Abstract
Melanoma associated antigen-A (MAGE-A) is an oncogene and correlated with tumor initiation and development. However the roles of MAGE-A9 in non-small cell lung cancer (NSCLC) are still unknown. We investigated MAGE-A9 mRNA expression in 18 tumor tissues of NSCLC by qRT-PCR and MAGE-A9 protein expression in 213 NSCLC samples of tissue arrays by immunohistochemical staining. We assessed the relationship between MAGE-A9 expression and clinical parameters. The results showed that the high expression of MAGE-A9 protein in NSCLC tumor cells were commonly present in squamous cell carcinomas (P = 0.030). It was also related to larger tumor diameter, lymph node metastasis and later stage grouping with TNM classification (all P < 0.05). Whereas the expression of MAGE-A9 in stromal cells was higher in squamous cell carcinomas as well. Cox regression univariate and multivariable analysis revealed that MAGE-A9 expression in tumor cells of NSCLC (P < 0.001) is an independent prognostic factor in five-year overall survival rate. We concluded that the molecular assessment of MAGEA9 could be considered to improve prognostic evaluation and to identify eligible patients for potential target therapy.
Keywords: Melanoma associated antigen-A 9, non-small cell lung cancer, tumor cell, stromal, immunohistochemistry, prognosis
Introduction
Lung cancer is the leading cause of cancer-related deaths in the United States and worldwide [1]. Non-small cell lung cancer (NSCLC), which arises from epithelial cells of the airways, accounts for 85% of lung cancer [2]. The overall 5-year survival rate for NSCLC is only about 17.1% [3]. In the last decades, remarkable progress has been made in the treatment of lung cancer as a result of combined chemotherapy, radiotherapy, development in surgical and diagnostic imaging techniques [4,5]. However, despite these advances, the number of deaths is over 160,000 per year, representing 25% of all cancer related deaths [2]. Thus, in the era of ‘personalized’ medicine, biomarkers play a critical role. Not only have biomarkers contributed greatly to early detection, they have also led to significant improvements in treatment outcomes [6-8].
Cancer/testis (CT) antigens are encoded by genes that have little or no expression in somatic adult tissues, but differentially expressed in a variety of human cancers. Its potent immunogenicity has led to intense research as therapeutic vaccines [9-12]. Melanoma-associated antigen (MAGE) genes are the best characterized members of the CTA family, which classified into type 1 (MAGE-A, MAGE-B, and MAGE-C) and type 2 (MAGE-D) based on differences in tissue-specific expression patterns and gene structure [13]. Type 2 MAGE genes are almost universally expressed [13]. While type 1 MAGE expression has been documented in a broad variety of malignancies [14-19]. MAGE-A is a multigene family consisting of 12 homologous genes MAGE-A1 to MAGE-A12 located at chromosome Xq28 [20,21], where they code for antigens recognized by cytolytic T lymphocytes. The promoters and the first exons of the MAGEA genes show considerable variability, suggesting that the existence of this gene family enables the same function to be expressed under different transcriptional controls.
Studies have shown some of the MAGE-A gene expressed in lung cancer and become the target of choice for NSCLC immune-therapy [22-25]. MAGE-A9 is frequently expressed in urinary tumor, can provide additional prognostic information in renal cell carcinoma and bladder cancer, besides, it was also overexpressed in cutaneous T-cell lymphomas, esophageal adenocarcinomas as well [26-30]. At present, there are only few reports about MAGE-A9 in NSCLC and the small size of the sample may limit the diagnostic and prognostic value [31]. As far as we known, MAGE-A9 expression in protein level and its correlation with clinical parameters have not been evaluated yet. Thus we detected MAGE-A9 gene and protein expression in NSCLC samples to analyze the associations between MAGE-A9 expression and clinicopathologic data in a group of patients with NSCLC. In contrast to previous study about MAGE-A9 expressed in renal cancer, we find out that MAGE-A9 expressed in stroma of NSCLC tissue as well. The tumor microenvironment is considered pro-oncogenic for reasons that, among others, involve the stimulation of cancer cell growth. Accumulating evidence indicates that the tumor immune microenvironment constitutes a robust prognostic indicator, recently, in early-stage lung adenocarcinoma, the immune microenvironment conveys robust prognostic information [32]. In this report, we also correlate them with clinic pathological parameters including patient outcome data by Kaplan-Meier survival and Cox regression analyses.
Material and methods
Patients and resources
NSCLC and matched adjacent non-cancerous tissues (n = 213; 141 squamous cell carcinomas, 60 adenocarcinomas, 12 other histological type carcinomas) and matched peritumoral tissue specimens (n = 114) were excised from fresh surgical samples at the Affiliated Hospital of Nangtong university from January 2004 to October 2009. The 5-year actuarial overall survival was calculated from the date of surgery until the date of death or last follow-up appointment. The diagnosis was confirmed according to the recently published guidelines from the recent national comprehensive cancer network (NCCN). All patients did not receive chemotherapy or radiotherapy prior to surgery.
The study was approved by the Hospital’s Ethical Research Committee. Informed consent waivers were obtained for all patients in this study to allow collection of retrospective clinical data and to conduct an analysis of archived paraffin-embedded specimens.
qRT-PCR
Total RNA was isolated from 18 lung carcinomas and matched adjacent non-neoplastic tissues using the TRIzol reagent (Invitrogen, USA), according to the manufacturer’s instructions. RNA concentration was determined using the NanoDrop ND-1000 spectrophotometer (Nano-Drop Technologies, USA). Following isolation of RNA, The RNA was reverse transcribed (Invitrogen) into combinational DNA (cDNA) at 16°C for 30 min, 42°C for 30 min, and 85°C for 5 min. MAGE-A9 specific oligonucleotide primers: sense, 5’-CTCTGGTAAAGTGGATATTGT-3’; reverse, 5’-GGTGGAATCATATTGGAACA-3’ (85bp). The quality of all cDNAs was assured by confirmed expression of the housekeeping genes GAPDH. The primers were as follows: sense: 5’-CTCTGGTAAAGTGGATATTGT-3’, reverse: 5’-GGTGGAATVATATTGGAACA-3’, (106 bp). PCR amplification was conducted with SYBR Green Master Mix (Applied Biosystems, Foster City, Calif). The reaction mixtures were incubated in an ABI 7500 Fast Real-Time PCR system. Thermal cycles were: 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. The cycle threshold (Ct) values were calculated by the SDS 2.0.1 software from ABI.
Immunohistochemical staining and evaluation
As for immunohistochemical analysis, we used Tissue Microarray System (Quick-Ray, UT06, UNITMA, Korea) in the department of clinical pathology, Nantong University Hospital, Jiangsu, China to produce 2 mm thick Paraffin-embedded NSCLC TMA sections. The TMA analysis was used as quality control for H & E staining. Tissue sections were deparaffinized and rehydrated in graded ethanol. Antigen retrieval was performed by boiling in ethylene diamine tetracetic acid buffer, pH 6.0, for 3 minutes in a pressure cooker. Endogenous peroxidase activity was quenched with 3% hydrogen peroxide for 30 minutes. Sections were then incubated with a polyclonal antibody specific to MAGEA9 (AP6170a, 2.5 μg/ml dilution; Abgent, San Diego, CA, USA) at 4°C overnight and following incubated with biotinylated anti-rabbit secondary antibody at 37°C for 30 min. The slides were then processed using horseradish peroxidase and colorized with 3, 3-diaminobenzidine (DAB) chromogen solution and counterstained with hematoxylin. The staining intensity of MAGE-A9 for each slide was evaluated and scored by 2 independent pathologists. Staining intensity was scored according to four grades: 0, 1, 2 or 3, ranging from negative and weak, to strong intensity. The percentage of MAGE-A9 positive cells was scored as follows: 0 for 0-20%, 1 for 21-50%, 2 for 51-75% and 3 for 76-100%. The product of the percentage and intensity scores was used as the final staining score as described previously [33]. The cutoff point for the MAGE-A9 expression score that was statistically significant in terms of overall survival (OS) was set using the X-tile software program (The Rimm Lab at Yale University; http://www.tissuearray.org/rimmlab) as described previously [34]. The degree of cytoplasm MAGE-A9 staining was quantified using a two-level grading system, and staining scores were defined as follows: 0-4, low and none expression, and 5-9, high expression.
Statistical analysis
The MAGE-A9 mRNA expression in fresh-frozen samples of NSCLC compared with the matched peritumoral tissue was analyzed with Wilcoxon signed rank nonparametric test. χ2 tests were performed in statistical analyses to evaluate whether MAGE-A9 expression was correlated with clinic-pathologic parameters. For TMA slides, the following clinical data were evaluated: gender, age, tumor diameter, and other clinicopathlogic information, as well as patients’ outcome survival curves were calculated using the Kaplane-meier method. Factors shown to be of prognostic significance in the univariate models were evaluated in a multivariable Cox regression model. For all analyses, a P value < 0.05 was regarded as statistically significant. Data were analyzed using SPSS20 statistic software (SPSS Inc, Chicago, IL) and STATA 12.0 (StataCorp, College Station, TX, USA).
Results
MAGE-A9 mRNA expression was detected in NSCLC and peritumoural tissues
Total RNA was extracted from the freshly frozen NSCLC tissues and subjected to qRT-PCR to investigate MAGE-A9 mRNA expression. We also investigated samples from adjacent matched tumor tissues. When normalized to GAPDH, the mean expression levels of MAGE-A9 mRNA in NSCLC and corresponding non-cancerous tissue were 438.049 and 5.954 (P = 0.0397), respectively. MAGE-A9 expression was 4.57-fold higher on average in the cancer samples than in non-malignant tissues (Figure 1).
Figure 1.
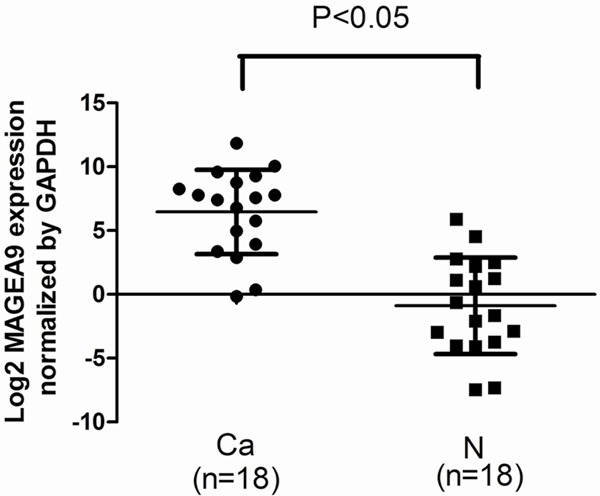
Expression of MAGE-A9 mRNA was detected in non-small cell lung cancer (NSCLC) and peritumoral tissues. Quantitative real-time polymerase chain reaction (qPCR) was performed to elucidate MAGE-A9 mRNA expression levels in NSCLC (cancer) tissues compared with peritumoral (normal) tissues. Normalized to β-Actin mRNA levels, the MAGE-A9 mRNA level in cancerous tissues is significantly higher than that in the corresponding noncancerous tissues (P < 0.05). Error bar is the standard error.
MAGE-A9 protein expression patterns were demonstrated in tissue arrays of NSCLC
To confirm MAGE-A9 expression in NSCLC at the tissue level, TMA-based immunohistochemical (IHC) studies were carried out to complement the gene expression studies. Positive staining for MAGE-A9 was mainly localized to tumor cells in the cytoplasm and interstitial at different levels (Figure 2). High MAGE-A9 expression was detected in 111 (52.11%) of the 213 NSCLC tumors, compared with only 35 (30.70%) of the 114 peritumoral lung tissue samples. The data showed statistical significance using χ2 test analysis (χ2 = 13.7744, P < 0.001) and was consistent with MAGE-A9 mRNA levels in NSCLCs. Besides, MAGE-A9 expression in stromal cells was observed in 36.84% of peritumoral lung tissue samples and 37% of the NSCLC tumors, respectively, which did not reach statistical significance.
Figure 2.
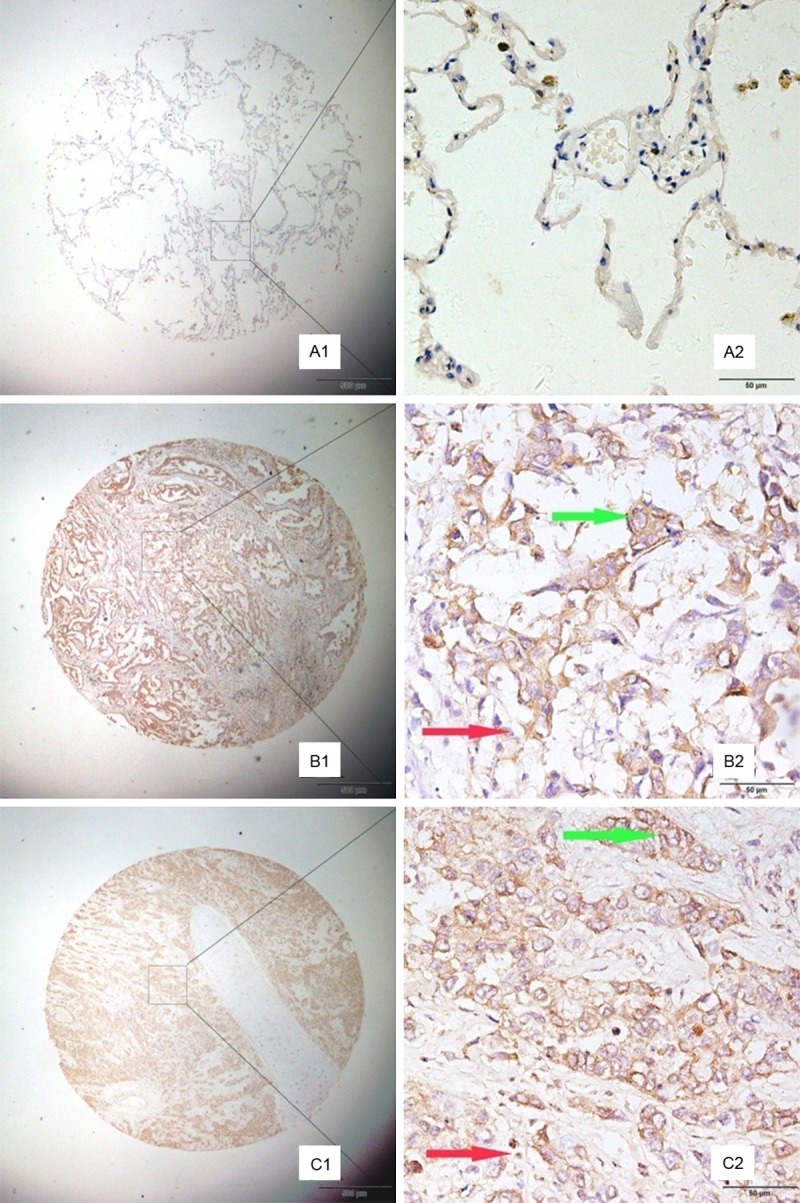
Representative patterns of MAGE-A9 protein expression showed in non-small cell lung cancer (NSCLC) and adjacent noncancerous tissues. Case A, the adjacent tissue, shows negative staining. Case B, the lung adenocarcinoma tissue, presents high expression level of MAGE-A9 (green arrow). The stromal tissue was also detected MAGE-A9expression (red arrow). Case C, lung squamous cell carcinoma tissue, shows strong MAGE-A9 protein expression in both cancer cells (green arrow) and stomal cells (red arrow). Original magnification: (A1-C1) × 40, (A2-C2) × 400.
Expression of MAGE-A9 antigens was correlated with clinical parameters
We analyzed the association of MAGE-A9 expression with clinic-pathologic variables in the study patients. We observed a statistically significant correlation between MAGE-A9 overexpression and outcome of these patients (Table 1) according to the cutoff point for the MAGE-A9 expression score as determined using the X-tile software program. High expression of MAGE-A9 in NSCLC tumors was significantly associated with histological type (χ2 = 4.7222, P = 0.030), tumor diameter (χ2 = 7.8410, P = 0.005), Lymph node metastasis (χ2 = 6.1128, P = 0.047), TNM classification (χ2 = 7.6263, P = 0.022). By contrast, no statistically significant association was found for gender, age, differentiation. Moreover, MAGE-A9 expression in interstitial according to age, positivity was significantly greater in patients of squamous carcinoma (χ2 = 4.5969, P = 0.032). The expression in interstitial of NSCLC tissues was not associated with other clinical parameters.
Table 1.
Association of MAGE-A9 expression with clinical characteristics of NSCLC
| Groups | No. | Cytoplasm staining of MAGE-A9 | Interstitial staining of MAGE-A9 | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Low or no expression (%) | High expression (%) | Pearson X2 | P value | Low or no expression (%) | High expression (%) | Pearson X2 | P value | ||
| Total | 213 | 102 (47.89) | 111 (52.11) | 115 | 98 (37.0) | ||||
| Gender | |||||||||
| Male | 160 | 72 (45.00) | 88 (55.00) | 2.1481 | 0.143 | 87 (54.37) | 73 (45.630) | 0.0382 | 0.845 |
| Female | 53 | 30 (56.60) | 23 (43.40) | 28 (52.83) | 25 (41.17) | ||||
| Age | |||||||||
| ≤ 60 years | 71 | 33 (46.48) | 38 (53.52) | 0.0847 | 0.771 | 45 (63.38) | 26 (36.62) | 3.7799 | 0.052 |
| > 60 years | 142 | 69 (48.59) | 73 (51.41) | 70 (49.30) | 72 (50.70) | ||||
| Differentiation | |||||||||
| Well and Moderately | 141 | 66 (46.81) | 75 (53.19) | 0.1130 | 0.737 | 75 (53.19) | 66 (46.81) | 0.0659 | 0.797 |
| Poorly | 69 | 34 (49.28) | 35 (50.72) | 38 (55.07) | 31 (44.93) | ||||
| Unknown | 3 | 2 | 1 | 2 | 1 | ||||
| Tumor size | |||||||||
| ≤ 3 cm | 74 | 44 (59.46 ) | 30 (40.54) | 7.8410 | 0.005* | 39 (52.70) | 35 (47.30) | 0.0024 | 0.961 |
| > 3 cm | 128 | 50 (39.06) | 78 (60.94) | 67 (52.34) | 61 (47.66) | ||||
| Unknown | 11 | 8 | 3 | 9 | 2 | ||||
| Histological type | |||||||||
| Squamous cell carcinoma | 141 | 61 (43.26) | 80 (56.74) | 4.7222 | 0.030* | 82 (58.16) | 59 (41.84) | 4.5969 | 0.032* |
| Adenocarcinoma | 60 | 36 (60.00) | 24 (40.00) | 25 (41.67) | 35 (58.33) | ||||
| Other | 12 | 5 | 7 | 8 | 4 | ||||
| Lymph node metastasis | |||||||||
| No regional lymph node metastasis | 128 | 69 (53.91) | 59 (46.09) | 6.1128 | 0.047* | 70 (54.69) | 58 (45.31) | 0.2436 | 0.885 |
| Metastasis in ipsilateral peribronchial | 49 | 20 (40.82) | 29 (59.18) | 26 (53.060) | 23 (46.94) | ||||
| Metastasis in mediastinal | 34 | 11 (32.35) | 23 (67.65) | 17 (50.00) | 17 (50.00) | ||||
| Unknown | 2 | 2 | 2 | ||||||
| TNM Classification | |||||||||
| I | 74 | 45 (60.81) | 29 (39.19) | 7.6263 | 0.022* | 39 (52.70) | 35 (47.30) | 0.2577 | 0.879 |
| II | 121 | 50 (41.32) | 71 (58.68) | 67 (55.37) | 54 (44.63) | ||||
| III and IV | 18 | 7 (38.89) | 11 (61.11) | 9 (50.00) | 9 (50.00) | ||||
NSCLC, non-small cell lung cancer.
P < 0.05.
MAGE-A9 expression was related to patient survival
Univariate analysis showed the correlations of TNM classification (P = 0.011), lymph node metastasis (P = 0.001), tumor diameter (P = 0.017), positive MAGE-A9 status in tumors cells (P < 0.001) and in interstitial tissue (P < 0.001) with lifespan of patients in NSCLC (Table 2). All these factors were included in a multivariable analysis. High MAGE-A9 expression in tumor tissue (P < 0.001), high MAGE-A9 expression in interstitial tissue (P < 0.001) and TNM classification (P = 0.021) were identified as independent predictive factors for poor outcome of NSCLC. Kaplan-Meier survival curves again showed that high MAGE-A9 expression in NSCLC tissues and stromal had a significantly shorter survival time compared with those with no or low MAGE-A9 protein expression (Figure 3).
Table 2.
Univariate and multivariable analyses of prognostic factors in NSLCL for 5-year survival
| Variable | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| HR | P value | 95% CI | HR | P value | 95% CI | |
| Cytoplasm expression of MAGE-A9 | ||||||
| High versus low | 3.104 | 0.001* | 2.263-4.257 | 2.334 | 0.001* | 1.664-3.274 |
| Stromal expression of MAGE-A9 | ||||||
| High versus low | 2.283 | 0.001* | 1.668-3.123 | 1.942 | 0.001* | 1.407-2.681 |
| Gender | ||||||
| Male versus female | 0.843 | |||||
| Age (years) | ||||||
| < 60 versus ≥ 60 | 0.645 | |||||
| Tumor size (cm) | ||||||
| ≤ 3 versus > 3 | 1.488 | 0.017* | 1.073-2.063 | 0.914 | 0.648 | 0.884-11.448 |
| Differentiation | ||||||
| Well and mod versus poorly | 0.252 | |||||
| Histological type | ||||||
| Squamous cell carcinoma versus adenocarcinoma | 0.256 | |||||
| Lymph node metastasis | ||||||
| No regional lymph node versus MIP versus MIM | 1.432 | 0.001* | 1.170-1.754 | 1.200 | 0.089 | 0.973-1.481 |
| TNM classification | ||||||
| stage I versus stage II versus stage III/IV) | 1.320 | 0.011* | 1.064-1.637 | 1.403 | 0.021* | 1.053-1.870 |
CI, confidence interval; HR, hazard ratio; MIM, metastasis in mediastinal; MIP, metastasis in ipsilateral peribronchial; NSCLC, non-small cell lung cancer.
Figure 3.
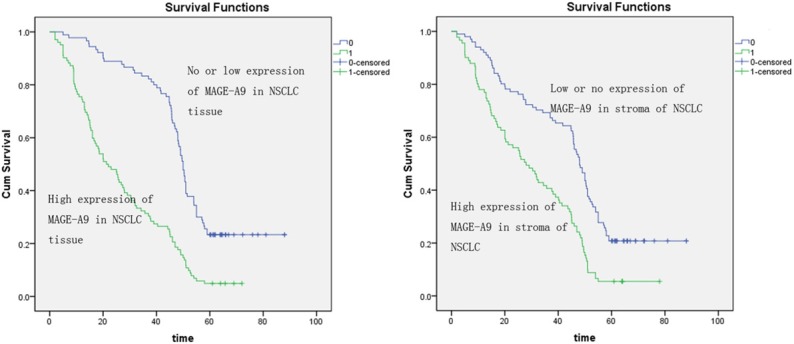
The Kaplan-Meier analysis was used for investigation of the relationship between clinicopathologic factors and overall survival of non-small cell lung cancer (NSCLC) patients. Curves show the calculation of MAGE-A9 expression in NSCLC tissues with the Kaplane-Meier method. A. The high expression of the MAGE-A9 high expression group (green line) has significantly less survival than the low expression of the MAGE-A9 low expression group (blue line). B. The curves show the calculation of MAGE-A9 expression in the stroma of NSCLC with the Kaplane-Meier method. The high expression of the MAGE-A9 high expression in the stroma of NSCLC group (blue line) has significantly less survival than the low expression of the MAGE-A9 low expression in stroma of NSCLC group (green line).
Discussion
The majority of lung cancer patients are not candidates for surgery because they have advanced diseases such as nodal and/or distant metastases. Despite the introduction of new chemotherapeutic agents and molecularly targeted drugs, the outcomes remain poor, emphasizing the need for new treatment approaches [35]. CTAs are currently major focus of cancer research due to their potential as targets for cancer-specific immunotherapy [9-12]. As the best characterized members of the CTA family, MAGEA-derived immunogens have been taken into clinical trials [36-38].
MAGEA genes are frequently expressed in non-small cell lung cancers [23,41-43]; MAGE-A3 expression was observed in 35% of lung cancer patients through a multi-center study [39]. Whereas MAGE-A4 was reported to be expressed in 48% of non-small cell lung carcinomas, moreover, 90% of lung carcinomas expressing MAGE-A4 were classified as squamous cell carcinomas [22]. Based on these findings, MAGE-A based vaccination has been developed as a promising treatment modality for lung cancer [40]. As another important member of MAGE-A family, overexpression of MAGE-A9 was reported in many malignancies except lung cancer. To our knowledge, this is the first investigation about both the expression of MAGE-A9 mRNA and protein in NSCLC tumors compared with the matched peritumoral lung tissue.
In the present study, we detected expression of MAGE-A9 mRNA in fresh NSCLC and adjacent noncancerous tissues using qRT-PCR. We found a significantly higher level in carcinoma tissues, which was in agreement with the high prevalence of MAGE-A9 gene in other tumors. For further proof, we using a TMA to emphasized the prognostic value of MAGE-A9 expression in NSCLC. Different from previous study which carried out that MAGE-A9 expressed in nuclear and cytoplasm of renal cell carcinoma, our immunohistochemistry demonstrated high MAGE-A9 protein expression in both NSCLC tumor cell and stromal cell. As we known, malignant tumors consist of neoplastic cancer cells and stromal cells [27]. During the development of malignancy, the tumor microenvironment progressively changes and becomes “fixed” in a cancer-associated state that ultimately favors and further promotes tumor growth [41,42]. Unlike tumor cells, stromal cell types within the tumor microenvironment are genetically stable and thus represent an attractive therapeutic target with reduced risk of resistance and tumor recurrence. In the present investigation, high MAGE-A9 expression was correlated significantly with large tumor diameter, regional lymph node metastasis and later TNM stage in NSCLC patients. Furthermore, high MAGE-A9 expression along with later TNM stage was identified to be an independent predictive factor for poor outcome in NSCLC. However, our results are corroborate the earlier studies of high prevalence of other MAGE-A genes and protein expression in non-small cell lung cancers as well as their more frequent presence in squamous cell carcinomas compared with adenocarcinomas [43-46]. These results and ours suggest that MAGE-A9 might be expressed in diverse types of cancers and will improve our understanding of MAGE-A9 association with cancer.
Although the physiologic function of MAGE-A proteins remains largely unknown, it was shown that MAGE-A1 transcription was induced in cell cultures treated with demethylating agent 5’-aza-2’-deoxycytidine, and other MAGE genes are presumed to be regulated in a similar manner [47,48]. DNA hypermethylation and histone deacetylation is responsible for the mechanism underlying MAGE-A9 gene silencing [49]. Evidence increasing suggests their involvements in early carcinogenesis of the lung [50], involving the regulation of apoptosis and cell cycle progression [51]. MAGE-A proteins are also more frequently expressed in chemotherapy (paclitaxel) resistant compared with chemotherapy susceptible ovarian cancer, melanoma and multiple myeloma cell lines [52]. Therefore, it can be speculated that the MAGE-A9 expression favors tumor cell survival and that MAGE-A9 proteins function as the oncoproteins. Better insights in the function of these genes may shed a light on the link between NSCLC and tumor growth. They could be the targets in anti-tumor therapies.
There are some limitations of this research, such as the study on inhibition or overexpression of the gene which hasn’t been carried on. It should be the subject of future study. Furthermore, the molecular mechanisms underlying MAGE-A9 involvement in cell proliferation, migration and chemosensitivity should be analyzed in vivo and in vitro. If the results from further investigations support our findings, the combination of an appropriate strategy with targeting MAGE-A9 might be expected as a high efficacy of the chemo-immunotherapeutic approach.
Acknowledgements
This study was supported by the Social Development and Applied Research Projects (HS 201407), Nantong City 226001, Jiangsu Province, China.
Disclosure of conflict of interest
None.
References
- 1.Hernandez BY, Green MD, Cassel KD, Pobutsky AM, Vu V, Wilkens LR. Preview of Hawaii Cancer Facts and Figures 2010. Hawaii Med J. 2010;69:223–224. [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 3.Siegel R, DeSantis C, Virgo K, Stein K, Mariotto A, Smith T, Cooper D, Gansler T, Lerro C, Fedewa S, Lin C, Leach C, Cannady RS, Cho H, Scoppa S, Hachey M, Kirch R, Jemal A, Ward E. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62:220–241. doi: 10.3322/caac.21149. [DOI] [PubMed] [Google Scholar]
- 4.Akin O, Brennan SB, Dershaw DD, Ginsberg MS, Gollub MJ, Schöder H, Panicek DM, Hricak H. Advances in oncologic imaging: update on 5 common cancers. CA Cancer J Clin. 2012;62:364–393. doi: 10.3322/caac.21156. [DOI] [PubMed] [Google Scholar]
- 5.McCloskey P, Balduyck B, Van Schil PE, Faivre-Finn C, O’Brien M. Radical treatment of non-small cell lung cancer during the last 5 years. Eur J Cancer. 2013;49:1555–1564. doi: 10.1016/j.ejca.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 6.Pal SK, Figlin RA, Reckamp K. Targeted therapies for non-small cell lung cancer: an evolving landscape. Mol Cancer Ther. 2010;9:1931–1944. doi: 10.1158/1535-7163.MCT-10-0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kelly K, Huang C. Biological agents in non-small cell lung cancer: a review of recent advances and clinical results with a focus on epidermal growth factor receptor and vascular endothelial growth factor. J Thorac Oncol. 2008;3:664–673. doi: 10.1097/JTO.0b013e3181758141. [DOI] [PubMed] [Google Scholar]
- 8.Parkinson DR, Johnson BE, Sledge GW. Making personalized cancer medicine a reality: challenges and opportunities in the development of biomarkers and companion diagnostics. Clin Cancer Res. 2012;18:619–624. doi: 10.1158/1078-0432.CCR-11-2017. [DOI] [PubMed] [Google Scholar]
- 9.Scanlan MJ, Gure AO, Jungbluth AA, Old LJ, Chen YT. Cancer/testis antigens: an expanding family of targets for cancer immunotherapy. Immunol Rev. 2002;188:22–32. doi: 10.1034/j.1600-065x.2002.18803.x. [DOI] [PubMed] [Google Scholar]
- 10.Caballero OL, Chen YT. Cancer/testis (CT) antigens: potential targets for immunotherapy. Cancer Sci. 2009;100:2014–2021. doi: 10.1111/j.1349-7006.2009.01303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brichard VG, Lejeune D. GSK’s antigen-specific cancer immunotherapy programme: pilot results leading to Phase III clinical development. Vaccine. 2007;25(Suppl 2):B61–71. doi: 10.1016/j.vaccine.2007.06.038. [DOI] [PubMed] [Google Scholar]
- 12.Bodey B. Cancer-testis antigens: promising targets for antigen directed antineoplastic immunotherapy. Expert Opin Biol Ther. 2002;2:577–584. doi: 10.1517/14712598.2.6.577. [DOI] [PubMed] [Google Scholar]
- 13.Barker PA, Salehi A. The MAGE proteins: emerging roles in cell cycle progression, apoptosis, and neurogenetic disease. J Neurosci Res. 2002;67:705–712. doi: 10.1002/jnr.10160. [DOI] [PubMed] [Google Scholar]
- 14.Sakata M. Expression of MAGE gene family in lung cancers. Kurume Med J. 1996;43:55. doi: 10.2739/kurumemedj.43.55. [DOI] [PubMed] [Google Scholar]
- 15.Otte M, Zafrakas M, Riethdorf L, Pichlmeier U, Loning T, Jänicke F, Pantel K. MAGE-A gene expression pattern in primary breast cancer. Cancer Res. 2001;61:6682–6687. [PubMed] [Google Scholar]
- 16.Li M, Yuan YH, Han Y, Liu YX, Yan L, Wang Y, Gu J. Expression profile of cancer-testis genes in 121 human colorectal cancer tissue and adjacent normal tissue. Clin Cancer Res. 2005;11:1809–1814. doi: 10.1158/1078-0432.CCR-04-1365. [DOI] [PubMed] [Google Scholar]
- 17.Yanagawa N, Tamura G, Oizumi H, Endoh M, Motoyama T. MAGE expressions mediated by demethylation of MAGE promoters induce progression of non-small cell lung cancer. Anticancer Res. 2011;31:171–175. [PubMed] [Google Scholar]
- 18.Kim J, Reber HA, Hines OJ, Kazanjian KK, Tran A, Ye X, Amersi FF, Martinez SR, Dry SM, Bilchik AJ, Hoon DS. The clinical significance of MAGEA3 expression in pancreatic cancer. Int J Cancer. 2006;118:2269–2275. doi: 10.1002/ijc.21656. [DOI] [PubMed] [Google Scholar]
- 19.Pereira CM, Gomes CC, De Fatima Correia Silva J, Bucciarelli Rodriguez M, Barbosa AA, Gomez RS. Evaluation of MAGE A1 in oral squamous cell carcinoma. Oncol Rep. 2012;27:1843–1848. doi: 10.3892/or.2012.1736. [DOI] [PubMed] [Google Scholar]
- 20.Chomez P, De Backer O, Bertrand M, De Plaen E, Boon T, Lucas S. An overview of the MAGE gene family with the identification of all human members of the family. Cancer Res. 2001;61:5544–5551. [PubMed] [Google Scholar]
- 21.Chen YT, Scanlan MJ, Sahin U, Tureci O, Gure AO, Tsang S, Williamson B, Stockert E, Pfreundschuh M, Old LJ. A testicular antigen aberrantly expressed in human cancers detected by autologous antibody screening. Proc Natl Acad Sci U S A. 1997;94:1914–1918. doi: 10.1073/pnas.94.5.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peikert T, Specks U, Farver C, Erzurum SC, Comhair SA. Melanoma antigen A4 is expressed in non–small cell lung cancers and promotes apoptosis. Cancer Res. 2006;66:4693–4700. doi: 10.1158/0008-5472.CAN-05-3327. [DOI] [PubMed] [Google Scholar]
- 23.Karimi S, Mohammadi F, Porabdollah M, Mohajerani SA, Khodadad K, Nadji SA. Characterization of melanoma-associated antigena genes family differential expression in non-small-cell lung cancers. Clin Lung Cancer. 2012;13:214–219. doi: 10.1016/j.cllc.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 24.Kim SH, Lee S, Lee CH, Lee MK, Kim YD, Shin DH, Choi KU, Kim JY, Park do Y, Sol MY. Expression of cancer-testis antigens MAGE-A3/6 and NY-ESO-1 in non-small-cell lung carcinomas and their relationship with immune cell infiltration. Lung. 2009;187:401–411. doi: 10.1007/s00408-009-9181-3. [DOI] [PubMed] [Google Scholar]
- 25.Tajima K, Obata Y, Tamaki H, Yoshida M, Chen YT, Scanlan MJ, Old LJ, Kuwano H, Takahashi T, Takahashi T, Mitsudomi T. Expression of cancer/testis (CT) antigens in lung cancer. Lung Cancer. 2003;42:23–33. doi: 10.1016/s0169-5002(03)00244-7. [DOI] [PubMed] [Google Scholar]
- 26.Bergeron A, Picard V, LaRue H, Harel F, Hovington H, Lacombe L, Fradet Y. High frequency of MAGE-A4 and MAGE-A9 expression in high-risk bladder cancer. Int J Cancer. 2009;125:1365–1371. doi: 10.1002/ijc.24503. [DOI] [PubMed] [Google Scholar]
- 27.Hatiboglu G, Pritsch M, Macher-Goeppinger S, Zoller M, Huber J, Haferkamp A, Pahernik S, Wagener N, Hohenfellner M. Prognostic value of melanoma-associated antigen A9 in renal cell carcinoma. Scand J Urol. 2013;47:311–322. doi: 10.3109/00365599.2012.740070. [DOI] [PubMed] [Google Scholar]
- 28.Eichmuller S, Usener D, Thiel D, Schadendorf D. Tumor-specific antigens in cutaneous T-cell lymphoma: expression and sero-reactivity. Int J Cancer. 2003;104:482–487. doi: 10.1002/ijc.10967. [DOI] [PubMed] [Google Scholar]
- 29.Lin J, Lin L, Thomas DG, Greenson JK, Giordano TJ, Robinson GS, Barve RA, Weishaar FA, Taylor JM, Orringer MB, Beer DG. Melanoma-associated antigens in esophageal adenocarcinoma: identification of novel MAGE-A10 splice variants. Clin Cancer Res. 2004;10:5708–5716. doi: 10.1158/1078-0432.CCR-04-0468. [DOI] [PubMed] [Google Scholar]
- 30.van Duin M, Broyl A, de Knegt Y, Goldschmidt H, Richardson PG, Hop WC, van der Holt B, Joseph-Pietras D, Mulligan G, Neuwirth R, Sahota SS, Sonneveld P. Cancer testis antigens in newly diagnosed and relapse multiple myeloma: prognostic markers and potential targets for immunotherapy. Haematologica. 2011;96:1662–1669. doi: 10.3324/haematol.2010.037978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsai JR, Chong IW, Chen YH, Yang MJ, Sheu CC, Chang HC, Hwang JJ, Hung JY, Lin SR. Differential expression profile of MAGE family in non-small-cell lung cancer. Lung Cancer. 2007;56:185–192. doi: 10.1016/j.lungcan.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 32.Kadota K, Nitadori JI, Adusumilli PS. Prognostic value of the immune microenvironment in lung adenocarcinoma. Oncoimmunology. 2013;2:e24036. doi: 10.4161/onci.24036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang J, Zhang X, Tang Q, Zhang F, Li Y, Feng Z, Zhu J. Prognostic significance and potential therapeutic target of VEGFR2 in hepatocellular carcinoma. J Clin Pathol. 2011;64:343–348. doi: 10.1136/jcp.2010.085142. [DOI] [PubMed] [Google Scholar]
- 34.Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10:7252–7259. doi: 10.1158/1078-0432.CCR-04-0713. [DOI] [PubMed] [Google Scholar]
- 35.Pfister DG, Johnson DH, Azzoli CG, Sause W, Smith TJ, Baker S Jr, Olak J, Stover D, Strawn JR, Turrisi AT, Somerfield MR American Society of Clinical Oncology. American Society of Clinical Oncology treatment of unresectable non-small-cell lung cancer guideline: Update 2003. J. Clin. Oncol. 2004;22:330–353. doi: 10.1200/JCO.2004.09.053. [DOI] [PubMed] [Google Scholar]
- 36.Escudier B, Dorval T, Chaput N, André F, Caby MP, Novault S, Flament C, Leboulaire C, Borg C, Amigorena S, Boccaccio C, Bonnerot C, Dhellin O, Movassagh M, Piperno S, Robert C, Serra V, Valente N, Le Pecq JB, Spatz A, Lantz O, Tursz T, Angevin E, Zitvogel L. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: results of thefirst phase I clinical trial. J Transl Med. 2005;3:10. doi: 10.1186/1479-5876-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Toungouz M, Libin M, Bulte F, Faid L, Lehmann F, Duriau D, Laporte M, Gangji D, Bruyns C, Lambermont M, Goldman M, Velu T. Transient expansion of peptide-specific lymphocytes producing IFN-gamma after vaccination with dendritic cells pulsed with MAGE peptides in patients with mage-A1/A3-positive tumors. J Leukoc Biol. 2011;69:937–943. [PubMed] [Google Scholar]
- 38.Nishiyama T, Tachibana M, Horiguchi Y, Nakamura K, Ikeda Y, Takesako K, Murai M. Immunotherapy of bladder cancer using autologous dendritic cells pulsed with human lymphocyte antigen-A24-specific MAGE-3 peptide. Clin Cancer Res. 2001;7:23–31. [PubMed] [Google Scholar]
- 39.Sienel W, Varwerk C, Linder A, Kaiser D, Teschner M, Delire M, Stamatis G, Passlick B. Melanoma associated antigen (MAGE)-A3 expression in Stages I and II non-small cell lung cancer: results of a multi-center study. Eur J Cardiothorac Surg. 2004;25:131–134. doi: 10.1016/j.ejcts.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 40.Atanackovic D, Altorki NK, Cao Y, Ritter E, Ferrara CA, Ritter G, Hoffman EW, Bokemeyer C, Old LJ, Gnjatic S. Booster vaccination of cancer patients with MAGE-A3 protein reveals long-term immunological memory or tolerance depending on priming. Proc Natl Acad Sci U S A. 2008;105:1650–1655. doi: 10.1073/pnas.0707140104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kiaris H, Chatzistamou I, Kalofoutis C, Koutselini H, Piperi C, Kalofoutis A. Tumour-stroma interactions in carcinogenesis: basic aspects and perspectives. Mol Cell Biochem. 2004;261:117–122. doi: 10.1023/b:mcbi.0000028746.54447.6c. [DOI] [PubMed] [Google Scholar]
- 42.Kiaris H, Trimis G, Papavassiliou AG. Regulation of tumor-stromal fibroblast interactions: implications in anticancer therapy. Curr Med Chem. 2008;15:3062–3067. doi: 10.2174/092986708786848596. [DOI] [PubMed] [Google Scholar]
- 43.Fischer C, Gudat F, Stulz P, Noppen C, Schaefer C, Zajac P, Trutmann M, Kocher T, Zuber M, Harder F, Heberer M, Spagnoli GC. High expression of MAGE-3 protein in squamous-cell lung carcinoma. Int J Cancer. 1997;71:1119–1121. doi: 10.1002/(sici)1097-0215(19970611)71:6<1119::aid-ijc34>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 44.Sugita M, Geraci M, Gao B, Powell RL, Hirsch FR, Johnson G, Lapadat R, Gabrielson E, Bremnes R, Bunn PA, Franklin WA. Combined use of oligonucleotide and tissue microarrays identifies cancer/testis antigens as biomarkers in lung carcinoma. Cancer Res. 2002;62:3971–3979. [PubMed] [Google Scholar]
- 45.Bolli M, Kocher T, Adamina M, Guller U, Dalquen P, Haas P, Mirlacher M, Gambazzi F, Harder F, Heberer M, Sauter G, Spagnoli GC. Tissue microarray evaluation of Melanoma antigen E (MAGE) tumor-associated antigen expression: potential indications for specific immunotherapy and prognostic relevance in squamous cell lung carcinoma. Ann Surg. 2002;236:785. doi: 10.1097/01.SLA.0000036266.09823.6C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gure AO, Chua R, Williamson B, Gonen M, Ferrera CA, Gnjatic S, Ritter G, Simpson AJ, Chen YT, Old LJ, Altorki NK. Cancer-testis genes are coordinately expressed and are markers of poor outcome in non-small cell lung cancer. Clin Cancer Res. 2005;11:8055–8062. doi: 10.1158/1078-0432.CCR-05-1203. [DOI] [PubMed] [Google Scholar]
- 47.De Smet C, Lurquin C, Lethé B, Martelange V, Boon T. DNA methylation is the primary silencing mechanism for a set of germ line-and tumor-specific genes with a CpG-rich promoter. Mol Cell Biol. 1999;19:7327–7335. doi: 10.1128/mcb.19.11.7327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Adair SJ, Hogan KT. Treatment of ovarian cancer cell lines with 5-aza-2′-deoxycytidine upregulates the expression of cancer-testis antigens and class I major histocompatibility complex-encoded molecules. Cancer Immunol Immunother. 2009;58:589–601. doi: 10.1007/s00262-008-0582-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hou S, Sang M, Geng C, Liu W, Lü W. Expressions of MAGE-A9 and MAGE-A11 in breast cancer and their mechanism of expression. Arch Med Res. 2014;45:44–51. doi: 10.1016/j.arcmed.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 50.Jang SJ, Soria JC, Wang L, Hassan KA, Morice RC, Walsh GL, Hong WK, Mao L. Activation of melanoma antigen tumor antigens occurs early in lung carcinogenesis. Cancer Res. 2001;61:7959–7963. [PubMed] [Google Scholar]
- 51.Sakurai T, Itoh K, Higashitsuji H, Nagao T, Nonoguchi K, Chiba T, Fujita J. A cleaved form of MAGE-A4 binds to Miz-1 and induces apoptosis in human cells. J Biol Chem. 2004;279:15505–15514. doi: 10.1074/jbc.M310437200. [DOI] [PubMed] [Google Scholar]
- 52.Duan Z, Duan Y, Lamendola DE, Yusuf RZ, Naeem R, Penson RT, Seiden MV. Overexpression of MAGE/GAGE genes in paclitaxel/doxorubicin-resistant human cancer cell lines. Clin Cancer Res. 2003;9:2778–2785. [PubMed] [Google Scholar]


