Abstract
Background and aim: Fentanyl is widely used for relieving pain and narcotizing in cancer patients. However, there are few published reports regarding the effects of fentanyl on tumor control and treatment. Here we investigated the effects of fentanyl on tumor growth and cell invasion in the human colorectal carcinoma (HCT116) cells. Methods: Nude mice xenografts of HCT116 cells were established to assess the inhibition effect on tumor growth by fentanyl. MTT and Transwell were employed to determine the cell survival rate and cell invasion, respectively. MicroRNAs and mRNAs expression were quantified by real-time PCR. β-catenin and matrix metalloproteinases (MMP-2 and MMP-9) expression were assayed by western blotting. β-Catenin-specific small interfering RNA (Si-β-catenin) and miR-182 mimics were transfected in cells to investigate the mechanism underlying the effects of fentanyl on the colorectal tumor and HCT116 cells. Results: Treatment with fentanyl inhibited the tumor growth and HCT116 cells invasion. Fentanyl also downregulated the expression of β-catenin and miR-182 in both xenograft tumors and HCT116 cells, and decreased the protein level of MMP-9 in HCT116 cells. Downregulation of β-Catenin resulted in the decrease of miR-182 expression in colorectal cells. In addition, the overexpression of miR-182 reversed the effect of fentanyl on MMP-9 expression and cell invasion of HCT116 cells. Conclusions: The current study demonstrated that the inhibition of tumor growth and cell invasion in colorectal cancer by fentanyl is probably due to downregulation of miR-182 and MMP-9 expression by β-catenin.
Keywords: Colorectal cancer, fentanyl, β-catenin, miR-182, HCT116 cells
Introduction
Colorectal cancer is one of the most common cancers and the leading cause of cancer death in developed country [1]. Colorectal cancers are largely due to lifestyle factors and increasing age with a minority of individuals due to genetic basis of inherited [2,3]. The treatment of colorectal cancer depends mostly on patient’s health and stage of the tumor [4].
Fentanyl, is an opioid agonist, has been extensively applied to breakthrough pain management and acts as a pain reliever as well as a narcotic [5,6]. It has been reported that fentanyl could suppress cell growth and proliferation and interfere with the cell cycle in gastric carcinoma cells, which suggests a potential therapeutic role in gastric cancer treatment [7]. However, few reports about fentanyl on cancer treatment or chemotherapy are known to date. Therefore, whether fentanyl inhibits cell invasion and metastasis in colorectal cancer is still unknown.
MicroRNAs (miRNAs), a new class of endogenous and non-coding RNAs, are implicated in regulation of target genes expression [8]. Currently, more than several hundred miRNAs are reported to be involved in tumorigenesis or tumor suppressors [9], suggesting a target strategy to prevent a variety of cancers. In colorectal cancer, four hundred miRNAs including miR-182, miR-150, miR-146a, miR-120 and miR-122 are in a deregulation [10,11], takes part in promoting the proliferation and metastasis of colorectal cancer.
It was reported that the signal pathway of miRNAs affecting invasion and metastasis of colorectal cancer via β-catenin regulation [12]. β-catenin is a subunit of the cadherin protein complex with regulating the cell-cell adhesion and gene transcription. Abnormal expression of contributes to tumor formation and participates in the mechanism of the cancer disease, including hepatocellular carcinoma, lung cancer, breast tumors, endometrial cancer and colorectal cancer [13]. In addition, it is found that in hepatocellular carcinoma cells, the increase of β-catenin upregulates the matrix metalloproteinases (MMPs) expression, which results in the regulation of hepatocellular carcinoma invasion and metastasis [14].
Our present studies investigated the effects of fentanyl on colorectal tumor growth and cell invasion in the human colorectal carcinoma (HCT116) cells and its underlying mechanism.
Materials and methods
Xenograft in nude mice
5 × 106 human colorectal carcinoma (HCT116) cells (Nanjing, China) were suspended in 100 μL PBS and subcutaneously injected into the flanks of 4-week-old BALB/c athymic nude mice (Gene-Cell, China). Five days after inoculation, 24 animals were randomly divided into four groups (injected with 0 mg/kg, 0.05 mg/kg, 0.1 mg/kg and 0.2 mg/kg fentanyl in tumor for every two days respectively). The mice were sacrificed and tumors were removed and weighted after the three weeks of fentanyl treatment. Tumor size was calculated according to the following formula: π×length×width2/6 [15].
All experiments with animals were approved by a local animal committee for ethics.
Cell culture
HCT116 cells were cultured in DMEM medium (contained 10% fetal bovine serum, 100 IU/mL penicillin, 100 IU/mL penicillin) at 37°C in humidified atmosphere of 5% CO2. Cells cultured in fresh medium containing fentanyl at clinically relevant concentrations: 0 ng/mL, 0.5 ng/mL and 2 ng/mL in DMEM medium. After 24 h, an MTT assay (Beyotime, China) was employed to determine the cell inhibitory effect of fentanyl (104 cells/ml was used).
Invasive assay
Transwell chambers with Matrigel (BD Bioscience, USA) were used to evaluate the cell invasion. Briefly: HCT116 cells were incubated in the upper chambers of a Transwell plate (Corning, USA) with serum-free medium. Lower chambers with polycarbonate membranes, received 10% FBS-containing medium, served as the attractant. After 24 h, the cells in upper chambers were removed; migrated cells on lower side were observed after being fixed with 4% paraformaldehyde and stained with crystal violet under a microscope. Migrating cells in five fields on each chamber were counted to calculate the average.
Quantitative real-time PCR
Total RNA and miRNAs from xenografts or cells were extracted using the TRIzol reagent (Invitrogen, USA) and 2 μg of RNA was used to reverse transcription-PCR with ImProm-II™ (Promega, USA) following the manufacturer’s instructions. The mRNA and miRNAs levels were quantified by real-time PCR using TransStartTM SYBR Green qPCR Supermix (TransGen Biotech, China), and with β-actin and U6 small nuclear RNA served as an internal normalized reference respectively.
Western blotting
In the logarithmic phase of cell growth, cells were harvested and prepared to do western blotting. Proteins were loaded onto the sodium dodecyl sulfate -polyacrylamide gels for electrophoresis and transferred to PVDF membranes, which were then blocked with 5% BSA prior to incubation with the indicated primary antibodies and the secondary antibodies (Cell signaling, USA). Immunoreactivity was determined using enhanced chemiluminescence (Millipore, USA) and observed using autoradiography (Protein Simple, USA). β-actin was served as a control of the amount of protein loading.
β-catenin by RNA interference and over-expression of miR-182
HCT116 cells were transiently transfected with siRNA using the Lipofectamine 2000 reagent (Invitrogen, USA) according to the manufacturer’s instructions. β-catenin-specific small interfering RNA (Si-β-catenin) and control siRNA were synthesized by RIBOBIO (Ribobio, China).
miR-182 was overexpressed by transfection with miRNA mimic using siPort Neo-FX (Ambion, USA). miR-182 mimics were synthesized by RIBOBIO (Ribobio, China).
ChIP-Quantitative real-time PCR analysis
Cells were prepared as above described. ChIP assays were performed with cells using chromatin immunoprecipitation assay kit (Upstate, USA) following the manufacturer’s instructions. Quantitative real-time PCR was used to analyze binding to the miR-182 enhancer.
Statistical analysis
All data were presented as means ± SD. Each experiment in vitro was performed at least in triplicate. Variance (ANOVA) or Student’s t-test by SPSS 17.0 software was performed to statistical analysis. P value < 0.05 was regarded as statistically significant.
Results
Effect of fentanyl on growth of tumor and expression of β-catenin and miR-182
We first evaluated the effect of fentanyl on growth of colorectal xenograft tumor. The results in Figure 1A, 1B showed that injection of fentanyl at 0.1 mg/kg and 0.2 mg/kg in xenograft tumor for 3 weeks decreased the size and weight of tumor in a dose-dependent manner, and 0.05 mg/kg fentanyl had no effect on the decrease of tumor growth. In addition, fentanyl at 0.1 mg/kg and 0.2 mg/kg concentration downregulated the expression of β-catenin protein by western blotting (Figure 1C), and reduced the level of miR-182 expression by quantitative real-time PCR (Figure 1D) in xenograft tumors. However, no changes of the expression of miR-150, miR-146a, miR-210 and miR-122 were observed in tumors treated with different concentrations fentanyl (Figure 1D).
Figure 1.
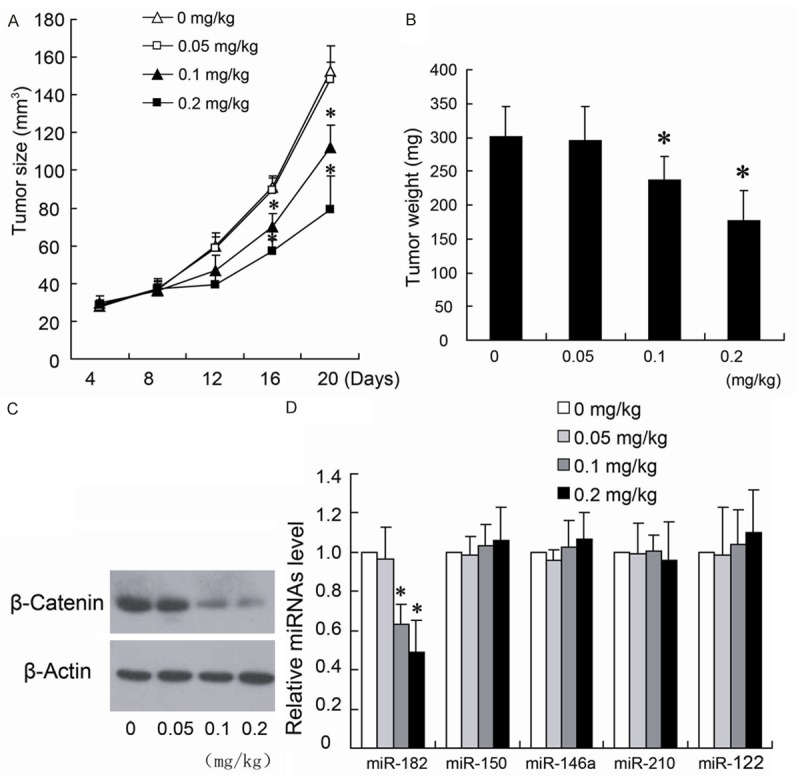
Fentanyl inhibit the growth of colorectal xenograft tumor and expression of β-catenin and miR-182. The mice were sacrificed after the three weeks with different concentrations of fentanyl injection. The tumor size (A) and weight (B) were measured. β-catenin and miRNAs expression were detected by Western blotting (C) and quantitative real-time PCR (D), respectively. *P < 0.05 by Student’s t-test, indicates a significant difference from the group without fentanyl treatment. All experiments were repeated three times.
Fentanyl inhibites the survival and invasion of HCT116 cells
To identify the mechanisms underlying the effects of fentanyl on the colorectal tumor, MTT assay and Transwell were performed to detect the inhibition rate and cell invasion in HCT116 cells. Cells treated with different concentrations (0.5 ng/ml, 2 ng/ml, 5 ng/ml and 10 ng/mL) of fentanyl resulted in a dose-dependent increase in inhibition rate of cell (Figure 2A), but 0.5 ng/ml and 2 ng/ml fentanyl treatment had no significant increase in inhibition rate of cell growth. However, 2 ng/mL fentanyl decreased the cell invasion to 62.3 ± 8.2% (Figure 2B).
Figure 2.
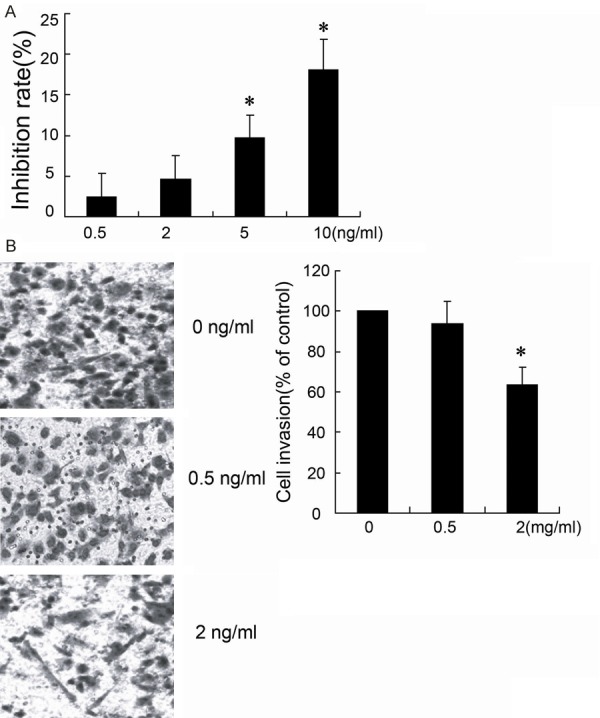
Fentanyl inhibites the proliferation and invasion of HCT116 cells. HCT116 cells were treated with different concentrations (0 ng/ml, 0.5 ng/ml, 2 ng/ml, 5 ng/ml and 10 ng/ml) of fentanyl for 24 hours. 104 cells per ml was used to determine the cell inhibition rate by MTT assays (A). The cell invasion was evaluated with Transwell (B). *P < 0.05 by Student’s t-test, indicates a significant difference from the group without fentanyl treatment. All experiments were repeated three times.
Fentanyl inhibites the expression of β-catenin protein and miR-182 of HCT116 cells
β-catenin expression was detected by Western blotting. The results in Figure 3A showed that HCT116 cells with 2 ng/mL fentanyl treatment decreased the expression of β-catenin. In addition, real-time PCR results (Figure 3B) also presented that miR-182 was downregulated in cells treated with 2 ng/mL fentanyl, but the similar effect on β-catenin and miR-182 expression was not observed in 0.5 ng/ml fentanyl group (Figure 3A, 3B). Therefore, the 2 ng/ml fentanyl was used in cell treatment to determine the binding of β-catenin on miR-182 promoter region. The ChIP data showed that 2 ng/mL fentanyl decreased the combination of β-catenin and miR-182 promoter in HCT116 cells.
Figure 3.
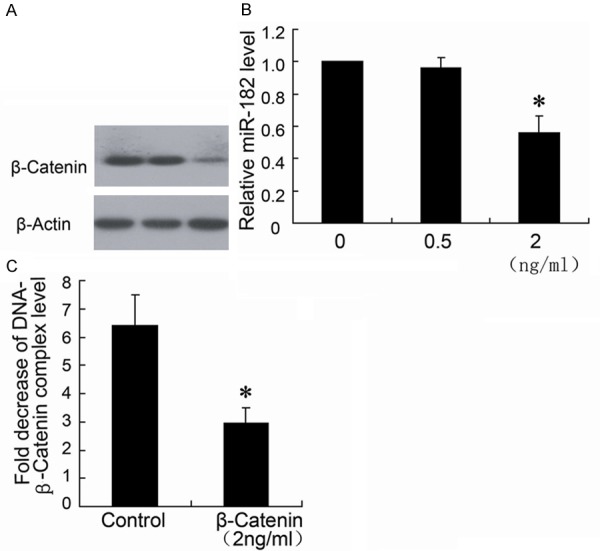
Fentanyl inhibits the expression of β-catenin protein and miR-182 of HCT116 cells. HCT116 cells were treated with different concentrations (0 ng/ml, 0.5 ng/ml and 2 ng/ml) of fentanyl for 24 hours. β-catenin expression was detected by Western blotting (A) and miR-182 was detected by real-time PCR (B). ChIP assay was performed to determine the binding of β-catenin on miR-182 promoter region (C). *P < 0.05 by Student’s t-test, indicates a significant difference from the group without fentanyl treatment. All experiments were repeated three times.
Knockdown of β-catenin protein decreased miR-182 expression of HCT116 cells
To evaluate the role of β-catenin in the effects on miR-182 expression, we used Si-β-catenin to downregulate the β-catenin expression. As shown in Figure 4A, the Si-β-catenin effectively silenced the β-catenin expression in HCT116 cells, which resulted in significant down regulation of the miR-182 level.
Figure 4.
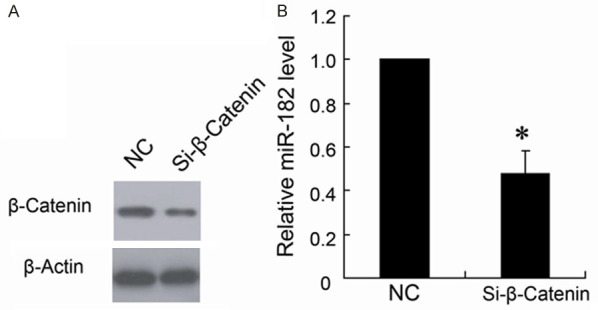
Knockdown of β-catenin expression decreased miR-182 expression of HCT116 cells. The HCT116 cells were transfected with Si-β-catenin (Si-β-catenin) or control siRNA (NC). Western blotting (A) was used to determine β-catenin expression and real-time PCR (B) was used to determine miR-182 expression. *P < 0.05 by Student’s t-test, indicates a significant difference from control (NC). All experiments were repeated three times.
Effect of fentanyl on MMP-2 and MMP-9 expression of HCT116 cells
The real-time PCR data showed that MMP-2 and MMP-9 expression levels in HCT116 cells treated with different concentrations of fentanyl had no difference with the group without fentanyl treatment (Figure 5A, 5B). However, 2 ng/ml fentanyl downregulated the MMP-9 expression in protein level, but had no effect on MMP-2 expression (Figure 5C).
Figure 5.
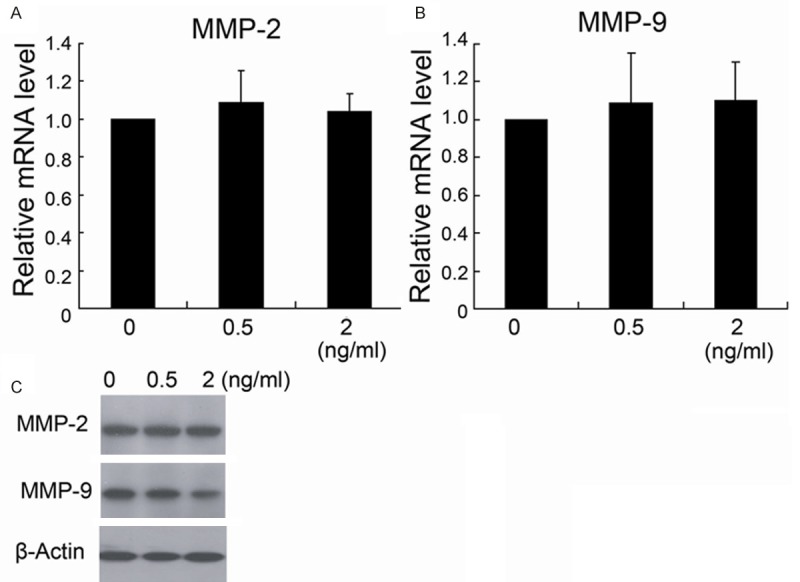
The effect of fentanyl on MMP-2 and MMP-9 expression of HCT116 cells. HCT116 cells were treated with different concentrations (0 ng/ml, 0.5 ng/ml and 2 ng/ml) of fentanyl for 24 hours. MMP-2 and MMP-9 expression were detected by real-time PCR (A, B) and Western blotting (C). *P < 0.05 by Student’s t-test, indicates a significant difference from the group without fentanyl treatment. All experiments were repeated three times.
Overexpression of miR-182 reversed the effect of fentanyl on HCT116 cells
To investigate whether miR-182 is involved in the inhibition effect of fentanyl on cell invasion. Cells treated by fentanyl transfected with miR-182 mimic to upregulate the miR-182 expression. The results demonstrated that overexpression of miR-182 significantly attenuated fentanyl-induced downregulation of MMP-9 expression (Figure 6A) and inhibition of cell invasion (Figure 6B).
Figure 6.
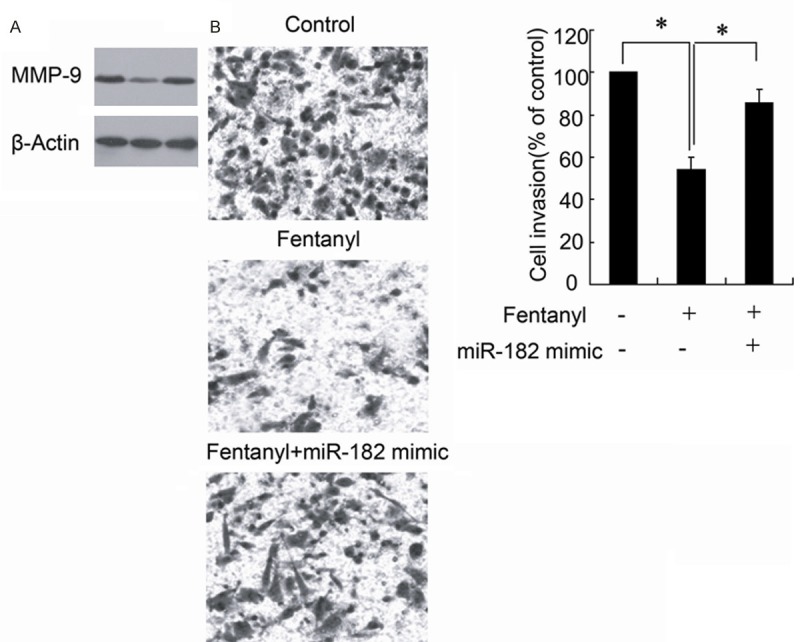
The overexpression of miR-182 reversed the effect of fentanyl in HCT116 cells. HCT116 cells treated by fentanyl were transfected with or without the miR-182 mimic. MMP-9 expression were detected by Western blotting (A). The cell invasion was evaluated with Transwell chamber (B). *P < 0.05 by Student’s t-test, indicates a significant difference. All experiments were repeated three times.
Discussion
Fentanyl acts as a potent analgesic, clinically used to relieve pain and narcotize in preoperative and postoperative. Qin et al has found that fentanyl inhibited gastric cancer progression in vitro experiment [7]. Colorectal cancer is one of the most common malignant tumors in the world, with high rate of recurrence and metastasis [16]. However, there has been very few reports on fentanyl in colorectal tumor inhibition. Current study demonstrated that fentanyl inhibits the colorectal tumor growth and HCT116 cell invasion via downregulating β-Catenin and miR-182 expression.
Pretreatment of fentanyl reduced colorectal cancer xenograft tumor in size and weight, and inhibited the invasion of HCT116 cells in this study. In vitro experiment, HCT116 cells treated with different concentrations of fentanyl resulted in a dose-dependent increase in cell inhibition rate and decrease in cell invasion. However, 2 ng/mL fentanyl had no effect on the inhibition rate of HCT116 cells, which was correspond with the report of literature [17]. This is probably concerned with the low fentanyl concentration in medium. We also illustrated that both xenograft tumor and HCT116 cells treated with fentanyl had a decrease in β-catenin protein expression by western blotting. The proposed mechanism of inhibition of HCT116 cells invasion by fentanyl was correlated with down regulation of β-catenin.
β-catenin, mainly located in cell membrane and plays a critical role in cell-cell communications, is identified as a multifunctional protein [12]. It also acts as a key transcriptional factor in Wnt pathway, which participates widely in regulation of cell proliferation and migration [18]. In many cancers, β-catenin plays pivotal role in malignant transformation of cells [13]. It has been reported that β-catenin mediated expression of matrix metalloproteinases (MMPs) enhance epithelial-mesenchymal transition in tumor development [19]. The key point of tumor invasion and metastasis is degrading extracellular matrix. MMPs take part in the degradation of extracellular matrix, so it was related closely with the tumor occurrence and development [20]. Western blotting results showed that fentanyl (2 ng/ml) downregulated the expression of β-catenin as well as MMP-9 protein in HCT116 cells. The research by Tutton et al concluded that MMP-2 and MMP-9 levels in plasma are potential indicators of invasion or metastasis in colorectal tumor [21]. The decreased expression of MMP-9 confirmed the fentanyl inhibition effect on human colorectal cancer cell invasion.
More than four hundred miRNAs were showed in colorectal cancer over the past decade. [9]. Although the mechanism of dysregulation of miRNAs in colorectal cancer remains not fully clear, a large number of research in vivo and in vitro have supported that the expression of miRNAs have close relationship with the pathophysiology cancer [11]. Quantitative real-time PCR was used to measure the expression of miR-150, miR-146a, miR-210 and miR-122 and miR-182 in colorectal cancer xenograft tumor, and the results showed that only miR-182 expression was downregulated by fentanyl injection. miR-182 is involved in carcinogenesis by targeting the tumor suppressor gene [22], and the up-regulation of miR-182 was significantly correlated with large tumor size, positive regional lymph node metastasis, and advanced TNM stage. It is also reported that miR-182 is upregulated in breast cancer [23], prostate Cancer [24] and colorectal cancer [10]. In vitro experiments, downregulation of miR-182 expression by fentanyl treatment was also observed in HCT116 cells, which indicated the mechanism of fentanyl on tumor inhibition.
To evaluate the role of β-catenin in the effects of miR-182 expression in HCT116 cells, we used Si-β-catenin to silence the β-catenin expression. As expected, miR-182 was downregulated by silence of β-catenin. The similar results were observed in breast cancer reported [25]. In addition, Our ChIP data demonstrated that binding of β-catenin on miR-182 promoter region was significantly decreased by fentanyl treatment, implying fentanyl inhibits invasion of colorectal cancer via down-regulation of miR-182 by β-catenin. On the other hand, overexpression of miR-182 attenuated the fentanyl-induced decrease in cell invasion and MMP-9 expression. Therefore, it was supposed that miR-182 participated in the effect of fentanyl on HCT116 cell invasion.
In conclusion, we revealed in the present study that fentanyl exerts the inhibition of colorectal tumor growth and invasion in human colorectal carcinoma cells. Our results also suggest that the inhibition effects associated with fentanyl treatment are due to downregulation of β-catenin and miR-182 expression. The role of fentanyl in the inhibition of colorectal tumor and more cancers should be further studied.
Disclosure of conflict of interest
None.
References
- 1.Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64:104–117. doi: 10.3322/caac.21220. [DOI] [PubMed] [Google Scholar]
- 2.Johnson CM, Wei C, Ensor JE, Smolenski DJ, Amos CI, Levin B. Meta-analyses of colorectal cancer risk factors. Cancer Causes Control. 2013;24:1207–1222. doi: 10.1007/s10552-013-0201-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yacoub G, Nagalla S, Aklilu M. Oncologic management of hereditary colorectal cancer. Clin Colon Rectal Surg. 2012;25:118–122. doi: 10.1055/s-0032-1313783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stein A, Atanackovic D, Bokemeyer C. Current standards and new trends in the primary treatment of colorectal cancer. Eur J Cancer. 2011;47:S312–314. doi: 10.1016/S0959-8049(11)70183-6. [DOI] [PubMed] [Google Scholar]
- 5.Davis MP. Fentanyl for breakthrough pain: a systematic review. Expert Rev Neurother. 2011;11:1197–1216. doi: 10.1586/ern.11.63. [DOI] [PubMed] [Google Scholar]
- 6.Kitamura T, Fujiwara H, Nagata O, Usui H, Suzuki T, Ogawa M. [Anesthetic management using propofol and fentanyl for transrectal ultrasound-guided prostatic biopsy] . Masui. 2001;50:1209–1212. [PubMed] [Google Scholar]
- 7.Qin Y, Li L, Chen J, Tang X, Liao C, Xie Y. Fentanyl inhibits progression of human gastric cancer MGC-803 cells by NF-kappaB down-regulation and PTEN upregulation in vitro. Oncol Res. 2012;20:61–69. doi: 10.3727/096504012x13473664562501. [DOI] [PubMed] [Google Scholar]
- 8.Felekkis K, Touvana E, Stefanou C, Deltas C. microRNAs: a newly described class of encoded molecules that play a role in health and disease. Hippokratia. 2010;14:236–240. [PMC free article] [PubMed] [Google Scholar]
- 9.Hogan NM, Joyce MR, Kerin MJ. MicroRNA expression in colorectal cancer. Cancer Biomark. 2012;11:239–243. doi: 10.3233/CBM-2012-00278. [DOI] [PubMed] [Google Scholar]
- 10.Liu H, Du L, Wen Z, Yang Y, Li J, Wang L. Up-regulation of miR-182 expression in colorectal cancer tissues and its prognostic value. Int J Colorectal Dis. 2013;28:697–703. doi: 10.1007/s00384-013-1674-0. [DOI] [PubMed] [Google Scholar]
- 11.Pizzini S, Bisognin A, Mandruzzato S, Biasiolo M, Facciolli A, Perilli L. Impact of microRNAs on regulatory networks and pathways in human colorectal carcinogenesis and development of metastasis. BMC Genomics. 2013;14:589. doi: 10.1186/1471-2164-14-589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li X, Zhang X, Liu X, Tan Z, Yang C, Ding X. Caudatin induces cell apoptosis in gastric cancer cells through modulation of Wnt/beta-catenin signaling. Oncol Rep. 2013;30:677–684. doi: 10.3892/or.2013.2495. [DOI] [PubMed] [Google Scholar]
- 13.Fu Y, Zheng S, An N, Athanasopoulos T, Popplewell L, Liang A. β-catenin as a potential key target for tumor suppression. Int J Cancer. 2011;129:1541–1551. doi: 10.1002/ijc.26102. [DOI] [PubMed] [Google Scholar]
- 14.Qu B, Liu BR, Du YJ, Chen J, Cheng YQ, Xu W. Wnt/beta-catenin signaling pathway may regulate the expression of angiogenic growth factors in hepatocellular carcinoma. Oncol Lett. 2014;7:1175–1178. doi: 10.3892/ol.2014.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cao Q, Mani RS, Ateeq B, Dhanasekaran SM, Asangani IA, Prensner JR, Kim JH, Brenner JC, Jing X, Cao X, Wang R, Li Y, Dahiya A, Wang L, Pandhi M, Lonigro RJ, Wu YM, Tomlins SA, Palanisamy N, Qin Z, Yu J, Maher CA, Varambally S, Chinnaiyan AM. Coordinated regulation of polycomb group complexes through microRNAs in cancer. Cancer Cell. 2011;20:187–199. doi: 10.1016/j.ccr.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kin C, Kidess E, Poultsides GA, Visser BC, Jeffrey SS. Colorectal cancer diagnostics: biomarkers, cell-free DNA, circulating tumor cells and defining heterogeneous populations by single-cell analysis. Expert Rev Mol Diagn. 2013;13:581–599. doi: 10.1586/14737159.2013.811896. [DOI] [PubMed] [Google Scholar]
- 17.Nomura Y, Kawaraguchi Y, Sugimoto H, Furuya H, Kawaguchi M. Effects of morphine and fentanyl on 5-fluorouracil sensitivity in human colon cancer HCT116 cells. J Anesth. 2014;28:298–301. doi: 10.1007/s00540-013-1717-2. [DOI] [PubMed] [Google Scholar]
- 18.Kim W, Kim M, Jho EH. Wnt/beta-catenin signalling: from plasma membrane to nucleus. Biochem J. 2013;450:9–21. doi: 10.1042/BJ20121284. [DOI] [PubMed] [Google Scholar]
- 19.Orlichenko LS, Radisky DC. Matrix metalloproteinases stimulate epithelial-mesenchymal transition during tumor development. Clin Exp Metastasis. 2008;25:593–600. doi: 10.1007/s10585-008-9143-9. [DOI] [PubMed] [Google Scholar]
- 20.Fagan-Solis KD, Schneider SS, Pentecost BT, Bentley BA, Otis CN, Gierthy JF. The RhoA pathway mediates MMP-2 and MMP-9-independent invasive behavior in a triple-negative breast cancer cell line. J Cell Biochem. 2013;114:1385–1394. doi: 10.1002/jcb.24480. [DOI] [PubMed] [Google Scholar]
- 21.Tutton MG, George ML, Eccles SA, Burton S, Swift RI, Abulafi AM. Use of plasma MMP-2 and MMP-9 levels as a surrogate for tumour ex-pression in colorectal cancer patients. Int J Cancer. 2003;107:541–550. doi: 10.1002/ijc.11436. [DOI] [PubMed] [Google Scholar]
- 22.Guo Y, Liao Y, Jia C, Ren J, Wang J, Li T. MicroRNA-182 promotes tumor cell growth by targeting transcription elongation factor A-like 7 in endometrial carcinoma. Cell Physiol Biochem. 2013;32:581–590. doi: 10.1159/000354462. [DOI] [PubMed] [Google Scholar]
- 23.Guttilla IK, White BA. Coordinate regulation of FOXO1 by miR-27a, miR-96, and miR-182 in breast cancer cells. J Biol Chem. 2009;284:23204–23216. doi: 10.1074/jbc.M109.031427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Casanova-Salas I, Rubio-Briones J, Calatrava A, Mancarella C, Masia E, Casanova J. Identification of miR-187 and miR-182 as biomarkers of Early Diagnosis and Prognosis in Patients with Prostate Cancer Treated with Radical Prostatectomy. J Urol. 2014;192:252–259. doi: 10.1016/j.juro.2014.01.107. [DOI] [PubMed] [Google Scholar]
- 25.Chiang CH, Hou MF, Hung WC. Up-regulation of miR-182 by beta-catenin in breast cancer increases tumorigenicity and invasiveness by targeting the matrix metalloproteinase inhibitor RECK. Biochim Biophys Acta. 2013;1830:3067–3076. doi: 10.1016/j.bbagen.2013.01.009. [DOI] [PubMed] [Google Scholar]


