Abstract
A high rate of glycolytic flux, even in the presence of oxygen, is a key metabolic hallmark of cancer cells. Lactate, the end product of glycolysis, decreases the extracellular pH and contributes to the proliferation, invasiveness and metastasis of tumor cells. CD147 play a crucial role in tumorigenicity, invasion and metastasis; and CD147 also interacts strongly and specifically with monocarboxylate transporter1 (MCT1) that mediates the transport of lactate. The objective of this study was to determine whether CD147 is involved, via its association with MCT1 to transport lactate, in glycolysis, contributing to the progression of thyroid carcinoma. The expression levels of CD147 in surgical specimens of normal thyroid, nodular goiter (NG), well-differentiated thyroid carcinoma (WDTC), and undifferentiated thyroid carcinoma (UDTC) were determined using immunohistochemical techniques. The effects of CD147 silencing on cell proliferation, invasiveness, metastasis, co-localization with MCT1, glycolysis rate and extracellular pH of thyroid cancer cells (WRO and FRO cell lines) were measured after CD147 was knocked-down using siRNA targeting CD147. Immunohistochemical analysis of thyroid carcinoma (TC) tissues revealed significant increases in signal for CD147 compared with normal tissue or NG, while UDTC expressed remarkably higher levels of CD147 compared with WDTC. Furthermore, silencing of CD147 in TC cells clearly abrogated the expression of MCT1 and its co-localization with CD147 and dramatically decreased both the glycolysis rate and extracellular pH. Thus, cell proliferation, invasiveness, and metastasis were all significantly decreased by siRNA. These results demonstrate in vitro that the expression of CD147 correlates with the degree of dedifferentiation of thyroid cancer, and show that CD147 interacts with MCT1 to regulate tumor cell glycolysis, resulting in the progression of thyroid carcinoma.
Keywords: Thyroid cancer-experimental, genetics, pathology-thyroid, CD147, glycolysis, RNA interference
Introduction
Unlike many differentiated cells in an adult organism, tumor cells preferentially utilize glycolysis to satisfy their increased energetic and biosynthetic requirements. The observation that tumor cells take up and catabolize glucose at a significantly higher rate than their tissue of origin was first reported by Otto Warburg in the 1950’s (also known as the Warburg effect) [1]. Tumor cells rely on glycolysis for several reasons including the excessive demand for rapid energy production and for the metabolites of glycolysis to support malignant behavior. Lactate, the end product of glycolysis, is excreted from cells through the monocarboxylate transporter (MCT), leading to a decrease in the extracellular pH of tumors [2]. Tumor acidity generates a more aggressive phenotype of cancer cells with a number of altered behaviors. Low pH is known to markedly increase the invasive behavior of tumor cells [3].
CD147, also known as extracellular matrix metalloproteinase inducer (EMMPRIN) or basigin, is widely expressed on many cell types and its expression is increased in various tumor cells [4]. There is emerging evidence that implicates CD147 in the invasion processes of tumor cells [5]. Interestingly, CD147 associates closely with MCT1 in lactate transport. The rapid transport of lactate by MCTs is of critical importance for almost all cells, especially tumor cells with their elevated glycolysis, resulting in a decrease in extracellular pH.
Thyroid carcinoma (TC) is the most common endocrine gland carcinoma with a 3:1 female/male ratio, and accounts for approximately 1% of all malignancies [6]. In immunohistochemical and immunocytochemical studies on papillary thyroid carcinoma (PTC), CD147 expression was found to correlate with the degree of dedifferentiation [7]. In addition, CD147 plays a critical role in TC invasiveness [8]. Because the role of CD147 in tumor progression is similar to that of low extracellular PH and CD147 is closely associated with MCT1, we hypothesized that it is involved in TC glycolysis and in the transport of lactate across the plasma membrane as well as playing a role in changing the behavior of TC cells. Therefore, we examined the expression levels of CD147 in surgical specimens of thyroid tissue using immunohistochemical techniques. After knocking down the expression of CD147 with RNAi technology in TC cells (WRO and FRO cell lines), we examined the co-localization of CD147 with MCT1 and investigated phenotypic changes including glycolysis rate, extracellular pH, proliferation, invasiveness and metastatic potential. We found significant increases in expression of CD147 in TC compared with normal tissue or nodular goiter (NG); further, undifferentiated thyroid carcinoma (UDTC) expressed remarkably higher CD147 levels compared with well-differentiated thyroid carcinoma (WDTC). In other words, there was a correlation between CD147 expression and the pathological grading of TC progression. We also found that both the environmental pH and cellular activities were significantly changed after inhibition of CD147 in TC cells. We report here the requirement for CD147 in the regulation of glycolysis in thyroid carcinoma cells which results in enhanced proliferation, invasiveness and metastasis of tumor cells, suggesting that this role may be the fundamental property of CD147 that affects tumorigenesis and tumor development.
Materials and methods
Clinical specimens and immunohistochemistry
The design of our study using human tissues was approved by the Department of Surgery, Xiangya Hospital, Central-South University. Surgical specimens were obtained from 57 patients (age: 19-65 years; sex: 13 males and 57 females), with thyroid tumors (20 with NG, 30 with WDTC including 20 papillary thyroid carcinoma/PTC and 10 follicular thyroid carcinoma/FTC; and 7 with UDTC including 5 medullary thyroid carcinoma/MTC and 2 anaplastic thyroid carcinoma/ATC) who had undergone resection of the primary thyroid neoplasm between 2008 and 2013. To use as negative control sections, we also isolated 20 samples of normal thyroid tissue from the surgical tissues of the above patients. There was no significant difference in age or sex between the normal, NG, WDTC, and UDTC groups (P > 0.05) by analysis of variance (ANOVA). Specimens obtained from the thyroid lesions and dissected lymph nodes were fixed in 10% formalin and were routinely processed for paraffin embedding. For morphological analysis, multiple 4-μm-thick sections were cut from each paraffin-embedded specimen used for immunohistochemical staining. For immunohistochemistry, sections were deparaffinized, rehydrated, quenched for 10 minutes at room temperature (RT) with 3% H2O2 to inhibit endogenous peroxidase activity, and rinsed in phosphate-buffered saline (PBS, pH 7.6). For unmasking of the antigens CD147 and MCT1, sections were processed by microwaving in citrate buffer (pH 6.0) then cooling at RT for 2 h. After washing with PBS, blocking serum was applied for 10 min. Sections were subsequently incubated overnight at 4°C with the antibodies to CD147 (1:200 dilution, Abcam, Cambridge, UK) and MCT1 (1:200 dilution, Santa Cruz Biotechnology, Santa Cruz, CA). After washing in PBS, a biotinylated secondary antibody was applied for 20 min, followed by peroxidase-conjugated streptavidin for an additional 20 min. 3, 3’-Diaminobenzidine tetrahydrochloride (DAB) was used as the chromogen, with hematoxylin as the counterstain. Sections were processed in the same way but with omission of the primary antibody as negative controls.
Cell culture
The human UDTC cell line (anaplastic thyroid carcinoma cells) FRO and the human DTC cell line (follicular thyroid carcinoma cells) WRO were originally provided by Dr. Xin-ying Li (Central-South University, Changsha, China). FRO cells and WRO cells were grown in RPMI 1640 medium (Gibco/Life Technologies, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS, Gibco) and 1% penicillin–streptomycin solution (Invitrogen, Carlsbad, CA) and incubated at 37°C in a humidified atmosphere containing 5% CO2.
Small interfering RNA (siRNA) transfection
The siRNA sequence we previously designed to target human CD147 mRNA was used in this study [9]. WRO and FRO cells (5 × 104 cells/well) were each seeded into two 24-well plates in 500 μL of growth medium without antibiotics. After 24 h incubation, they reached 50-80% confluence and were transfected with 0.4 μg recombinant plasmid pSUPER containing CD147 siRNA using Lipofectamine reagent (Invitrogen) in 25 μL medium without serum, as recommended by the manufacturers. After 3 h incubation, the medium was replaced with RPMI 1640 containing 20% FBS and the cells were incubated for another 72 h at 37°C. Stably transfected WRO and FRO clones were established by selection with 0.5 μg/mL puromycin (Sigma, St Louis, MO). Clones of WRO cells and FRO cells transfected with recombinant plasmid containing siRNA1 were established and designated siWRO and siFRO, respectively.
Western blot analysis
Total protein was isolated from the cultured cells. Briefly, after washing three times with ice-cold PBS, the cells were suspended in RIPA buffer (Beyotime Institute of Biotechnology, Shanghai, China) and incubated on ice for 30 min. After removing cell debris by centrifugation (4°C, 3,000 rpm, 10 min), the concentration of protein in the supernatant was measured using the bicinchoninic acid protein assay reagent (Pierce, Rockford, IL) according to the manufacturer’s instructions. Equal amounts (10 μg) of protein were heated at 95°C for 5 min in sample buffer containing β-mercaptoethanol, separated by 12% SDS-PAGE and electrotransferred onto polyvinylidene difluoride membranes. Non-specific binding sites on the membrane were blocked in 5% skimmed milk for 1 h at RT. The blots were then incubated with primary antibodies against human CD147 and MCT1 diluted at 1:1000 overnight at 4°C and then with anti-rabbit IgG for 1 h at RT. After washing, the signals were visualized using an ECL plus western blotting detection system (Amersham Biosciences, Chalfont St Giles, UK).
Confocal immunofluorescence microscopy
The cells were cultured for 1 h on Labtek chamber slides until they reached 60-70% confluence, fixed with 4% paraformaldehyde for 10 min, gently washed in PBS and then in 100% methanol. After two washes with PBS, nonspecific binding was blocked with 3% skimmed milk. The cells were then incubated overnight at 4°C with primary antibodies against MCT1 (1:100) and CD147 (1:50). The slides were washed 3 times with PBS and incubated for 1 h at RT with the secondary antibody, FITC-conjugated anti-IgG for MCT1 (1:100, Sigma) and TRITC-conjugated anti-rabbit IgG for CD147 (1:100, Sigma). Images were captured using a confocal laser scanning microscope (Zeiss LSM 510 Meta, Carl Zeiss Microscopy GmbH, Jena, Germany).
Lactate concentration measurement
WRO cells and FRO cells were allowed to grow in supplemented 1640 medium until they reached 60% confluence. The cells were then preincubated with 6 μM oligomycin (Sigma) in culture medium for 30 min. After replacing the culture medium with medium supplemented with mitochondrial H+-ATP synthase inhibitor for 4 h, 100 μL aliquots were collected to determine the lactate concentration. The samples were precipitated in cold 6% perchloric acid, neutralized with 3 M KOH, centrifuged at 1,500 rpm (4°C, 10 min) to remove the sediment, and the supernatant was used for the enzymatic determination of lactate as previously described [10].
pH measurement
Cells were cultured as described above for lactate concentration measurement. Medium (2 mL) was collected and pH was measured with an electronic pH meter (PB-11 Basic Meter; Sartorius AG, Goettingen, Germany). Measure- ments were made within 2 min of sample collection.
CCK-8 cell proliferation assay
Cell proliferation rates were measured using a Cell Counting Kit (CCK-8) (Beyotime Institute of Biotechnology). Twenty-four hours after cells were transfected with siRNA, the cells were plated into 96-well plates at 500 cells per well and incubated for 24, 48, 72 or 96 h. Ten microliters of CCK-8 reagent were added to each well one hour prior to detection. The absorbance at 450 nm of each well was determined by a microplate reader.
Invasion and metastasis assays
The invasiveness of FRO or WRO cells was assayed using modified transwell Boyden chambers. The polycarbonate filter (pore size, 8 μm) separating the upper and lower compartments was coated with 50 μg of reconstituted basement membrane (Matrigel™, BD Biosciences, Franklin Lakes, NJ). Serum-free 1640 medium containing 1.0 × 105 cells in 50 μL was introduced into the upper compartment; the lower compartment contained 250 μL 1640 medium supplemented with 10% FBS. After 48 h of incubation at 37°C, cells on the polycarbonate filter were fixed with formaldehyde and stained with 0.1% crystal violet. Cells that had penetrated the Matrigel were counted under a microscope. The metastasis assay was performed in the same way as the invasiveness assay but without Matrigel™ on the polycarbonate filter.
Statistical analysis
For statistical analyses we used SPSS17.0 software. All data are expressed as means ± SD and ANOVA was used to compare mean values. P < 0.05 was defined as statistically significant.
Results
CD147 expression correlates with the degree of dedifferentiation of TC tissues
In comparison to normal thyroid tissue sections used as negative control sections, immunohistochemical staining with a CD147-specific antibody showed the presence of CD147 protein, mainly localized in the cell membrane and cytoplasm of carcinomatous tissues (Figure 1A). CD147 staining was only very weak or absent in the normal tissue samples (Figure 1Aa) and in the NG group (Figure 1A, 1Ab), but was somewhat more intense in the WDTC (Figure 1Ac, 1Ad) and UDTC (Figure 1Ae, 1Af) groups. Analysis of the proportion of positive cells revealed a significant difference in CD147 immunoreactivity between normal thyroid tissues and TC specimens, NG and TC specimens, and between WDTC and UDTC specimens, *P < 0.005 (Figure 1B, 1C).
Figure 1.
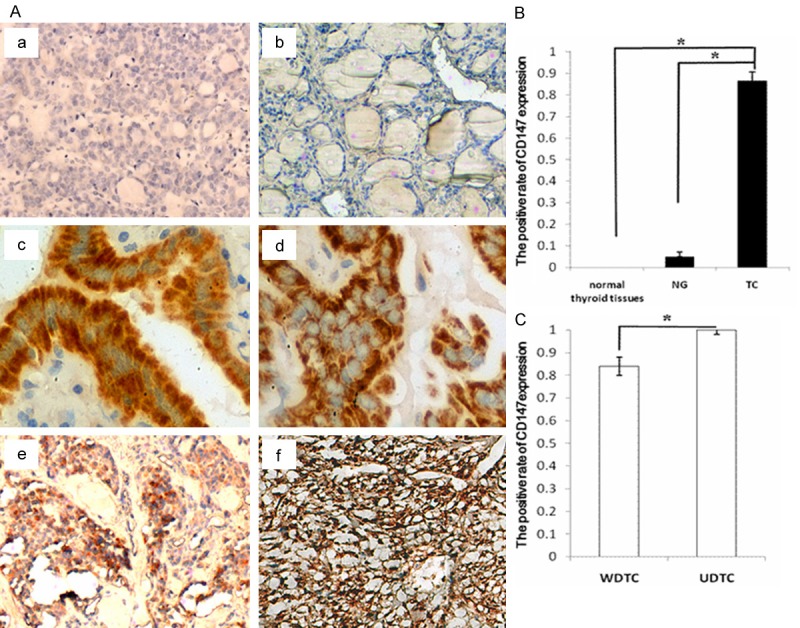
Immunohistochemical staining demonstrating the correlations between CD147 expression and the pathological grading of TC tissues. A: CD147 expressed at various levels in normal thyroid tissues (a, ×200), NG (b, ×200), WDTC (c, papillary thyroid carcinoma (PTC), ×400; d, FTC, ×400) and UDTC (e, MTC, ×200; f, ATC, ×200). Staining is weak or absent in normal tissues and in NG specimens, whereas CD147 is strongly expressed in WDTC and UDTC samples. B: Comparison of the positive rate of CD147 expression. Normal thyroid tissues and NG compared with TC, *P < 0.005. C: Comparison of the positive rate of CD147 expression between WDTC and UDTC samples, *P < 0.005.
siRNA targeting CD147 mRNA specifically down-regulatesCD147 expression
Specific siRNA targeting CD147 mRNA was used to knock down CD147 expression in TC cells. The knock-down efficiency of CD147-specific siRNA in TC cells was first evaluated using western blotting. As shown in Figure 2A, western blot analysis showed a reduced expression of CD147 protein in WRO cells transfected with pSUPER/CD147 siRNA, or in FRO cells transfected with pSUPER/CD147 siRNA. These results demonstrated that the expression of CD147 could be down-regulated specifically and effectively by the specific CD147 siRNA.
Figure 2.
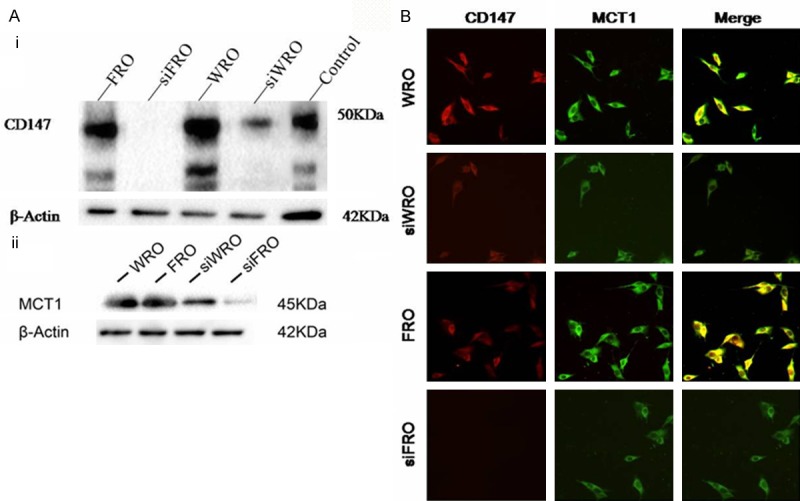
siRNA-mediated silencing of CD147 expression. A: Western blotting analysis of the CD147 (i) and MCT1 (ii) protein level after the transient transfection of WRO and FRO cells with pSUPER⁄CD147 siRNA (si-CD147). Expression of CD147 and MCT1 were both significantly decreased in siWRO and siFRO samples. B: Immunofluorescence confocal microscopy. WRO or FRO cells were co-labeled with anti-CD147 and anti-MCT1 antibodies. Co-localization of MCT1 with CD147 can be observed in the plasma membrane of WRO and FRO cells. The co-localized expression of CD147 and MCT1 was abrogated in siWRO and siFRO cells.
Co-localization of MCT1 with CD147 in the TC cell lines WRO and FRO
To investigate whether there was an association between CD147 and MCT1 in WRO and FRO cells, the cells were double-labeled with anti-CD147 and anti-MCT1 antibodies and observed under an immunofluorescence confocal microscope. CD147 and MCT1 manifested a similar distribution in the plasma membrane; their co-localization is illustrated in the merged view (Figure 2B).
Effects of CD147 silencing on the expression of MCT1
Next we evaluated the effect of CD147 silencing, because CD147 acts as a chaperone of MCT1, ensuring its correct expression in the plasma membrane [11]. Western blots revealed decreased expression of MCT1 as well as CD147 in siWRO and siFRO cells (Figure 2A), confirming that MCTs are targeted for degradation in the absence of CD147 [12]. Under an immunofluorescence confocal microscope, we observed a significant decrease in MCT1 expression and loss of the co-localized expression of CD147 in the plasma membrane in siWRO and siFRO cells (Figure 2B).
Involvement of CD147 in the regulation of glycolysis
Glycolysis is significantly enhanced in tumor cells compared with their non-tumori genic counterparts [13] and increased glycolysis leads to excessive glucose consumption and lactate production. Since the lactate produced is secreted across the plasma membrane and CD147 is a molecular chaperone of MCTs, we measured the extracellular lactate concentration and pH in siWRO and siFRO cells and their wild-type counterparts. As CD147 regulates lactate transport, inhibition of CD147 would result in a blocking of lactate transport and the consequent accumulation of lactate in the cytoplasm and the restoration of extracellular pH. As shown in Figure 3, extracellular pH was lower in WRO cells than in siWRO ells (Figure 3A), and it was also lower in FRO cells than in siFRO cells; the extracellular lactate concentration was also significantly increased decreased in siWRO and siFRO cells compared with their wild-type counterparts (Figure 3B). Compared to wild-type cells, the extracellular lactate concentration in siWRO and siFRO cells was remarkably reduced to 68.7% and 41.5% respectively, *P < 0.005, data not shown.
Figure 3.
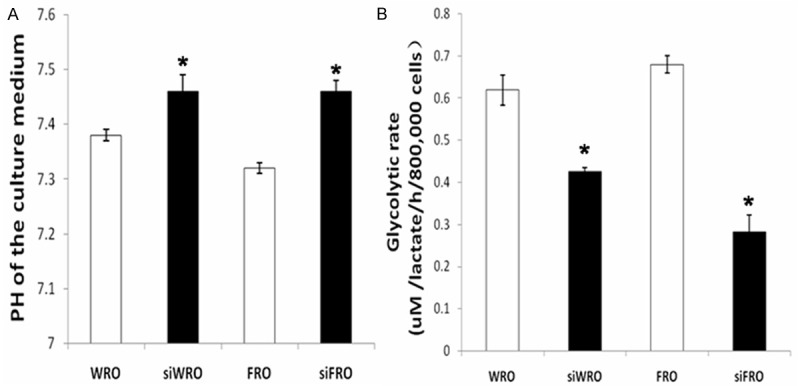
Effects of CD147 in the regulation of glycolysis. A: After CD147 silencing, extracellular pH was higher in siWRO or siFRO cells than in wild-type cells. *P < 0.005 in the comparison between siWRO or siFRO and the respective wild-type cells. B: The extracellular lactate concentration was significantly decreased in siWRO or siFRO cells compared with the respective wild-type cells after CD147 silencing. Compared to wild-type cells, in siWRO and siFRO it was markedly reduced to 68.7% and 41.5% respectively, *P < 0.005.
CD147 silencing reduces the proliferation of TC cells
The proliferation of WRO, siWRO, FRO, and siFRO cells was determined by CCK-8 assay. As shown in Figure 4, compared with WRO, the viability of siWRO cells was reduced to 51.52% (P < 0.005), 52.78% (P < 0.005), and 52.63% (P < 0.005) at 72, 96 and 120 h respectively; compared with FRO, the viability of siFRO was reduced to 51.61% (P < 0.005), 54.29% (P < 0.005), and 50.05% (P < 0.005) at 72, 96 and 120 h, respectively. In other words, CD147 silencing effectively reduces the proliferation of TC cells.
Figure 4.
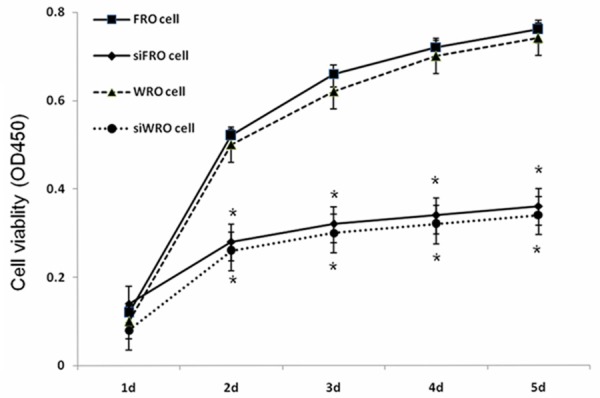
Knockdown of CD147 inhibits WRO and FRO cell proliferation. Effect of siRNA transfection of CD147 on WRO or FRO cell proliferation measured by CCK8 assay, *P < 0.005 in comparison between siWRO or siFRO and their respective wild-type cells.
CD147 silencing reduces the invasive and metastatic capacity of TC cells in vitro
To examine whether the down-regulation of CD147 in TC cells affected their invasive and metastatic capacity, we performed an in vitro Matrigel™ transwell analysis. The results showed that WRO and FRO cells had a similar ability to pass through the Matrigel™ coated filter, because the numbers of invading cells were roughly equal (Figure 5A, 5B). There was a significant difference between the invasive and metastatic ability of WRO cells and siWRO cells, and this was also observed between FRO cells and siFRO cells (P < 0.005). The number of invading cells was reduced in siWRO cells by 56.67% compared with WRO cells, and in siFRO cells by 70.97% compared with FRO cells (Figure 5C); similarly metastasis was inhibited in siWRO cells by 59.46% compared with WRO cells, and in siFRO cells by 66.67% compared with FRO cells (Figure 5D).
Figure 5.
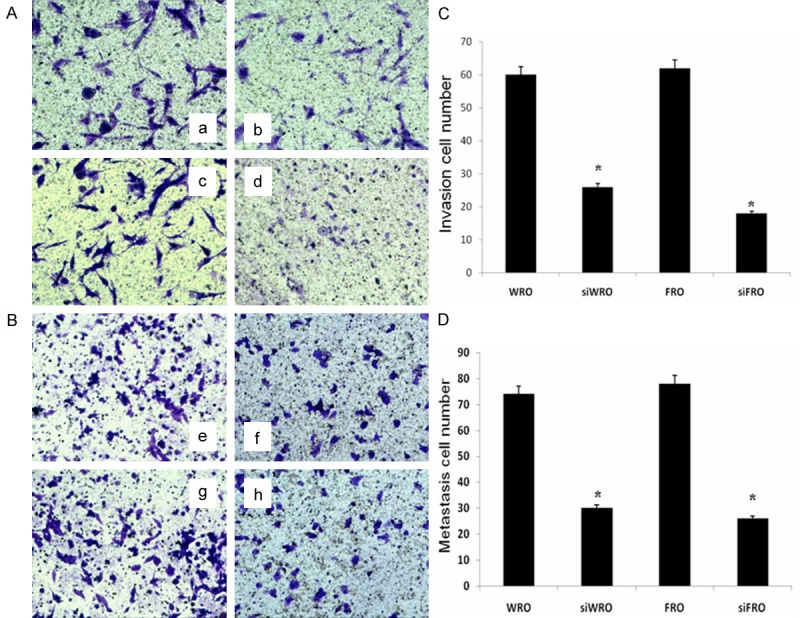
Effects of specific siRNA transfection of CD147 on Matrigel™ invasion and metastasis. A: RNA interference targeting CD147 inhibits the invasiveness of TC cells: (a) WRO, (×400); (b) siWRO, (×400); (c) FRO, (×400); (d) siFRO, (×400). B: siRNA transfection of CD147 inhibits the metastatic activity of TC cells: (e) WRO, (×200); (f) siWRO, (×200); (g) FRO, (×200); (h) siFRO, (×200). C, D: Number of cells migrated or invaded was evaluated in three fields for each experimental group and the mean ± SD is shown. *P < 0.005 in comparison between siWRO or siFRO and their respective wild-type cells.
Discussion
This study shows that CD147 expression in TC tissues correlates with the degree of dedifferentiation of the TC tissues. Our results also show that the expression of both CD147 and MCT1 is enhanced and that CD147 and MCT1 colocalize on the plasma membrane of WRO and FRO cells. Silencing of CD147 by means of siRNA technology down-regulates the expression of MCT1 and reduces the co-localization of CD147 with MCT1. We also demonstrate that glycolysis is activated and extracellular pH is lower in both WRO and FRO cells compared with the same cells transfected with siRNA, and that the glycolysis rate and extracellular pH recover upon siRNA silencing. Cell proliferation, invasiveness and metastasis are all inhibited after CD147 knockdown.
Unlike many differentiated cells in an adult organism, the high rate of glycolysis in tumor cells has recently been characterized as one of the major metabolic alterations of tumor cells [14]. Tumor cells preferentially utilize glycolysis in order to satisfy their increased energetic and biosynthetic requirements. Indeed, tumor cells, which have an altered carbohydrate metabolism, produce ATP from glucose through oxidative phosphorylation and anaerobic glycolysis even under normal oxygen pressures. The Warburg effect having been recently revisited, a more realistic description of cancer cell metabolism suggests that anaerobic glycolysis is the main way to sustain energy needs during tumorigenesis [15]. The high rate of glycolysis in tumors results in excessive lactate production and secretion. As lactate is transported out of the cells, its extracellular concentration increases and extracellular pH decreases [2]. Low extracellular pH is correlated with cell proliferation [16], tumor cell invasion [17], and metastasis [12], and affects the outcome in patients with tumors [18]. Previous studies revealed that the metastatic potential of experimental pulmonary tumors in athymic nude mice was increased at acidic pH [19]. These earlier findings clearly demonstrated that the pH in the microenvironment plays an essential role in tumor cell proliferation, invasiveness, and tumorigenesis.
The transport of lactate across the plasma membrane is mediated by a family of proton-linked MCTs. MCT1 is the most widely expressed member of this family and has recently been shown to be elevated in a variety of cancers [20]. MCT1, as a facilitator of lactate transport, participated in mediating the transport of lactate across the plasma membrane [21]. However, it has been shown both in vitro and in vivo that MCT1 requires an accessory protein, CD147, for trafficking to the cell surface [22]. CD147 has been characterized as a type I transmembrane glycoprotein and a member of the immunoglobulin superfamily [23]. MCT1 requires association with CD147 in the endoplasmic reticulum (ER) for trafficking to the plasma membrane, and MCT1 is targeted for degradation in the absence of CD147 [24]. It has been reported that CD147 is enriched on thyroid carcinoma (TC) cells as well as on other tumor cells [25,26], but the molecular mechanisms involved and the role of CD147 in TC remain poorly understood.
In the present study, we used RNAi technology to construct the CD147 shRNA expression vector pSUPER/CD147 siRNA for transfection into the TC cell lines WRO and FRO. We established a stable pSUPER/CD147 siRNA clone that effectively and stably inhibited CD147 mRNA expression [27]. Taken together, our results using immunohistochemical staining demonstrate that CD147 expression correlates with the degree of dedifferentiation of TC tissues. In addition, we found that glycolysis and cellular activities, up-regulated in WRO and FRO cells, were significantly inhibited following silencing of CD147 by siRNA. This study strongly suggests that CD147, by its close association with MCT1, plays a dominant role in glycolysis, reflected by the transmembrane transport of lactate, and that this results in regulation of cell proliferation, invasiveness and metastasis. We first confirmed the involvement of CD147 in thyroid carcinoma glycolysis and then lowered the extracellular pH, resulting in tumor progression. Since we did not use an animal model in this study, it remains to be determined whether silencing of CD147 will modulate the activity of thyroid carcinoma. Better understanding of the function of CD147 may provide new insights into the possible prevention of progression of thyroid carcinoma.
Disclosure of conflict of interest
None.
References
- 1.Cai Q, Lin T, Kamarajugadda S, Lu J. Regulation of glycolysis and the Warburg effect by estrogen-related receptors. Oncogene. 2013;32:2079–2086. doi: 10.1038/onc.2012.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cardone RA, Casavola V, Reshkin SJ. The role of disturbed pH dynamics and the Na+/H+ exchanger in metastasis. Nat Rev Cancer. 2005;5:786–795. doi: 10.1038/nrc1713. [DOI] [PubMed] [Google Scholar]
- 3.Le A, Cooper CR, Gouw AM, Dinavahi R, Maitra A, Deck LM, Royer RE, Vander JD, Semenza GL, Dang CV. Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression. Proc Natl Acad Sci U S A. 2010;107:2037–2042. doi: 10.1073/pnas.0914433107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ishibashi Y, Matsumoto T, Niwa M, Suzuki Y, Omura N, Hanyu N, Nakada K, Yanaga K, Yamada K, Ohkawa K, Kawakami M, Urashima M. CD147 and matrix metalloproteinase-2 protein expression as significant prognostic factors in esophageal squamous cell carcinoma. Cancer. 2004;101:1994–2000. doi: 10.1002/cncr.20593. [DOI] [PubMed] [Google Scholar]
- 5.Nabeshima K, Inoue T, Shimao Y, Sameshima T. Matrix metalloproteinases in tumor invasion: role for cell migration. Pathol Int. 2002;52:255–264. doi: 10.1046/j.1440-1827.2002.01343.x. [DOI] [PubMed] [Google Scholar]
- 6.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 7.Aratake Y, Marutsuka K, Kiyoyama K, Kuribayashi T, Miyamoto T, Yakushiji K, Ohno S, Miyake Y, Sakaguchi T, Kobayashi TK, Okayama A, Tamura K, Ohno E. EMMPRIN (CD147) expression and differentiation of papillary thyroid carcinoma: implications for immunocytochemistry in FNA cytology. Cytopathology. 2010;21:103–110. doi: 10.1111/j.1365-2303.2009.00716.x. [DOI] [PubMed] [Google Scholar]
- 8.Omi Y, Shibata N, Okamoto T, Obara T, Kobayashi M. The role of CD147 in the invasiveness of follicular thyroid carcinoma cells. Thyroid. 2012;22:383–394. doi: 10.1089/thy.2010.0426. [DOI] [PubMed] [Google Scholar]
- 9.Chen X, Lin J, Kanekura T, Su J, Lin W, Xie H, Wu Y, Li J, Chen M, Chang J. A small interfering CD147-targeting RNA inhibited the proliferation, invasiveness, and metastatic activity of malignant melanoma. Cancer Res. 2006;66:11323–11330. doi: 10.1158/0008-5472.CAN-06-1536. [DOI] [PubMed] [Google Scholar]
- 10.Cuezva JM, Burkett ES, Kerr DS, Rodman HM, Patel MS. The newborn of diabetic rat. I. Hormonal and metabolic changes in the postnatal period. Pediatr Res. 1982;16:632–637. doi: 10.1203/00006450-198208000-00009. [DOI] [PubMed] [Google Scholar]
- 11.Kirk P, Wilson MC, Heddle C, Brown MH, Barclay AN, Halestrap AP. CD147 is tightly associated with lactate transporters MCT1 and MCT4 and facilitates their cell surface expression. Embo J. 2000;19:3896–3904. doi: 10.1093/emboj/19.15.3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gallagher SM, Castorino JJ, Wang D, Philp NJ. Monocarboxylate transporter 4 regulates maturation and trafficking of CD147 to the plasma membrane in the metastatic breast cancer cell line MDA-MB-231. Cancer Res. 2007;67:4182–4189. doi: 10.1158/0008-5472.CAN-06-3184. [DOI] [PubMed] [Google Scholar]
- 13.Mirebeau-Prunier D, Le Pennec S, Jacques C, Fontaine JF, Gueguen N, Boutet-Bouzamondo N, Donnart A, Malthiery Y, Savagner F. Estrogen-related receptor alpha modulates lactate dehydrogenase activity in thyroid tumors. PLoS One. 2013;8:e58683. doi: 10.1371/journal.pone.0058683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11:85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- 15.Koppenol WH, Bounds PL, Dang CV. Otto Warburg’s contributions to current concepts of cancer metabolism. Nat Rev Cancer. 2011;11:325–337. doi: 10.1038/nrc3038. [DOI] [PubMed] [Google Scholar]
- 16.Vander HM. Targeting cancer metabolism: a therapeutic window opens. Nat Rev Drug Discov. 2011;10:671–684. doi: 10.1038/nrd3504. [DOI] [PubMed] [Google Scholar]
- 17.Gatenby RA, Gawlinski ET, Gmitro AF, Kaylor B, Gillies RJ. Acid-mediated tumor invasion: a multidisciplinary study. Cancer Res. 2006;66:5216–5223. doi: 10.1158/0008-5472.CAN-05-4193. [DOI] [PubMed] [Google Scholar]
- 18.Pellegrini P, Strambi A, Zipoli C, Hagg-Olofsson M, Buoncervello M, Linder S, De Milito A. Acidic extracellular pH neutralizes the autophagy-inhibiting activity of chloroquine: Implications for cancer therapies. Autophagy. 2014;10:562–71. doi: 10.4161/auto.27901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rofstad EK, Mathiesen B, Kindem K, Galappathi K. Acidic extracellular pH promotes experimental metastasis of human melanoma cells in athymic nude mice. Cancer Res. 2006;66:6699–6707. doi: 10.1158/0008-5472.CAN-06-0983. [DOI] [PubMed] [Google Scholar]
- 20.Koukourakis MI, Giatromanolaki A, Harris AL, Sivridis E. Comparison of metabolic pathways between cancer cells and stromal cells in colorectal carcinomas: a metabolic survival role for tumor-associated stroma. Cancer Res. 2006;66:632–637. doi: 10.1158/0008-5472.CAN-05-3260. [DOI] [PubMed] [Google Scholar]
- 21.Halestrap AP. Monocarboxylic Acid transport. Compr Physiol. 2013;3:1611–1643. doi: 10.1002/cphy.c130008. [DOI] [PubMed] [Google Scholar]
- 22.Deora AA, Philp N, Hu J, Bok D, Rodriguez-Boulan E. Mechanisms regulating tissue-specific polarity of monocarboxylate transporters and their chaperone CD147 in kidney and retinal epithelia. Proc Natl Acad Sci U S A. 2005;102:16245–16250. doi: 10.1073/pnas.0504419102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Biswas C, Zhang Y, DeCastro R, Guo H, Nakamura T, Kataoka H, Nabeshima K. The human tumor cell-derived collagenase stimulatory factor (renamed EMMPRIN) is a member of the immunoglobulin superfamily. Cancer Res. 1995;55:434–439. [PubMed] [Google Scholar]
- 24.Philp NJ, Ochrietor JD, Rudoy C, Muramatsu T, Linser PJ. Loss of MCT1, MCT3, and MCT4 expression in the retinal pigment epithelium and neural retina of the 5A11/basigin-null mouse. Invest Ophthalmol Vis Sci. 2003;44:1305–1311. doi: 10.1167/iovs.02-0552. [DOI] [PubMed] [Google Scholar]
- 25.Aratake Y, Marutsuka K, Kiyoyama K, Kuribayashi T, Miyamoto T, Yakushiji K, Ohno S, Miyake Y, Sakaguchi T, Kobayashi TK, Okayama A, Tamura K, Ohno E. EMMPRIN (CD147) expression and differentiation of papillary thyroid carcinoma: implications for immunocytochemistry in FNA cytology. Cytopathology. 2010;21:103–110. doi: 10.1111/j.1365-2303.2009.00716.x. [DOI] [PubMed] [Google Scholar]
- 26.Tan H, Ye K, Wang Z, Tang H. CD147 expression as a significant prognostic factor in differentiated thyroid carcinoma. Transl Res. 2008;152:143–149. doi: 10.1016/j.trsl.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 27.Su J, Chen X, Kanekura T. A CD147-targeting siRNA inhibits the proliferation, invasiveness, and VEGF production of human malignant melanoma cells by down-regulating glycolysis. Cancer Lett. 2009;273:140–147. doi: 10.1016/j.canlet.2008.07.034. [DOI] [PubMed] [Google Scholar]


