Abstract
Improving the dysfunction of endothelial progenitor cell (EPCs) in patients with diabetes mellitus is important for preventing vascular complication. Vaspin, an adipocytokine, has the anti-atherogenic properties rely on its positive effect on nitric oxide (NO) bioavailability. We hypothesis that vaspin may ameliorate high glucose induced dysfunction of EPCs. In rat bone morrow derived EPCs, glucose treatment results in a decrease in the proliferation and migration capacity in a dose dependent manner. These detrimental effects can be alleviated by vaspin. Furthermore, vaspin increased the production of NO and the effect of vaspin on EPCs can be diminished partly by the eNOS inhibitor (L-NAME). We assessed total eNOS protein expression and Ser1177-phospho-eNOS expression and found that vaspin not only induced eNOS protein expression but also up regulate the eNOS activation. Subsequently, we investigated protein kinase B (Akt) activation in the presence and absence of phosphatidylinositol 3-kinase (PI 3-kinase) inhibitor (LY-2940002). Vaspin increased total Akt and Ser473-phospho-Akt expression and these effects can be blocked by LY-2940002. The results of our study indicate a novel effect of vaspin to regulate eNOS expression and function in EPCs via a PI3K/Akt/eNOS pathway; vaspin may have a protective effect in patients with diabetes to prevent the occurrence of vascular complication.
Keywords: Vaspin, endothelial progenitor cells, diabetes mellitus, protein kinase B, atherosclerosis, phosphatidylinositol 3-kinase, endothelial nitric oxide synthase
Introduction
Vaspin (visceral adipose tissue-derived serine protease inhibitor) is a 392-395-amino acid adipocytokine belonging to the serine protease inhibitor family. It is identified firstly in visceral white adipose tissues of a rat model of abdominal obesity with type 2 diabetes- the Otsuka Long-Evans Tokushima Fatty rat [1]. Prior reports showed that vaspin has the anti-atherogenic properties rely on its positive effect on Nitric Oxide (NO) bioavailability [2].
Endothelial progenitor cells (EPCs) originate from bone marrow. These cells can migrate to the peripheral circulation and participate in the process of endothelial repairing by the capacity of differentiating into mature cells. Dysfunction of EPCs is correlation with many cardiovascular risk factors such as hypercholesteremia, smoke and diabetic mellitus [3]. In patients with type 1 or type 2 diabetes, the number of circulating EPCs is decreased significantly as well as the migration and proliferation capacity of EPCs [4,5]. Results in vitro experiment showed that high glucose reduced the proliferation and migration of EPCs by modifying eNOS-NO bioavailability [6].
It is generally accepted that keep the integrity of the endothelial nitric oxide synthase (eNOS) pathway is important for EPCs function. The major upstream effectors of the eNOS pathway include phosphatidylinositol-3 kinase (PI3K) and protein kinase B (PKB/Akt) [7]. Given the role of vaspin on NO bioavailability and high glucose on EPCs dysfunction. In this report, we tested the hypothesis that vaspin may alleviate the negative effect on EPCs function of high glucose by the PI3K/Akt/eNOS pathway. The approach was to give cultured rat bone marrow-derived EPCs high glucose and/or human recombinant vaspin. The number as well as proliferation and migration capacity of EPCs was observed and immunoblotting techniques were used to detect the variation of PI3K/Akt/eNOS pathway.
Materials and methods
Isolation and identification of EPCs
EPCs were isolated and identified as previously described [8]. Briefly, bone marrow obtained from Sprague-Dawley rats was subjected to density gradient centrifugation to isolate mononuclear cells (MNCs). The MNCs were re suspended in EGM-2MV bullet Kit medium (Lonza, Switzerland) containing endothelial basal medium (EBM-2), 5% foetal bovine serum and supplemented with recombinant human (rh) EGF, rhFGF-B, rhVEGF, rhIGF-1, ascorbic acid and heparin. The glucose concentration of this medium is 5 mM. Cells were seeded in six-well or 96-well tissue culture plates (precoated with fibronectin) at a density of 4 × 106/cm2 and cultured at 37°C in 5% CO2 and humidified incubator. In order to identify the characteristic of EPCs, cells were processed by immunofluroscence staining for the expression of VEGF receptor 2, CD34, and CD133 (Santa Cruz, USA). In addition, cells which double positive for 1,1’-dioctadecyl-3,3,3’,3’-tetramethylindocarbocyanine-labeled acetylated LDL (DiI-acLDL; Molecule Probe) and Ulex europaeus agglutinin-1 (UEA-1) lectins (Sigma, USA) by direct fluorescent staining were identified as EPCs.
Cell treatment
After seeding MNCs on wells, cells were incubated with different concentrations (10 mM, 15 mM, 20 mM) of glucose and/or human recombinant vaspin (100 ng/mL, Sigma, USA) for 4 days. After 96 h of culture, changed the medium for the first time, and the medium was changed each 3 days. In another set experiments, EPCs were incubated with vaspin with or without NG-nitro-L-arginine methyl ester (L-NAME, 0.1 mM).
EPCs number and proliferation assay
After seeding MNCs on wells, cells were incubated with different concentrations (10 mM, 15 mM, 20 mM) of glucose with or without human recombinant vaspin (100 ng/mL, Sigma, USA) for 4 days. The number of EPCs was decided by direct counting six random high-power microscope fields (×100). The WST-8 assay was used to assess the proliferation of EPCs. The cells were cultured in a 96 well culture plate with various concentrations of glucose with or without vaspin (100 ng/mL) underwent further culture for 3 days. Before the measurement, cells placed in serum-starved conditions for 6 h. Subsequently, 10 μl WST-8 dye (Beyotime, China) was added to each well, cells were incubated at 37°C for 4 h, and then the absorbance was determined at 450 nm using a microplate reader.
EPCs migration assay
The migration of EPCs was evaluated by a modified Boyden chamber (Transwell, Corning) assay. In brief, EPCs were treated with trypsin/EDTA, and then 4 × 104 EPCs which pretreatment with high glucose (20 mM) with or without human recombinant vaspin (100 ng/ml) were placed in the upper chamber with serum-free endothelial growth medium; the lower chamber filling with conditional growth medium supplemented with VEGF (50 ng/ml). After incubation for 24 h, the membrane was washed with PBS and fixed in 4% paraformaldeyde. The upper side of the membrane was wiped with a cotton ball. The membrane was then stained using crystal violet solution. The migration of EPCs was evaluated by direct counting six random high-power (×100) microscope fields. In another set of experiment, cells were incubated with vaspin (100 ng/ml) with or without NG-nitro-L-arginine methyl ester (L-NAME, 0.1 mM) in the up chamber.
Western blot analysis
EPCs were lysed in a Cell lysis buffer (Beyotime, China) supplemented with 0.5 mM PMSF and 2 mM Sodium orthovanadate. The protein lysates was subjected to SDS-PAGE, followed by electroblotting onto PVDF membrane. Membranes were probed with antibodies against eNOS, phospho-eNOS (Ser1177), Akt, phospho-Akt (Ser473), and actin (Santa Cruz, USA). For detecting Phospho-eNOS and phospho-Akt levels, cells were stimulated with vaspin (100 ng/ml) for 30 min with or without PI 3-kinase inhibitor (LY-294002, Sigma, USA) and Akt kinase inhibitor (GSK690693, Sigma, USA). Bands were detected by enhanced chemiluminescence (Pierce, USA). Densitometry signals were quantified by ImageQuant TL software.
Statistical analysis
Data is presented as means ± SE. Inter group comparisons were performed by one-way ANOVA test accompanied by post hoc Tukey’s test. Comparisons between two treatment groups were analyzed by the Student’s t-test. Probability values of P < 0.05 were considered statistically significant.
Results
Culture and identification of EPCs
After 48 h of plating, part of the cell attached to the culture plate and the adherent cells gradually stretched and enlarged. After 4-7 days cultured, adherent cells grew as colonies. Cells were spindly, oval or irregular. After a 7 day cultures, cells which were double positive of uptake of DiILDL and binding of UEA-1 defined as EPCs (Figure 1A-C). Furthermore, immunofluorescence assay showed that EPCs were positive expression of CD34, VEGFR2 and CD133 (Figure 1D-F).
Figure 1.
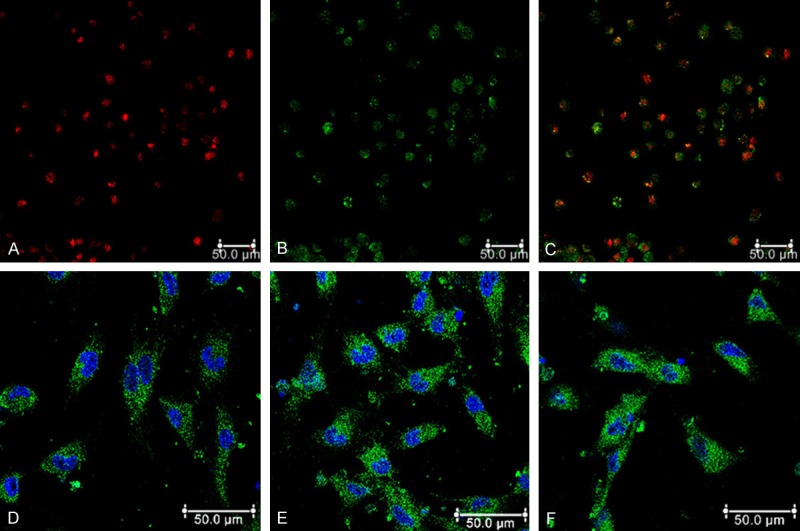
Phenotypes of endothelial characteristics of EPCs. (A) Most cells were shown to endocytose DiI-acLDL (red). (B) EPCs bind fluorescein isothiocyanate UEA-1 (lectin) (green). (C) Double positive for DiI-acLDL and UEA-1. Immunofluorescence detection (green) of CD34 (D), VEGFR2 (E) and CD133 (F) for EPCs. Cells were counterstained with DAPI for nucleus (blue).
Vaspin alleviates EPCs dysfunction induced by high glucose
After seeding MNCs on wells, cells were incubated with different concentrations of glucose for 4 days. The number of differentiated, adherent EPCs was decreased in a dose dependent manner. As compared with that in control medium (5 mM glucose), the amount of EPCs assessed by DiI-acLDL and UEA-1 staining was significantly reduced at 15, 20 and 25 mM glucose medium (17.1, 31.4 and 38.3% inhibition, respectively, P < 0.05) (Figure 2A). The effect of glucose on EPCs proliferation was analyzed by WST-8 assay. Consistently, glucose concentration dependently inhibited EPC proliferation activity, which became apparent at 20 and 25 mM glucose (23.2 and 40.1% inhibition, respectively, P < 0.05) (Figure 2B). The migration capacity of EPCs was impaired by high-glucose incubation (20 mM, 35% inhibition, P < 0.05) (Figure 2C).
Figure 2.
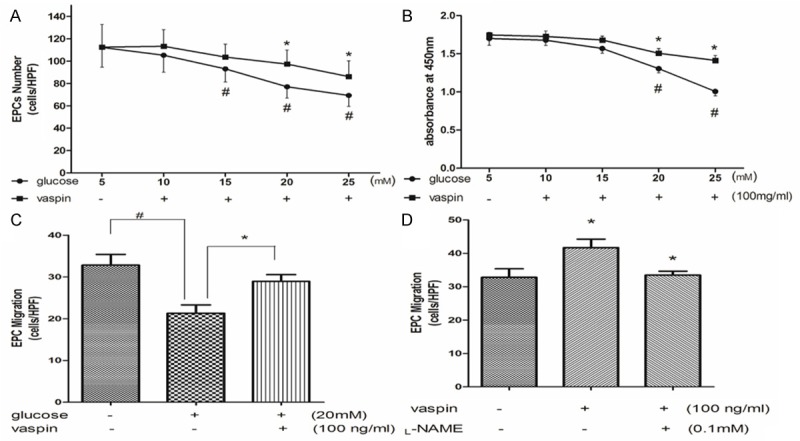
Vaspin alleviates dysfunction of endothelial progenitor cells induced by high glucose. A: Vaspin alleviates EPCs number decrease induced by high glucose. B: Vaspin alleviates EPCs proliferation activity decrease induced by high glucose. C: Vaspin alleviates EPCs migration activity decrease induced by high glucose. D: The enhancement of EPCs migration activity induced by vaspin can be inhabited by L-NAME. #P < 0.05 vs. control, *P < 0.05 vs. glucose alone.
The negative impact of high glucose on EPCs function can be partly rescued by vaspin. As compared with cells that incubation with high glucose, supplemented with vaspin increased EPCs number in different degree. With 20 and 25 mM glucose medium, vaspin increased number of EPCs by 17.9 and 14.3% respectively (P < 0.05) (Figure 2A). The proliferation activity of EPCs increased by 11.9 and 24% in 20 and 25 mM glucose medium respectively (P < 0.05) (Figure 2B). As well as, the migration capacity of EPCs was increased by 35.4% (P < 0.05) (Figure 2C) and the effect of vaspin on migration can be diminished by L-NAME (Figure 2D).
Vaspin stimulates eNOS expression and phosphorylation in part via PI3K/Akt activation
After being incubated with vaspin, the expression of eNOS protein was significantly enhanced in a time dependent manner (Figure 3A). To determine if vaspin stimulation of eNOS requires eNOS phosphorylation at Ser1177, EPCs were incubated with vaspin (100 ng/ml) in a further 30 min. The result showed that eNOS phosphorylation at Ser1177 of EPCs appeared 10 min after vaspin was added and increased over time (Figures 3B and 4).
Figure 3.
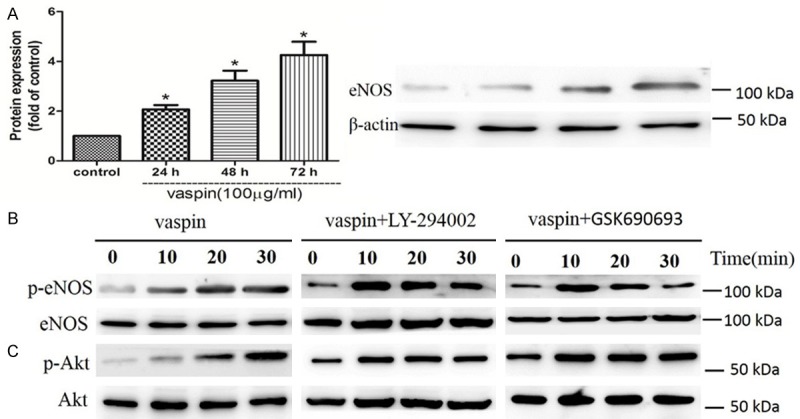
Vaspin-induced eNOS phosphorylation is dependent on protein kinase B (Akt) phosphorylation that are in turn regulated upstream by phosphatidylinositol 3-kinase (PI 3-kinase). (A) Vaspin induced eNOS protein expressing in a time dependent manner. (B, C) Representative Western blots for phospho (p)-eNOS (B), p-Akt (C) from EPCs that were stimulated with vaspin (100 ng/ml) alone, with vaspin following incubation with the PI 3-kinase inhibitor LY-294002 (50 μM), or with vaspin post treatment with the Akt kinase inhibitor GSK690693 (10 μM) for 30 min.
Figure 4.
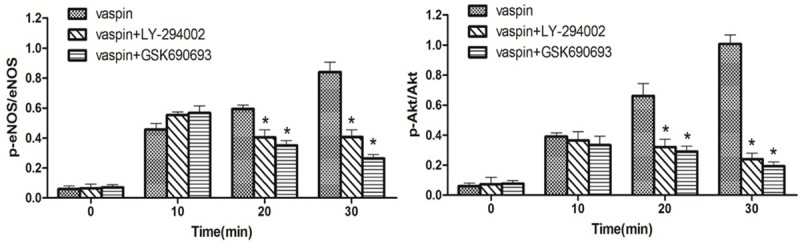
Quantitative temporal analyses of vaspin-induced eNOS and Akt phosphorylation following LY-294002 and GSK690693 treatments. Data are presented as means ± SE; *P < 0.05 vs. control (n = 3).
Given that Akt mediated phosphorylation of the enzyme is critical for activating eNOS, furthermore, Akt is activated by PI 3-kinase through phosphorylated at Ser473 and Ser308. We evaluated the contribution of Akt and PI 3-kinase in the phosphorylation of eNOS by vaspin. Akt phosphorylation at Ser473 was observed at 10 min post treatment with vaspin (Figures 3C and 4). Akt kinase inhibitor (GSK690693) inhibited Akt and eNOS phosphorylation, revealing that Akt kinase is necessary to activate eNOS. In addition, PI 3-kinase inhibitor (LY-294002), inhibited vaspin induced eNOS and Akt phosphorylation (Figures 3B, 3C and 4), reaffirming that PI 3-kinase is the upstream modulator of Akt activation.
Discussion
EPCs dysfunction is an early event in the development of atherosclerosis. High plasma concentrations of glucose impair EPCs function and are associated with the process of atherosclerosis. In this study, we investigated the effects of vaspin on high glucose-induced dysfunction in EPCs. We found that the negative effect of high glucose on EPCs was partly rescued by vaspin treatment via PI3K/Akt/eNOS pathway, indicating a protective effect of vaspin in high glucose stimulated EPCs.
There has been a paradigm shift from the traditional notion that adipose tissue is an energy store through its ability to release fatty acid to that adipose tissue is an endocrine or paracrine tissue through its ability to release adipokines [9]. Adipokines released from adipocytes, act as paracrine agents and hormones, play a pivotal role in inflammatory and metabolic responses [10]. Vaspin is one of the adipokines which first defined in a rat model of type 2 diabetes mellitus and be regarded as an insulin-sensitizing adipokines [1].
Although the role of vaspin in obesity and diabetes is unclear, the previous study support that increased vaspin secretion may be an adaptive response to protect against obesity, insulin resistance and their comorbidities such as atherosclerosis. Data from Aust et al. showed that low vaspin serum concentrations correlate with recently experienced ischemic events in patients with carotid stenosis despite the lack of an association between circulating vaspin and parameters of atherosclerosis severity [11]. Li et al. [12] found that decreased vaspin plasma levels and mRNA levels in peripheral blood mononuclear cells were observed in patients with unstable angina pectoris. Low vaspin concentrations correlate with coronary artery disease severity. In vitro experiment, vaspin exhibited a protective effect on endothelial cells. The mechanism of the protective effect of vaspin including up regulation of the PI3-kinase/Akt signaling pathway [13], increasing eNOS activity by reducing ADMA level through STAT3-mediated regulation of DDAH II expression [2] and by attenuating the cytokine-induced expression of adhesion molecule genes by inhibiting NF-kappaB following AMPK activation [14]. Consistently, our results in the current study found that vaspin treatment increased EPCs number and enhanced proliferation and migration function of EPCs in a dose dependent manner, indicating the role of vaspin in anti-atherosclerosis by enhancing the endothelial repairment by EPCs.
EPCs originated and resided within the bone marrow, under stimulation such as pro-inflammatory cytokines, EPCs would be mobilizing out of the bone marrow. Then circulating EPCs undergoing homing, further proliferation and differentiation to repair the endothelial injury. Under certain pathophysiologic conditions such as diabetes mellitus this process seems to be blunted, resulting in a dysfunction of EPCs. Tepper et al. [15] found that EPCs isolated from human type 2 diabetics exhibited less proliferation capacity compared to control subjects and this condition was inversely correlated with patient levels of hemoglobin A1C. The potential of diabetic EPCs adhesion to human umbilical vein endothelial cells and tubule formation also decreased compared with controls. Hamed et al. [16] reported that the ability of diabetic EPCs to integrate into vascular networks was reduced as well as circulating EPCs and EPCs colony formation was found to be reduced in these patients. Data from Voo et al. [17] showed that type 2 diabetes mellitus not only limits the abundance of EPCs following acute myocardial infarction, but also limit their activation and the resistance to oxidative stress.
In line with clinical data, studies to investigate EPCs biology in vitro experiment under hyperglycemic conditions also demonstrated a significant reduction in EPC function [16]. The mechanism associated with this loss of function may explain as follows. First of all, high glucose treatment lead to excess generation of reactive oxygen species (ROS) and a reduction in NO bioavailability in EPCs [16]. Excessive ROS formation impacted hypoxia signaling pathways and this process may account for the reduced EPCs mobilization and migration capacity observed in diabetic mouse models [18,19]. Furthermore, hyperglycemic culture conditions accelerated the onset of EPCs senescence leading to the impairment of proliferative activity, which might be mediated by the action of the p38 MAPK pathway [20]. At last, high glucose conditions decreased eNOS, FoxO1, and Akt phosphorylation, which inhibit the bioavailability of NO leading to reduce the number and migration capacity of EPCs. The effects of high glucose could be ameliorated by coincubation with NO donor sodium nitroprusside or p38 mitogen-activated protein kinase inhibitor [6]. In accordance with evidences above-mentioned, our data shows that high glucose treatment reduced the number and migration activity of EPCs companied with a decline of NO production. Furthermore, incubation with vaspin increased the number of EPCs and promoted the migration capacity of EPCs by a dose dependent manner; this positive effect of vaspin can be partially eliminated by eNOS inhibitor L-NAME. These results indicated that vaspin can alleviate the detrimental effect of high glucose on EPCs by elevated NO production.
In addition to regulating the NO synthesis, eNOS also play a critical role in regulating EPCs function. Powerful evidence of eNOS effect on EPCs was that mice deficient in eNOS (Nos3(-/-)) show reduced vascular endothelial growth factor (VEGF)-induced mobilization of EPCs [7]. Excepting the mobilization activity, circulating EPC number, colony-forming and migratory capacities were reduced and which was accompanied by a reduction in the activity and phosphorylation of eNOS [21]. In the current study, we make the novel observation that vaspin regulate endothelial cell function via unregulated eNOS expression. The mechanism associated with eNOS bioavailability regulating including transcriptional up-regulation, posttranscriptional activation. Our data show that vaspin affects NO production by increasing eNOS protein expression. Given that phosphorylation of Ser1177 within eNOS by Akt is critical for activation of eNOS, we investigated the effects of vaspin in this regard. The results show that vaspin can activate protein kinase Akt, which leads to activate eNOS via phosphorylation of the amino acid Ser1177. Because of PI 3-kinase pathway is known to play a pivotal role in the regulation of Akt activation, we investigated the role of PI 3-kinase on Akt phosphorylation by the specific inhibitor LY-294002. Our data show that inhibition of PI 3-kinase by LY-294002 can inhibit vaspin induced phosphorylation of Akt, indicating that vaspin participate in the activation of the PI3K /Akt pathway.
In conclusion, the present study demonstrated that vaspin ameliorates high glucose induced dysfunction of EPCs via activation the PI3K/Akt/eNOS pathway. These results indicate the role of vaspin in anti-atherosclerosis by enhancing the endothelial repairment by EPCs. Further studies are needed to explain the mechanism by which vaspin activates the PI-3 kinase/Akt pathway.
Acknowledgements
This study was supported in part by Tianjin Municipal Natural Science Foundation (13JCYBJC36400) and Tianjin Municipal Health Bureau Science foundation (2011KE113).
Disclosure of conflict of interest
None.
References
- 1.Hida K, Wada J, Eguchi J, Zhang H, Baba M, Seida A, Hashimoto I, Okada T, Yasuhara A, Nakatsuka A, Shikata K, Hourai S, Futami J, Watanabe E, Matsuki Y, Hiramatsu R, Akagi S, Makino H, Kanwar YS. Visceral adipose tissue-derived serine protease inhibitor: a unique insulin-sensitizing adipocytokine in obesity. Proc Natl Acad Sci U S A. 2005;102:10610–10615. doi: 10.1073/pnas.0504703102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jung CH, Lee WJ, Hwang JY, Lee MJ, Seol SM, Kim YM, Lee YL, Kim HS, Kim MS, Park JY. Vaspin increases nitric oxide bioavailability through the reduction of asymmetric dimethylarginine in vascular endothelial cells. PLoS One. 2012;7:e52346. doi: 10.1371/journal.pone.0052346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 4.Fadini GP, Miorin M, Facco M, Bonamico S, Baesso I, Grego F, Menegolo M, de Kreutzenberg SV, Tiengo A, Agostini C, Avogaro A. Circulating endothelial progenitor cells are reduced in peripheral vascular complications of type 2 diabetes mellitus. J Am Coll Cardiol. 2005;45:1449–1457. doi: 10.1016/j.jacc.2004.11.067. [DOI] [PubMed] [Google Scholar]
- 5.van den Oever IA, Raterman HG, Nurmohamed MT, Simsek S. Endothelial dysfunction, inflammation, and apoptosis in diabetes mellitus. Mediators Inflamm. 2010;2010:792393. doi: 10.1155/2010/792393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen YH, Lin SJ, Lin FY, Wu TC, Tsao CR, Huang PH, Liu PL, Chen YL, Chen JW. High glucose impairs early and late endothelial progenitor cells by modifying nitric oxide-related but not oxidative stress-mediated mechanisms. Diabetes. 2007;56:1559–1568. doi: 10.2337/db06-1103. [DOI] [PubMed] [Google Scholar]
- 7.Aicher A, Heeschen C, Mildner-Rihm C, Urbich C, Ihling C, Technau-Ihling K, Zeiher AM, Dimmeler S. Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells. Nat Med. 2003;9:1370–1376. doi: 10.1038/nm948. [DOI] [PubMed] [Google Scholar]
- 8.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 9.Szasz T, Webb RC. Perivascular adipose tissue: more than just structural support. Clin Sci (Lond) 2012;122:1–12. doi: 10.1042/CS20110151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wozniak SE, Gee LL, Wachtel MS, Frezza EE. Adipose tissue: the new endocrine organ? A review article. Dig Dis Sci. 2009;54:1847–1856. doi: 10.1007/s10620-008-0585-3. [DOI] [PubMed] [Google Scholar]
- 11.Aust G, Richter O, Rohm S, Kerner C, Hauss J, Kloting N, Ruschke K, Kovacs P, Youn BS, Bluher M. Vaspin serum concentrations in patients with carotid stenosis. Atherosclerosis. 2009;204:262–266. doi: 10.1016/j.atherosclerosis.2008.08.028. [DOI] [PubMed] [Google Scholar]
- 12.Li HL, Peng WH, Cui ST, Lei H, Wei YD, Li WM, Xu YW. Vaspin plasma concentrations and mRNA expressions in patients with stable and unstable angina pectoris. Clin Chem Lab Med. 2011;49:1547–1554. doi: 10.1515/CCLM.2011.236. [DOI] [PubMed] [Google Scholar]
- 13.Jung CH, Lee WJ, Hwang JY, Seol SM, Kim YM, Lee YL, Park JY. Vaspin protects vascular endothelial cells against free fatty acid-induced apoptosis through a phosphatidylinositol 3-kinase/Akt pathway. Biochem Biophys Res Commun. 2011;413:264–269. doi: 10.1016/j.bbrc.2011.08.083. [DOI] [PubMed] [Google Scholar]
- 14.Jung CH, Lee MJ, Kang YM, Lee YL, Yoon HK, Kang SW, Lee WJ, Park JY. Vaspin inhibits cytokine-induced nuclear factor-kappa B activation and adhesion molecule expression via AMP-activated protein kinase activation in vascular endothelial cells. Cardiovasc Diabetol. 2014;13:41. doi: 10.1186/1475-2840-13-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tepper OM, Galiano RD, Capla JM, Kalka C, Gagne PJ, Jacobowitz GR, Levine JP, Gurtner GC. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation. 2002;106:2781–2786. doi: 10.1161/01.cir.0000039526.42991.93. [DOI] [PubMed] [Google Scholar]
- 16.Hamed S, Brenner B, Aharon A, Daoud D, Roguin A. Nitric oxide and superoxide dismutase modulate endothelial progenitor cell function in type 2 diabetes mellitus. Cardiovasc Diabetol. 2009;8:56. doi: 10.1186/1475-2840-8-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Voo S, Dunaeva M, Eggermann J, Stadler N, Waltenberger J. Diabetes mellitus impairs CD133+ progenitor cell function after myocardial infarction. J Intern Med. 2009;265:238–249. doi: 10.1111/j.1365-2796.2008.02011.x. [DOI] [PubMed] [Google Scholar]
- 18.Ceradini DJ, Yao D, Grogan RH, Callaghan MJ, Edelstein D, Brownlee M, Gurtner GC. Decreasing intracellular superoxide corrects defective ischemia-induced new vessel formation in diabetic mice. J Biol Chem. 2008;283:10930–10938. doi: 10.1074/jbc.M707451200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gallagher KA, Liu ZJ, Xiao M, Chen H, Goldstein LJ, Buerk DG, Nedeau A, Thom SR, Velazquez OC. Diabetic impairments in NO-mediated endothelial progenitor cell mobilization and homing are reversed by hyperoxia and SDF-1 alpha. J Clin Invest. 2007;117:1249–1259. doi: 10.1172/JCI29710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuki S, Imanishi T, Kobayashi K, Matsuo Y, Obana M, Akasaka T. Hyperglycemia accelerated endothelial progenitor cell senescence via the activation of p38 mitogen-activated protein kinase. Circ J. 2006;70:1076–1081. doi: 10.1253/circj.70.1076. [DOI] [PubMed] [Google Scholar]
- 21.Thum T, Hoeber S, Froese S, Klink I, Stichtenoth DO, Galuppo P, Jakob M, Tsikas D, Anker SD, Poole-Wilson PA, Borlak J, Ertl G, Bauersachs J. Age-dependent impairment of endothelial progenitor cells is corrected by growth-hormone-mediated increase of insulin-like growth-factor-1. Circ Res. 2007;100:434–443. doi: 10.1161/01.RES.0000257912.78915.af. [DOI] [PubMed] [Google Scholar]


