMC Cancer. 2010;10:617. doi: 10.1186/1471-2407-10-617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wong CM, Ng YL, Lee JM, Wong CC, Cheung OF, Chan CY, Tung EK, Ching YP, Ng IO. Tissue factor pathway inhibitor-2 as a frequently silenced tumor suppressor gene in hepatocellular carcinoma. Hepatology. 2007;45:1129–1138. doi: 10.1002/hep.21578. [DOI] [PubMed] [Google Scholar]
- 14.Ran Y, Pan J, Hu H, Zhou Z, Sun L, Peng L, Yu L, Sun L, Liu J, Yang Z. A novel role for tissue factor pathway inhibitor-2 in the therapy of human esophageal carcinoma. Hum Gene Ther. 2009;20:41–49. doi: 10.1089/hum.2008.129. [DOI] [PubMed] [Google Scholar]
- 15.Miraglia S, Godfrey W, Yin AH, Atkins K, Warnke R, Holden JT, Bray RA, Waller EK, Buck DW. A novel five-transmembrane hematopoietic stem cell antigen: isolation, characterization, and molecular cloning. Blood. 1997;90:5013–5021. [PubMed] [Google Scholar]
- 16.Yin AH, Miraglia S, Zanjani ED, Almeida-Porada G, Ogawa M, Leary AG, Olweus J, Kearney J, Buck DW. AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood. 1997;90:5002–5012. [PubMed] [Google Scholar]
- 17.Shmelkov SV, St Clair R, Lyden D, Rafii S. AC133/CD133/Prominin-1. Int J Biochem Cell Biol. 2005;37:715–719. doi: 10.1016/j.biocel.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 18.Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, De Maria R. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 19.Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–5828. [PubMed] [Google Scholar]
- 20.Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 21.Shimada M, Sugimoto K, Iwahashi S, Utsunomiya T, Morine Y, Imura S, Ikemoto T. CD133 expression is a potential prognostic indicator in intrahepatic cholangiocarcinoma. J Gastroenterol. 2010;45:896–902. doi: 10.1007/s00535-010-0235-3. [DOI] [PubMed] [Google Scholar]
- 22.Leelawat K, Thongtawee T, Narong S, Subwongcharoen S, Treepongkaruna SA. Strong expression of CD133 is associated with increased cholangiocarcinoma progression. World J Gastroenterol. 2011;17:1192–1198. doi: 10.3748/wjg.v17.i9.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fan L, He F, Liu H, Zhu J, Liu Y, Yin Z, Wang L, Guo Y, Wang Z, Yan Q, Huang G. CD133: a potential indicator for differentiation and prognosis of human cholangiocarcinoma. BMC Cancer. 2011;11:320. doi: 10.1186/1471-2407-11-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hao XP, Willis JE, Pretlow TG, Rao JS, MacLennan GT, Talbot IC, Pretlow TP. Loss of Fragile Histidine Triad Expression in colorectal carcinomas and premalignant lesions. Cancer Res. 2000;60:18–21. [PubMed] [Google Scholar]
- 25.Iino M, Foster DC, Kisiel W. Quantification and characterization of human endothelial cell-derived tissue factor pathway inhibitor-2. Arterioscler Thromb Vasc Biol. 1998;18:40–46. doi: 10.1161/01.atv.18.1.40. [DOI] [PubMed] [Google Scholar]
- 26.Hubé F, Reverdiau P, Iochmann S, Rollin J, Cherpi-Antar C, Gruel Y. Transcriptional silencing of the TFPI-2 gene by promoter hypermethylation in choriocarcinoma cells. Biol Chem. 2003;384:1029–1034. doi: 10.1515/BC.2003.115. [DOI] [PubMed] [Google Scholar]
- 27.Gessler F, Voss V, Seifert V, Gerlach R, Kögel D. Knockdown of TFPI-2 promotes migration and invasion of glioma cells. Neurosci Lett. 2011;497:49–54. doi: 10.1016/j.neulet.2011.04.027. [DOI] [PubMed] [Google Scholar]
- 28.Konduri SD, Tasiou A, Chandrasekar N, Rao JS. Overexpression of tissue factor pathway inhibitor-2 (TFPI-2), decreases the invasiveness of prostate cancer cells in vitro. Int J Oncol. 2001;18:127–131. [PubMed] [Google Scholar]
- 29.Tang Z, Geng G, Huang Q, Xu G, Hu H, Chen J, Li J. Expression of tissue factor pathway inhibitor 2 in human pancreatic carcinoma and its effect on tumor growth, invasion, and migration in vitro and in vivo. J Surg Res. 2011;167:62–69. doi: 10.1016/j.jss.2009.06.015. [DOI] [PubMed] [Google Scholar]
- 30.Iochmann S, Bléchet C, Chabot V, Saulnier A, Amini A, Gaud G, Gruel Y, Reverdiau P. Transient RNA silencing of tissue factor pathway inhibitor-2 modulates lung cancer cell invasion. Clin Exp Metastasis. 2009;26:457–467. doi: 10.1007/s10585-009-9245-z. [DOI] [PubMed] [Google Scholar]
- 31.Sova P, Feng Q, Geiss G, Wood T, Strauss R, Rudolf V, Lieber A, Kiviat N. Discovery of novel methylation biomarkers in cervical carcinoma by global demethylation and microarray analysis. Cancer Epidemiol Biomarkers Prev. 2006;15:114–123. doi: 10.1158/1055-9965.EPI-05-0323. [DOI] [PubMed] [Google Scholar]
- 32.Santin AD, Zhan F, Bignotti E, Siegel ER, Cané S, Bellone S, Palmieri M, Anfossi S, Thomas M, Burnett A, Kay HH, Roman JJ, O’Brien TJ, Tian E, Cannon MJ, Shaughnessy J Jr, Pecorelli S. Gene expression profiles of primary HPV16-and HPV18-infected early stage cervical cancers and normal cervical epithetlium: identification of novel candidate molecular markers for cervical cancer diagnosis and therapy. Virology. 2005;331:269–291. doi: 10.1016/j.virol.2004.09.045. [DOI] [PubMed] [Google Scholar]
- 33.Qin Y, Zhang S, Gong W, Li J, Jia J, Quan Z. Adenovirus-mediated gene transfer of tissue factor pathway inhibitor-2 inhibits gallbladder carcinoma growth in vitro and in vivo. Cancer Sci. 2012;103:723–30. doi: 10.1111/j.1349-7006.2012.02218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu Y, Qin X, Zhou J, Tu Z, Bi X, Li W, Fan X, Zhang Y. Tissue factor pathway inhibitor-2 inhibits the growth and invasion of hepatocellular carcinoma cells and is inactivated in human hepatocellular carcinoma. Oncol Lett. 2011;2:779–783. doi: 10.3892/ol.2011.340. [DOI] [PMC free article] [PubMed] [Google Scholar]


