Abstract
Hydrogen sulfide (H2S), produced by cystanthionine-γ-lysase (CSE) in the cardiovascular system, is an endogenous gaseous mediator exerting pronounced physiological effects as the third gasotransmitter in addition to nitric oxide (NO) and carbon monoxide (CO). Accumulating evidence indicated that H2S could mediate the cardioprotective effects in myocardial ischemia model. Ventricular arrhythmia is the most important risk factor for cardiac mortality and sudden death after acute myocardial infarction (AMI). The potential impact of H2S on cardiomyocytes electrical remodeling post ischemic insult is not fully explored now. Present study investigated the role of H2S on cardiomyocytes electrical remodeling in rats with ischemia/reperfusion injury. H2S concentration was reduced and arrhythmia score was increased in this model. CSE mRNA level was also upregulated in the ischemic myocardium. Exposure to exogenous NaHS reduced the action potential duration (APD), inhibited L-type Ca2+ channels and activated KATP channels in cardiomyocytes isolated from ischemic myocardium Exogenous H2S application improves electrical remodeling in cardiomyocytes isolated from ischemic myocardium. These results indicated that reduced H2S level might be linked to ischemia/reperfusion induced arrhythmias.
Keywords: Hydrogen sulfide, CSE, ischemia/reperfusion, action potential duration, L-type Ca2+ channels, KATP channels
Introduction
Hydrogen sulphide (H2S), like nitric oxide and carbon monoxide, is an endogenously generated gaseous mediator exerting pronounced physiological effects on various organs including cardiovascular system [1]. Endogenous H2S is produced enzymatically by the cysteine metabolic enzymes cystathionine b-synthase (CBS), cystathionine c-lyase (CSE), and 3-mercaptopyruvate sulfurtransferase1 [2]. In the cardiovascular system, H2S is predominantly generated by CSE, Previous studies demonstrated that endogenously synthesized H2S could protect vascular tissues from atherogenic damage by reducing vessel intimal proliferation and inhibiting adhesion molecule expression [3]. The deficiency of CSE in mice leads to a decreased endogenous H2S level, an age-dependent increase in blood pressure, and impaired endothelium-dependent vasorelaxation [4] and decreased endogenous H2S production predisposes the animals to vascular remodeling and early development of atherosclerosis. Besides, reduced endogenous H2S in plasma and myocardial tissue was shown in isoproterenol-induced myocardial injury on male Wistar rats [4]. Previous study showed that endogenous H2S might mediate the cardioprotection effects exterted by ischemic postconditioning (IPO) in isolated rat hearts [5].
Additionally, exogenous administration of H2S (in the form of NaHS) could decrease blood pressure in spontaneously hypertensive rats and in anaesthetized normotensive rats [6,7]. Exogenous administration of H2S also reduced infarct size in a rat model of coronary artery ligation [8]. Moreover, H2S levels were reduced in heart failure mice and oral H2S therapy prevented the transition from compensated to decompensated heart failure via upregulation of endothelial nitric oxide synthase and increase of nitric oxide bioavailability [9].
Myocardial ischemia is characterized by ionic and biochemical alterations, which could result in an unstable electrical substrate capable of initiating and sustaining arrhythmias [11]. At least 75% AMI patients suffered from arrhythmia in the peri-infarct period, which served as a major cause of mortality post AMI [10]. Previous study demonstrated that exogenous administration of H2S could significantly decrease the duration and severity of ischemia/reperfusion-induced arrhythmias and increase the viability of cardiomyocytes isolated from isolated perfused rat hearts and cultured in simulated ischemia solution [11]. Above evidences suggest that there might be a close association between H2S and arrhythmias induced by myocardial ischemia. Till now, the relationship between H2S and ischemia/reperfusion-induced arrhythmia as well as cardiomyocytes electrical remodeling post ischemia is not fully explored. In this study, we investigated the relationship between H2S and ischemia/reperfusion-induced arrhythmia and cardiomyocytes electrical remodeling post ischemia/reperfusion injury.
Material and methods
Animals
Wistar rats (20 ± 2 weeks old, weighing 200-250 g) were obtained from the Department of Experimental Animals, Chinese Academy of Sciences in Shanghai. The investigation conformed to the “Guide for the Care and Use of Laboratory Animals” published by the National Institutes of Health (NIH) of the United States and was approved by the Ethics Committee of the Xinhua Hospital. A total of 85 rats were used for the experiment, ischemia/reperfusion model mortality is about 15%.
Ischemia/reperfusion injury model in rats
Anaesthesia was induced with 10 g/L chloral hydrate by intraperitoneal injection. Rats were intubated and mechanically ventilated using a rodent ventilator (TKR-200 C Shanghai Xinman). A continuous electrocardiogram (ECG) telemetry transmitter was implanted in the abdominal cavity. A saline filled PE50 catheter, which was connected with a pressure transducer, was inserted through the carotid artery into the left ventricle to measure the left ventricular end-systolic pressure. A left thoracotomy and pericardiotomy were performed, and left anterior descending coronary artery was ligated with 5-0 silk thread. The artery was occluded for 30 min by a knot, and followed by reperfusion, the success was confirmed by observing the color changing of myocardium and monitoring with an electrocardiogram, left ventricular pressure quickly reduction and increasing of myocardial enzyme.
Arrhythmia score
Arrhythmias were recorded by ECG during the 30 minutes ischemia and 120 minutes reperfusion period. Arrhythmia scores were reference to the following Lambeth Conference standard: 0 points: no arrhythmia; 1 point: occasional ventricular premature beats (VPBs); 2 points: frequent VPBs (bigeminal or trigeminal rhythm); 3 points: occasional ventricular tachycardia (VT); 4 points: sustained VT or occasional ventricular flbrillation; and 5 points: ventricular flbrillation or death.
Plasma H2S level measurement
Immediately after ischemia/reperfusion, plasma was collected from femoral artery of rats before sacrifice and centrifuged (4000 rpm, 10 min, Room Temperature). Seventy-five microliters plasma was mixed with 250 ml 1% (w/v) zinc acetate and 425 ml distilled water in a tube to trap H2S. Subsequently, N-dimethyl-p-phenylenediamine sulphate (20 µM; 133 µl) in 7.2 mM HCl was added followed by FeCl3 (30 µM; 133 µl) in 1.2 M HCl. The protein in the plasma was removed by adding 250 ml of 10% tricholoacetic acid to the reaction mixture and pelleted by centrifugation at 12000 g (15 min). The absorbance of the resulting solution at 670 nm was measured with a spectrophotometer in a 96-well plate.
CSE expression assay
Immediately after blood collection, rats were sacrificed under deep anesthesia and chest opened, heart excised, washed with PBS buffer, Myocardium around ischemic area was obtained (about 100 mg) and quick frozen in liquid nitrogen and kept at -80°C till further examinations. RNA was extracted using TRIzol reagent as in the manufacturer’s instructions (Invitrogen, CA, USA). The reverse transcription from mRNA to cDNA was performed with PrimeScript™ One Step RT-PCR Kit (TAKARA, JAP) with the manufacturer’s instructions. Rat β-actin served as an internal control gene. The primer sequences of CSE and β-actin were as following: CSE (forward): 5’-GTG TCT GTT ACT TCC GAT GAC CTC-3’; CSE (reverse): 5’-CCT CGG CAG CAG AGG TAA CA-3’; β-actin (forward): 5’-CCG TAA AGA CCT CTA TGC CAA CA-3’; β-actin (reverse): 5’-CGG ACT CAT CGT ACT CCT GCT-3’.
Isolation of cardiomyocytes
Ventricular myocytes were obtained from hearts of male Wistar rats (200-250 g) by enzymatic dissociation according to the method described by Bian et al. [12] with some modifications. In brief, the heart was removed, mounted in a Langendorff apparatus, and perfused retrogradely through the aorta with a Krebs’ solution containing 117 mM NaCl, 5 mM KCl, 1.2 mM MgSO4, 1.2 mM KH2PO4, 1.25 mM CaCl2, 25 mM NaHCO3, and 11 mM glucose and bubbled with 95% O2/5% CO2 (pH 7.4, 34°C) at a constant flow rate of 13 ml/min. Cardiomyocytes were isolated from the myocardium around the ischemic area using a collagenase perfusion method as described previously. After separation of cardiomyocytes, cells were allowed to stabilize for 30 minutes before further experiment was commenced. Cardiomyocytes were treated with 50, 100, 200 μM NaHS or DMEM solution for 5 minutes, respectively.
Electrophysiological measurements
Cardiomyocytes were placed in a chamber on the stage of an inverted microscope (Leica, DMIL, Wetzlar, Germany) and continuously perfused at a constant rate (1.8 ml/min) with 35°C Tyrode’s solution. The cells were patch-clamped in the whole-cell configuration using a patch-clamp amplifier (Axopatch 200B, Axon instruments, Burlingham, CA, USA). The signals were recorded and analyzed with pClamp 6.0 and Clampfit 9.0 softwares (Axon instruments). Briefly, patch electrodes were fabricated from borosilicate glass tubes and filled with a pipette solution containing (in mM) CsCl 120, MgCl2 2, CaCl2 1, Na2ATP 5, EGTA 11, and HEPES 10 (pH adjusted to 7.4 with CsOH). Filled electrodes had a tip resistance of 3-5 MΩ. A period of 10-15 min was allowed after the whole-cell configuration was achieved. I Ca, L, action potential and the KATP current were measured as described [13,14].
Statistical analysis
All experimental data are presented as means ± SEM. Differences between groups were analyzed by one-way ANOVA followed by post hoc Tukey’s test. A value of P < 0.05 was considered statistically significant.
Results
Plasma H2S level was related with the development of post- ischemia/reperfusion arrhythmias
As results in Figure 1A, the plasma H2S level was significantly lower in group with arrhythmia score ≥ 3 compared to control group with arrhythmia score = 0. CSE mRNA expression in infarct border zone was detected and the CSE mRNA expression was significantly upregulated in infarct border zone compared with tissue from normal control group (Figure 1B). These data indicated the potential relationship between plasma H2S level and the development of post-ischemia/reperfusion arrhythmia.
Figure 1.
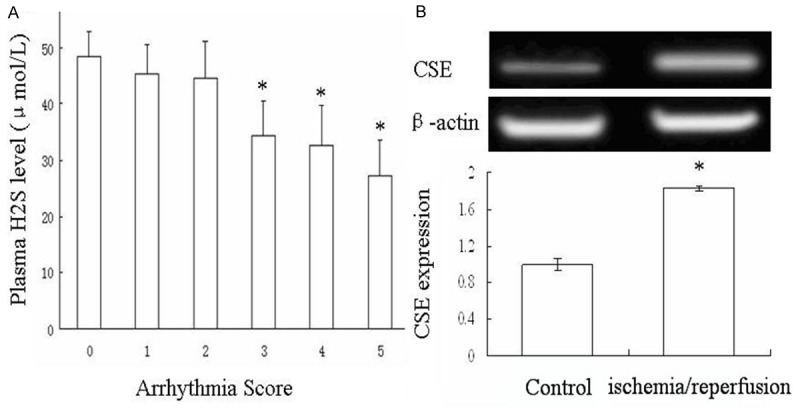
The plasma H2S level and CSE expression of rats after ischemia/reperfusion. The ischemia/reperfusion rat model was set up and arrhythmia score was assessed according to Lambeth Conference standard. Plasma were collected to test the level of H2S level in different group with different arrhythmia score. A. The tissue from ischemic area was collected to test the CSE expression by RT-PCR. B. Data are the mean ± SD of five mice per group. *P < 0.05 vs control group.
Exogenous NaHS reduced the action potential duration in isolated ischemic cardiomyocytes
To test the role of exogenous H2S on the electrical remodeling of cardiomyocytes, cardiomyocytes from infarct border zone were isolated. Resting potential (RP) and action potential duration (APD) of cardiomyocytes were measured post treatment for 5 minutes with various concentrations of NaHS. NaHS at concentration of 100 and 200 µM significantly reduced the APD compared with control cardiomyocytes (Figure 2A, 2B). However, the expose of NaHS had no impact on the resting potential (Figure 2C).
Figure 2.
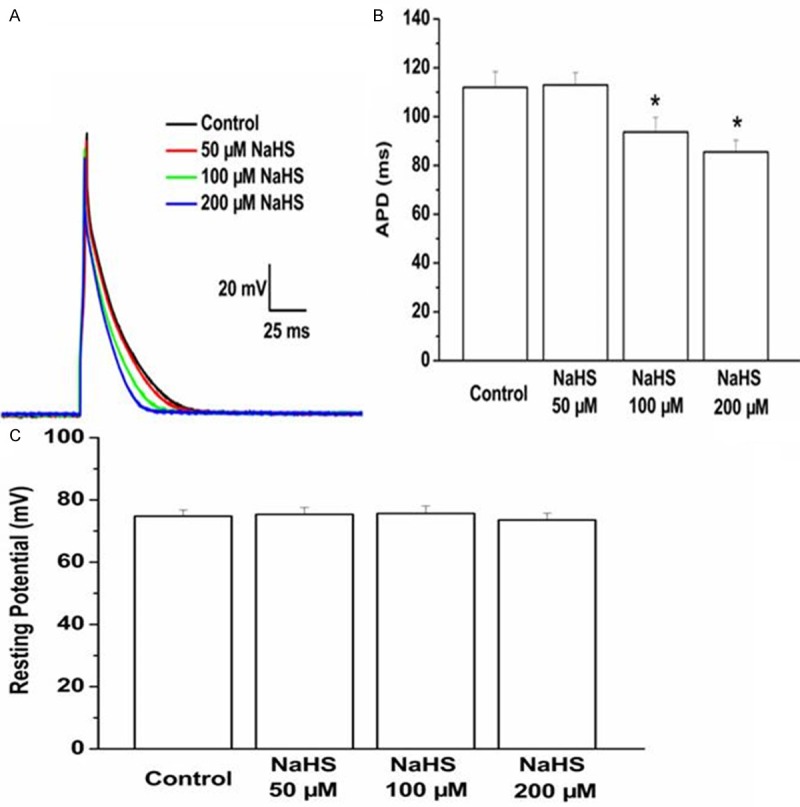
Effect of exogenous NaHS on the action potential duration and resting potential cardiomyocytes from myocardium around ischemic area were isolated and treated with NaHS. The NaHS levels were 50, 100, 200 μM. The action potential duration (A, B) and resting potential (C) were tested by patch-clamp amplifier. Data are the mean ± SD of six cells. *P < 0.05 vs control group.
Exogenous NaHS inhibited the L-type Ca2+ channels
To indentify the role of H2S on the activity of L-type Ca2+ channels, cardiomyocytes from infarct border zone were isolated ex vivo. The L-type Ca2+ current was assessed by voltage clamp. As showed in Figure 3A, 3B, the L-type Ca2+ current was reduced in a dose-dependent manner post treatment with NaHS (50, 100, 200 μM NaHS) for 5 minutes.
Figure 3.
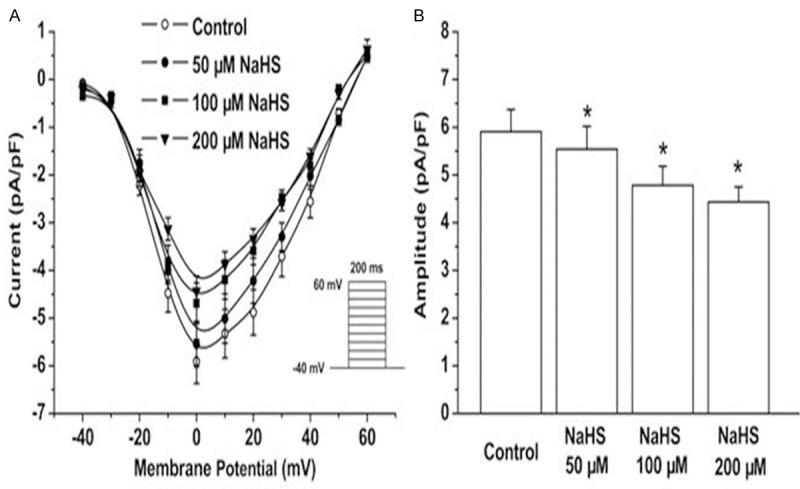
Effect of exogenous NaHS on L-type Ca2+ channels. Cardiomyocytes from myocardium around ischemic area were isolated and treated with 50, 100, 200 μM NaHS respectively. The L-type Ca2+ current was assessed by voltage clamp. The data were displayed with current pA/pF (A) and amplitude pA/pF (B). All data were calculated with the mean ± SD of six cells. *P < 0.05 vs control group.
Exogenous NaHS acitvated the ATP sensitive K+ channels
The opening of KATP channels in the myocardium plays a pivotal role in cardioprotection during ischemia and reperfusion injury. To investigate the activity of H2S on the KATP channels post-AMI, KATP current on cardiomyocytes isolated from infarct border zone was assessed by voltage clamp. The KATP current in isolated cardiomyocytes was significantly increased post 2 minutes treatment with NaHS (100, 200 μM) (Figure 4A, 4B). Thus, exogenous NaSH could activate KATP current of post-AMI cardiomyocytes.
Figure 4.
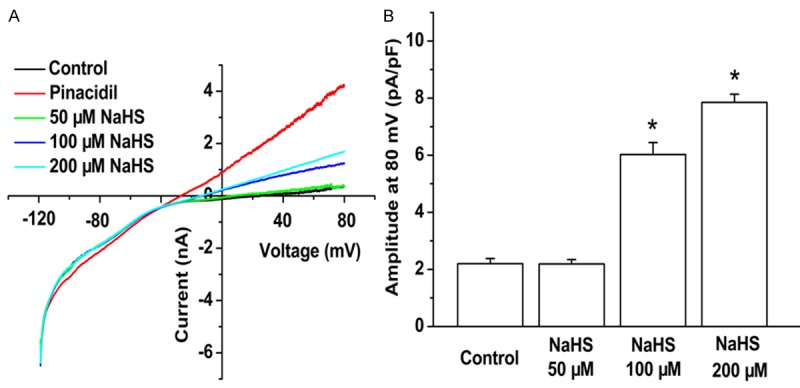
Effect of exogenous NaHS on ATP sensitive K+ channels. Cardiomyocytes from myocardium around ischemic area were isolated and treated with 50, 100, 200 μM NaHS respectively. The ATP sensitive K+ channels were assessed by voltage clamp. The data were displayed with current at different mV pA/pF (A) and amplitude at 80 mV pA/pF (B). All data were calculated with the mean ± SD of six cells. *P < 0.05 vs. control group.
Discussion
Cardiac electrical and structural remodeling plays an important role in both cardiovascular health and disease [15]. Myocardial ischemia usually accompanies with cardiomyocytes electrophysiology alterations, resulting in arrhythmias and conduction abnormalities. Exogenous H2S has been reported to be cardioprotective in various disease models. In this study, we tested serum H2S concentration in rat with different arrhythmia score post ischemic insult. The results showed that the H2S concentration was reduced with increasing arrhythmia score, which indicated that the reduction of endogenous H2S might be linked with the development of arrhythmia post ischemia/reperfusion injury. In cardiomyocytes isolated from myocardium around ischemic area, exogenous NaHS reduced the action potential duration and L-type Ca2+ current while increased KATP current. Moreover, mRNA expression of CSE in myocardium around ischemic area was upregulated.
In the cardiovascular system, H2S is predominantly generated by CSE [16]. Mice lacking the H2S-producing enzyme cystathionine γ-lyase (CSE) exhibit elevated oxidative stress, and exacerbated myocardial and hepatic I/R injury [17]. In the heart, reducing the level of H2S by inhibiting CSE increased myocardial infarct size [18]. Additionally, treatment with exogenous H2S or genetic overexpression of CSE resulted in increased endogenous H2S production, which was associated with profound protection against ischemia-induced heart failure and decreased mortality in mice with myocardial ischemia-reperfusion injury [19]. In this study, we demonstrated upregulated CSE mRNA expression in the myocardium around ischemic area. This phenomenon might reflect a compensatory local CSE expression upregulation to protection against ischemia-induced injury in this model.
In this study, cardiomyocytes from myocardium around ischemic area and were isolated and cultured. Exogenous H2S was co-incubation with these cardiomyocytes to observe the role of H2S on cardiomyocytes electrophysiology post ischmia/reperfusion injury. It is known that one of the hallmarks of secondary electrical remodeling post ischemic insult is repolarization abnormalities, specifically a prolongation of action potential duration (APD). The ionic mechanisms responsible for remodeling of the cardiac action potential involve a complex interplay between K+, Ca2+ and Na+ current [15]. These changes could markedly alter normal repolarization gradients in the heart and thus contribute to the development of abnormal heart rhythms (arrhythmogenesis) [20]. In this study, exogenous H2S treatment of cardiomyocytes isolated from myocardium around ischemic area shortens the APD compared with control group, suggesting that H2S treatment attenuated cardiomyocytes electrophysiology after ischemia-induced injury in this model.
The interplay among outward K+ currents (Ik), inward Ca2+ currents (ICa) and the late component of the inward Na+ current (INa) were changed after acute ischemic insult, which could also result in abnormal heart rhythms [15]. The opening of KATP channels in the myocardium plays a pivotal role in cardioprotection during ischemia and reperfusion injury and is a crucial component of the phenomenon termed cardiac ischemic preconditioning [21]. It was shown that sarcolemmal KATP channels were important determinants in H2S-mediated cardioprotection in an isolated cardiac myocyte model [11]. It was shown that opening of sarcolemmal KATP could lead to potassium efflux, cell membrane depolarization and shortening of the action potential duration [22]. These events might reduce calcium influx, leading to a reduction of mechanical contraction and resulting in energy sparing in the myocardium during early reperfusion injury [23,24]. Increasing evidence demonstrates that increased L-type Ca2+ density is an important mechanism for APD prolongation in mild to moderate hypertrophy [25]. Harbin Medical University, Harbin, Heilongjiang Hydrogen sulfide has been shown to inhibit L-type Ca2+ channels in the cardiomyocyte [26]. In line with above findings, H2S administration also inhibited the L-type Ca2+ channels and activated the KATP channels in this ischemia/reperfusion model. These results indicated that exogenous H2S could improve the cardiomyocytes electrical remodeling by activation of KATP channels post ischemic insult.
Understanding the cellular and molecular mechanisms of electrical remodeling might contribute to find out potential therapeutic targets for post-ischemic arrhythmias. This study suggested the relationship between endogenous H2S and post-ischemic arrhythmia. Lower level of H2S is negatively related to increased risk of arrhythmia after ischemia/reperfusion injury while exogenous H2S treatment improved the post-ischemic cardiomyocytes electrical remodeling as shown by inhibited L-type Ca2+ channels and activated KATP channels.
Acknowledgements
This work was supported by the National Science Foundation for Distinguished Young Scholars of China (Grant No. 81100097).
Disclosure of conflict of interest
None.
References
- 1.Calvert JW, Coetzee WA, Lefer DJ. Novel insights into hydrogen sulfide--mediated cytoprotection. Antioxid Redox Signal. 2010;12:1203–1217. doi: 10.1089/ars.2009.2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martelli A, Testai L, Breschi MC, Blandizzi C, Virdis A, Taddei S, Calderone V. Hydrogen sulphide: novel opportunity for drug discovery. Med Res Rev. 2012;32:1093–1130. doi: 10.1002/med.20234. [DOI] [PubMed] [Google Scholar]
- 3.Szabo C. Hydrogen sulphide and its therapeutic potential. Nat Rev Drug Discov. 2007;6:917–935. doi: 10.1038/nrd2425. [DOI] [PubMed] [Google Scholar]
- 4.Geng B, Chang L, Pan C, Qi Y, Zhao J, Pang Y, Du J, Tang C. Endogenous hydrogen sulfide regulation of myocardial injury induced by isoproterenol. Biochem Biophys Res Commun. 2004;318:756–763. doi: 10.1016/j.bbrc.2004.04.094. [DOI] [PubMed] [Google Scholar]
- 5.Yong QC, Lee SW, Foo CS, Neo KL, Chen X, Bian JS. Endogenous hydrogen sulphide mediates the cardioprotection induced by ischemic postconditioning. Am J Physiol Heart Circ Physiol. 2008;295:H1330–H1340. doi: 10.1152/ajpheart.00244.2008. [DOI] [PubMed] [Google Scholar]
- 6.Shi YX, Chen Y, Zhu YZ, Huang GY, Moore PK, Huang SH, Yao T, Zhu YC. Chronic sodium hydrosulfide treatment decreases medial thickening of intramyocardial coronary arterioles, interstitial fibrosis, and ROS production in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2007;293:H2093–2100. doi: 10.1152/ajpheart.00088.2007. [DOI] [PubMed] [Google Scholar]
- 7.Zhao W, Zhang J, Lu Y, Wang R. The vasorelaxant effect of H(2)S as a novel endogenous gaseous K(ATP) channel opener. Embo J. 2001;20:6008–6016. doi: 10.1093/emboj/20.21.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu YZ, Wang ZJ, Ho P, Loke YY, Zhu YC, Huang SH, Tan CS, Whiteman M, Lu J, Moore PK. Hydrogen sulfide and its possible roles in myocardial ischemia in experimental rats. J Appl Physiol (1985) 2007;102:261–268. doi: 10.1152/japplphysiol.00096.2006. [DOI] [PubMed] [Google Scholar]
- 9.Kondo K, Bhushan S, King AL, Prabhu SD, Hamid T, Koenig S, Murohara T, Predmore BL, Gojon G Sr, Gojon G Jr, Wang R, Karusula N, Nicholson CK, Calvert JW, Lefer DJ. H(2)S protects against pressure overload-induced heart failure via upregulation of endothelial nitric oxide synthase. Circulation. 2013;127:1116–1127. doi: 10.1161/CIRCULATIONAHA.112.000855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghuran AV, Camm AJ. Ischaemic heart disease presenting as arrhythmias. Br Med Bull. 2001;59:193–210. doi: 10.1093/bmb/59.1.193. [DOI] [PubMed] [Google Scholar]
- 11.Bian JS, Yong QC, Pan TT, Feng ZN, Ali MY, Zhou S, Moore PK. Role of hydrogen sulfide in the cardioprotection caused by ischemic preconditioning in the rat heart and cardiac myocytes. J Pharmacol Exp Ther. 2006;316:670–678. doi: 10.1124/jpet.105.092023. [DOI] [PubMed] [Google Scholar]
- 12.Bian JS, Pei JM, Cheung CS, Zhang WM, Wong TM. kappa-opioid receptor stimulation induces arrhythmia in the isolated rat heart via the protein kinase C/Na(+)-H(+) exchange pathway. J Mol Cell Cardiol. 2000;32:1415–1427. doi: 10.1006/jmcc.2000.1175. [DOI] [PubMed] [Google Scholar]
- 13.Liu S, Melchert RB, Kennedy RH. Inhibition of L-type Ca2+ channel current in rat ventricular myocytes by terfenadine. Circ Res. 1997;81:202–210. doi: 10.1161/01.res.81.2.202. [DOI] [PubMed] [Google Scholar]
- 14.Oketani N, Kakei M, Ichinari K, Okamura M, Miyamura A, Nakazaki M, Ito S, Tei C. Regulation of K(ATP) channels by P(2Y) purinoceptors coupled to PIP(2) metabolism in guinea pig ventricular cells. Am J Physiol Heart Circ Physiol. 2002;282:H757–765. doi: 10.1152/ajpheart.00246.2001. [DOI] [PubMed] [Google Scholar]
- 15.Cutler MJ, Jeyaraj D, Rosenbaum DS. Cardiac electrical remodeling in health and disease. Trends Pharmacol Sci. 2011;32:174–180. doi: 10.1016/j.tips.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pan LL, Liu XH, Gong QH, Yang HB, Zhu YZ. Role of cystathionine gamma-lyase/hydrogen sulfide pathway in cardiovascular disease: a novel therapeutic strategy? Antioxid Redox Signal. 2012;17:106–118. doi: 10.1089/ars.2011.4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.King AL, Polhemus DJ, Bhushan S, Otsuka H, Kondo K, Nicholson CK, Bradley JM, Islam KN, Calvert JW, Tao YX, Dugas TR, Kelley EE, Elrod JW, Huang PL, Wang R, Lefer DJ. Hydrogen sulfide cytoprotective signaling is endothelial nitric oxide synthase-nitric oxide dependent. Proc Natl Acad Sci U S A. 2014;111:3182–3187. doi: 10.1073/pnas.1321871111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sivarajah A, McDonald MC, Thiemermann C. The production of hydrogen sulfide limits myocardial ischemia and reperfusion injury and contributes to the cardioprotective effects of preconditioning with endotoxin, but not ischemia in the rat. Shock. 2006;26:154–161. doi: 10.1097/01.shk.0000225722.56681.64. [DOI] [PubMed] [Google Scholar]
- 19.Calvert JW, Elston M, Nicholson CK, Gundewar S, Jha S, Elrod JW, Ramachandran A, Lefer DJ. Genetic and pharmacologic hydrogen sulfide therapy attenuates ischemia-induced heart failure in mice. Circulation. 2010;122:11–19. doi: 10.1161/CIRCULATIONAHA.109.920991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spragg DD, Akar FG, Helm RH, Tunin RS, Tomaselli GF, Kass DA. Abnormal conduction and repolarization in late-activated myocardium of dyssynchronously contracting hearts. Cardiovasc Res. 2005;67:77–86. doi: 10.1016/j.cardiores.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 21.Gross GJ, Fryer RM. Sarcolemmal versus mitochondrial ATP-sensitive K+ channels and myocardial preconditioning. Circ Res. 1999;84:973–979. doi: 10.1161/01.res.84.9.973. [DOI] [PubMed] [Google Scholar]
- 22.Cole WC, McPherson CD, Sontag D. ATP-regulated K+ channels protect the myocardium against ischemia/reperfusion damage. Circ Res. 1991;69:571–581. doi: 10.1161/01.res.69.3.571. [DOI] [PubMed] [Google Scholar]
- 23.Pan TT, Feng ZN, Lee SW, Moore PK, Bian JS. Endogenous hydrogen sulfide contributes to the cardioprotection by metabolic inhibition preconditioning in the rat ventricular myocytes. J Mol Cell Cardiol. 2006;40:119–130. doi: 10.1016/j.yjmcc.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 24.Ji Y, Pang QF, Xu G, Wang L, Wang JK, Zeng YM. Exogenous hydrogen sulfide postconditioning protects isolated rat hearts against ischemia-reperfusion injury. Eur J Pharmacol. 2008;587:1–7. doi: 10.1016/j.ejphar.2008.03.044. [DOI] [PubMed] [Google Scholar]
- 25.Yang B, Lin H, Xiao J, Lu Y, Luo X, Li B, Zhang Y, Xu C, Bai Y, Wang H, Chen G, Wang Z. The muscle-specific microRNA miR-1 regulates cardiac arrhythmogenic potential by targeting GJA1 and KCNJ2. Nat Med. 2007;13:486–491. doi: 10.1038/nm1569. [DOI] [PubMed] [Google Scholar]
- 26.Sun YG, Cao YX, Wang WW, Ma SF, Yao T, Zhu YC. Hydrogen sulphide is an inhibitor of L-type calcium channels and mechanical contraction in rat cardiomyocytes. Cardiovasc Res. 2008;79:632–641. doi: 10.1093/cvr/cvn140. [DOI] [PubMed] [Google Scholar]


