Abstract
Aims: To investigate the clinical significance of receptor tyrosine kinase-like orphan receptor 2 (ROR2) in cervical cancer. Methods: We examined ROR2 levels in 8 pairs of surgically resected cervical cancer and adjacent normal cervical tissues by real-time PCR. Moreover, we performed immunohistochemistry to examine ROR2 expression in 94 paraffin-embedded cervical cancer samples and analyzed the association between ROR2 expression, clinicopathologic factors and prognosis. Results: ROR2 expression was up-regulated in cervical cancer tissues compared with adjacent normal cervix. In paraffin-embedded cervical cancer samples, high expression of ROR2 was shown in 40 (42.6%) of 94 cases, also, it was significantly associated with tumor stage (P = 0.018) and lymph nodes metastasis (P = 0.013). Moreover, survival analysis showed that ROR2 expression was an independent prognostic factor of poor overall and recurrent free survival (P = 0.045 and 0.001, respectively). Conclusion: These results indicate that ROR2 is significantly correlated with cancer progression and poor prognosis in cervical cancer.
Keywords: Cervical cancer, ROR2, prognosis, progression
Introduction
In addition to ovarian and endometrial cancer, cervical carcinoma is the third most common gynecologic cancer and accounting for approximately 13% of female malignancies [1]. Squamous cell carcinoma is the most common subtype of cervical cancer and accounts for approximately 80-90% of these tumors [2]. Both surgical resection and radiotherapy are curative treatment, while recurrent cervical cancer in general responds poorly to chemotherapy and radiotherapy [3]. If detected early, cervical cancer can be treated surgically, and 5-year survival rates approaching 90% can be achieved for patients with early stage disease, whereas the prognosis of patients with advanced tumors remains poor with a 5-year survival of less than 40% [4]. Therefore, identification of new biomarkers would enable prediction of tumor progression and the development of new targeted therapies for cervical cancer.
Receptor tyrosine kinase-like orphan receptor 2 (ROR2), also known as neurotrophic tyrosine kinase, belongs to the ROR subfamily of cell surface receptors. The receptor is encoded by the ROR2 gene, which is located on the long arm of chromosome 9 at position 22 [5]. ROR2 may be involved in the early formation of the chondrocytes and may be required for cartilage and growth plate development, and mutations in the ROR2 gene have been found to cause several diseases such as the autosomal recessive form of Robinow syndrome and brachydactyly type B1 [6]. Besides, ROR2 has been recently reported to be involved in tumorigenesis. Wright et al. demonstrated that ROR2 expression correlated with expression of genes involved at the extracellular matrix, and it promotes tumor growth potential in renal cell carcinoma [7]. Recent study also demonstrated that ROR2 has plays an important role in conveying a tonic signal to stabilize soluble β-catenin and create a poised state of enhanced responsiveness to Wnt3a exogenous signals in renal cell carcinoma [8]. By restoring ROR2 activity in colon cancer cells harboring ROR2 promoter hypermethylation, Lara et al. showed that the role of ROR2 in colon cancer is mediated by canonical Wnt and that its epigenetic repression can be pro-oncogenic [9]. Edris found that ROR2 is a useful prognostic indicator in the clinical management of these soft-tissue sarcomas [10]. However, the clinical significance of ROR2 in cervical cancer remains unclear.
In the present study, we aimed to investigate the expression of ROR2 in cervical cancer and further explore the clinical significance and biological functions of ROR2 in cervical cancer. We first examined the expression level of ROR2 in cervical cancer tissues by using Real-time PCR. Next, we analyzed its correlations with clinicopathological characters in order to determine the clinical significance of ROR2 in cervical cancer. Taken together, our research revealed a novel molecule involved in the progression and prognosis of cervical cancer.
Materials and methods
Patients and tissue specimens
A total of 94 paraffin-embedded primary cervical squamous cell carcinoma tissues were obtained from the First Affiliated Hospital of Shenzhen University from January 2004 to December 2007. Clinicopathological data were collected from impatient medical records and presented in Table 1. The median follow-up period was 46 months (range, 0.5-60 months). In addition, 8 pairs of cervical cancer tissues and matched adjacent normal tissues were dissected and frozen liquid nitrogen until further use. For the use of these clinical materials for research purposes, written informed consent from all patients and approval from the Institutional Research Ethics Committee were obtained.
Table 1.
Relationship between ROR2 expression level and clinicopathologic factors
| Variable | Category | No. | ROR2 | P | |
|---|---|---|---|---|---|
|
| |||||
| + | - | ||||
| Age (y) | ≤ 50 | 69 | 27 | 42 | 0.265 |
| > 50 | 25 | 13 | 12 | ||
| FIGO stage | IB | 62 | 21 | 41 | 0.018 |
| > IB | 32 | 19 | 13 | ||
| Differentiation | 1/2 | 40 | 16 | 24 | 0.148 |
| 3 | 54 | 14 | 40 | ||
| Tumor size | ≤ 4 cm | 65 | 26 | 39 | 0.454 |
| > 4 cm | 29 | 14 | 15 | ||
| LN Metastasis | No | 81 | 30 | 51 | 0.013 |
| Yes | 13 | 10 | 3 | ||
RNA extraction and Real-time PCR
Approximate 100 mg tissues from cervical cancer and matched cervical tissues were used for RNA extraction using the Trizol Reagent (Invitrogen) according to manufacturer’s instructions. The RNA was pretreated with RNase-free DNase (Promega), and 1 μg RNA was used for cDNA synthesis. Real-time PCR was performed using a Thermal Cycler Dice® Real-time System TP800 (Takara Bio Inc., Otsu, Japan) system. Sequences of the primers are: ROR2 forward primer 5’-GGCAGAACCCATCCTCGTG-3’, backward primer 5’-CGACTGCGAATCCAGGACC-3’; Actin forward primer 5’-GCACCCAGCACAATGAAGA-3’, backward primer 5’-CGATCCACACGGAGTACTTG-3’.
Immunohistochemical staining
Paraffin-embedded samples were obtained from 94 patients for immunohistochemical analysis. In brief, slides were cut to a thickness of 4 μm, deparaffinized in xylene, and hydrated in a graded series of alcohol, followed by boiling in 10 mmol/L of citrate buffer (pH 6.0) for antigen retrieval. Then sections were incubated with 5% serum to avoid the non-specific binding. The sections were incubated overnight at 4°C with primary mouse antibody at dilutions for ROR2 (Abcam, USA; 1:200). After washing with phosphate-buffered saline (PBS), the slides were incubated with hoseradishperoxidase-conjugated goat-anti-mouse secondary antibody for 30 min, followed by reaction with diaminobenzidine, and counterstaining with Mayer hematoxylin. For blank controls, the primary antibody was omitted. For negative controls, the primary antibody was replaced by nonimmune serum.
Evaluation of immunohistochemical staining
The e valuation of the immunohistochemical staining was performed independently by two pathologists blinded to clinical data. The proportion of positive tumor cells was scored as follows: 0 (no positive tumor cells); 1 (< 10% positive tumor cells); 2 (10-50% positive tumor cells); 3 (51-80% positive tumor cells), and 4 (> 80% positive tumor cells). Staining intensity was graded according to the following criteria: 1 (weak staining = light yellow); 2 (moderate staining = yellow brown) and 3 (strong staining = brown). Staining index (SI) was calculated as the product of staining intensity score and the proportion of positive tumor cells. The cut-off value for distinguishing high and low ROR2 expression was set as an SI of 6.
Statistical analysis
All statistical analyses were performed using SPSS version 16.0 (SPSS, Chicago, IL). The Chi-square test was performed to examine the associations of ROR2 expression with clinicopathological factors. Survival curves were generated using the Kaplan-Meier method and compared using the log -rank test. All results were expressed as means ± standard deviation (S. D.), where P values less than 0.05 were considered statistically significant.
Results
Expression of ROR2 in human cervical cancer specimens
Real-time PCR was performed to analyze ROR2 expression levels in 8 cervical cancer patients. As shown in Figure 1, ROR2 was dramatically upregulated in cervical cancer samples compared with the matched adjacent normal cervical tissues. The representative immunostaining of ROR2 in cervical cancer was shown in Figure 2.
Figure 1.
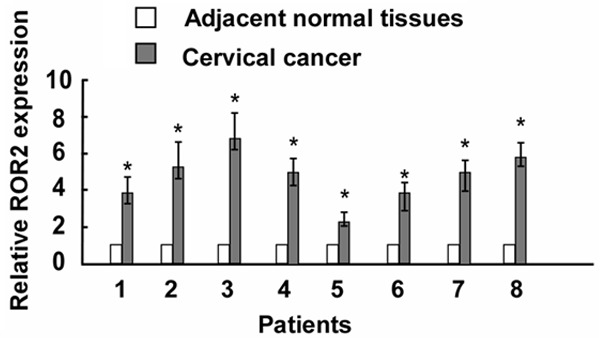
The ROR2 expression in cervical cancer specimens was detected by Real-time PCR (n = 8) compared with matched adjacent normal tissues. Asterisks, P < 0.05.
Figure 2.
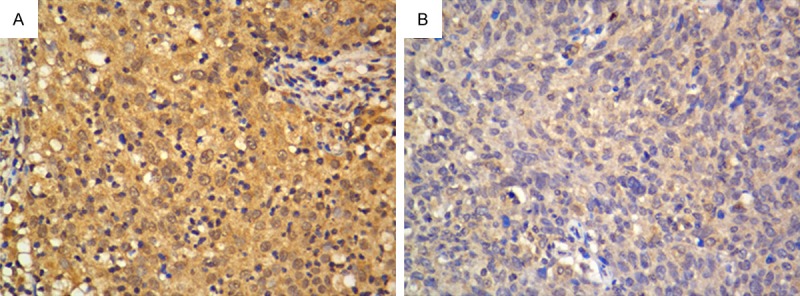
High (A) and low (B) expression of ROR2 in cervical cancer tissues by immunohistochemistry (400 × magnification).
ROR2 association with clinicopathological parameters
Expression of ROR2 was associated with various clinicopathological parameters (summarized in Table 1) using logistic regression modeling. In our analysis, ROR2 was positively correlated with tumor stage (P = 0.018) and lymph nodes metastasis (P = 0.013). However, no significant relationships were found between ROR2 expression and age or tumor grade.
Expression of ROR2 in cervical cancer patients is associated with decreased clinical survival
The prognostic impact of ROR2 in cervical cancer was evaluated using the Kaplan-Meier survival curve analysis. As shown in Figure 3, patients with high expression of ROR2 had significantly decreased overall and recurrent free survival compared to those with low expression (P = 0.045, and P = 0.001, respectively). The Cox proportional hazard model showed that ROR2 could be used as a potential prognostic marker for cervical cancer patients (Table 2).
Figure 3.
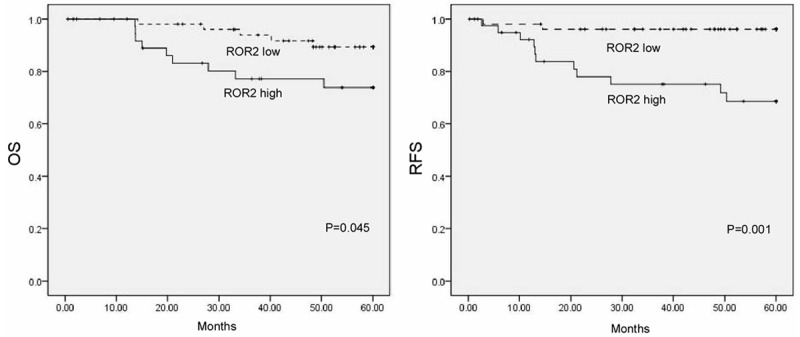
Survival analysis of ROR2. Patients with higher ROR2 expression in cervical cancer were closely correlated with poorer overall and recurrent free survival than patients with tumor with lower ROR2 expression.
Table 2.
Multivariate Cox regression analysis of OS and RFS in cervical cancer patients
| Prognostic variables | OS | RFS | ||
|---|---|---|---|---|
|
|
|
|||
| HR (95% CI) | P | HR (95% CI) | P | |
| Age (> 50 vs. ≤ 50) | 1.102 (0.200-5.154) | 0.962 | 1.273 (0.269-6.029) | 0.736 |
| FIGO Stage (> IB vs. IB) | 1.923 (0.422-7.425) | 0.067 | 1.825 (0.376-7.254) | 0.752 |
| Differentiation (Grade 3 vs. 1/2) | 1.284 (0.873-4.163) | 0.921 | 1.175 (0.562-4.432) | 0.858 |
| Tumor size (> 4 cm vs. ≤ 4 cm) | 1.411 (0.341-4.249) | 0.063 | 1.511 (0.445-6.197) | 0.087 |
| LN Metastasis (+ vs. -) | 2.942 (0.165-8.752) | 0.025 | 2.048 (0.875-6.213) | 0.031 |
| ROR2 expression (high vs. low) | 3.142 (1.008-13.543) | 0.047 | 4.075 (1.73-15.124) | 0.005 |
Discussion
In the present study, we show that the expression of ROR2 was upregulated in cervical cancer tissues in compared with the matched adjacent normal cervical tissues by Real-time PCR. There is correlation between ROR2 expression and tumor stage and lymph nodes metastasis. In addition, statistical analysis showed that high ROR2 expression predicts poor prognosis in patients with cervical cancer. Multivariate Cox regression analysis showed that ROR2 is an independent prognostic marker for cervical cancer patients. Our study may lead to improvement in the diagnosis and treatment of cervical cancer.
It has been previously demonstrated that ROR2 is part of a family of proteins known as receptor protein kinases, which have been reported to play a key role in lots of biological processes, such as cell growth and division, differentiation, cell survival, and cell movement, mainly due to its implication in chemical signaling within cells [11]. Among receptor protein kinases members, ROR2 has been demonstrated to be involved in several cancers, including colon cancer, melanoma, gastric cancer, breast cancer, and renal cancer [9,11]. Recent studies have shown that ROR2 is a receptor of Wnt5a by comparing expression levels and loss-of-function phenotypes between ROR2 and Wnt5a homologs [12], ROR2 and Wnt5a may be involved with the non-canonical
Wnt pathway [13]. It begins when Wnt5a binds to its receptor ROR2, which activated CamKII, a serine/threonine-specific protein kinase that is regulated by the Ca2+/calmodulin complex. Upon activation, CamKII negatively regulates the canonical Wnt/β-catenin signaling through the MAPK pathway. These inhibitory pathways may prevent aberrant Wnt-induced gene expression from cancer-promoting activity. In our previous study, we found that Wnt5a expression is upregulated in cervical cancer. However, whether the Wnt5a/ROR2 pathway is activated in cervical cancer remains unknown.
In our study, we for the first time investigated the clinical significance of ROR2 in cervical cancer patients. By using Real-time PCR, we showed that ROR2 was significantly up-regulated in cervical cancer tissues in compared with the matched adjacent normal cervical tissues. Immunohistological data showed that the ROR2 protein was mainly localized in the cytoplasm in cervical cancer tissues, while the nucleus and membrane showed few staining with ROR2 positive staining. Moreover, high ROR2 expression was correlated with poorer overall and recurrent free survival. Furthermore, multivariate analysis implied that ROR2 could be an independent prognostic factor in patients with cervical cancer. These results indicated the cancer-promoting activity of ROR2 in cervical cancer, which was in lined with previous studies. Mei et al. suggested that ROR2 expression is correlated with malignant attributes of CRC and may serve as an indicator for poor prognosis in patients with colorectal cancer [14]. Wright et al. reported that ROR2 expression correlated with expression of genes involved at the extracellular matrix, and that ROR2 could promote tumor growth potential in renal cell carcinoma [7]. Edris et al. revealed that ROR2 is a novel prognostic indicator in the clinical management of leiomyosarcoma and gastrointestinal stromal tumor and may represent a novel therapeutic target [10]. However, Ford et al. demonstrated that ROR2 appears to possess dual roles as a tumor suppressor or activator depending on tumor type [15], which was supported by Geng et al, who proposed that Wnt5a and ROR2 may serve as tumor suppressor genes in the development of hepatocellular carcinoma [16]. Taken together, these studies imply the tissue specific roles of ROR2 in different cancers.
Interestingly, we found that high expression of ROR2 was associated with tumor stage and lymph nodes metastasis of cervical cancer. Our result is in agreement with recent discovery that ROR2 is a novel Wnt receptor and may play a critical role in driving cell proliferation and migration [17]. O’Connell et al. showed that ROR2 is necessary for the Wnt5a-mediated metastasis of melanoma cells [18]. Lara et al. demonstrated that epigenetic alteration of ROR2 has a Wnt-mediated, pro-tumourigenic role in colon cancer [9]. Lu et al. reported that expression of Wnt5a and ROR2 correlates with disease severity in osteosarcoma patients [19]. These findings imply the tumor-promoting role of ROR2 in cancers.
Conclusion
In conclusion, our data for the first time showed that ROR2 was increased in cervical cancer and indicated that the up-regulation of ROR2 is closely related with the tumor progression and metastasis, and can be an independent marker for predicting the clinical outcome of patients with cervical cancer. These results indicated that ROR2 is a promising biomarker and a potential therapeutic target for cervical cancer in the future.
Disclosure of conflict of interest
None.
References
- 1.Colombo N, Carinelli S, Colombo A, Marini C, Rollo D, Sessa C. Cervical cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(Suppl 7):vii27–32. doi: 10.1093/annonc/mds268. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Forman D, O’Brien M, Ferlay J, Center M, Parkin DM. Cancer burden in Africa and opportunities for prevention. Cancer. 2012;118:4372–4384. doi: 10.1002/cncr.27410. [DOI] [PubMed] [Google Scholar]
- 3.Vici P, Mariani L, Pizzuti L, Sergi D, Di Lauro L, Vizza E, Tomao F, Tomao S, Mancini E, Vincenzoni C, Barba M, Maugeri-Sacca M, Giovinazzo G, Venuti A. Emerging biological treatments for uterine cervical carcinoma. J Cancer. 2014;5:86–97. doi: 10.7150/jca.7963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arbyn M, Castellsague X, de Sanjose S, Bruni L, Saraiya M, Bray F, Ferlay J. Worldwide burden of cervical cancer in 2008. Ann Oncol. 2011;22:2675–2686. doi: 10.1093/annonc/mdr015. [DOI] [PubMed] [Google Scholar]
- 5.Masiakowski P, Carroll RD. A novel family of cell surface receptors with tyrosine kinase-like domain. J Biol Chem. 1992;267:26181–26190. [PubMed] [Google Scholar]
- 6.Oldridge M, Fortuna AM, Maringa M, Propping P, Mansour S, Pollitt C, DeChiara TM, Kimble RB, Valenzuela DM, Yancopoulos GD, Wilkie AO. Dominant mutations in ROR2, encoding an orphan receptor tyrosine kinase, cause brachydactyly type B. Nat Genet. 2000;24:275–278. doi: 10.1038/73495. [DOI] [PubMed] [Google Scholar]
- 7.Wright TM, Brannon AR, Gordan JD, Mikels AJ, Mitchell C, Chen S, Espinosa I, van de Rijn M, Pruthi R, Wallen E, Edwards L, Nusse R, Rathmell WK. Ror2, a developmentally regulated kinase, promotes tumor growth potential in renal cell carcinoma. Oncogene. 2009;28:2513–2523. doi: 10.1038/onc.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rasmussen NR, Wright TM, Brooks SA, Hacker KE, Debebe Z, Sendor AB, Walker MP, Major MB, Green J, Wahl GM, Rathmell WK. Receptor tyrosine kinase-like orphan receptor 2 (Ror2) expression creates a poised state of Wnt signaling in renal cancer. J Biol Chem. 2013;288:26301–26310. doi: 10.1074/jbc.M113.466086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lara E, Calvanese V, Huidobro C, Fernandez AF, Moncada-Pazos A, Obaya AJ, Aguilera O, Gonzalez-Sancho JM, Sanchez L, Astudillo A, Munoz A, Lopez-Otin C, Esteller M, Fraga MF. Epigenetic repression of ROR2 has a Wnt-mediated, pro-tumourigenic role in colon cancer. Mol Cancer. 2010;9:170. doi: 10.1186/1476-4598-9-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edris B, Espinosa I, Muhlenberg T, Mikels A, Lee CH, Steigen SE, Zhu S, Montgomery KD, Lazar AJ, Lev D, Fletcher JA, Beck AH, West RB, Nusse R, van de Rijn M. ROR2 is a novel prognostic biomarker and a potential therapeutic target in leiomyosarcoma and gastrointestinal stromal tumour. J Pathol. 2012;227:223–233. doi: 10.1002/path.3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rebagay G, Yan S, Liu C, Cheung NK. ROR1 and ROR2 in Human Malignancies: Potentials for Targeted Therapy. Front Oncol. 2012;2:34. doi: 10.3389/fonc.2012.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Green JL, Inoue T, Sternberg PW. The C. elegans ROR receptor tyrosine kinase, CAM-1, non-autonomously inhibits the Wnt pathway. Development. 2007;134:4053–4062. doi: 10.1242/dev.005363. [DOI] [PubMed] [Google Scholar]
- 13.McDonald SL, Silver A. The opposing roles of Wnt-5a in cancer. Br J Cancer. 2009;101:209–214. doi: 10.1038/sj.bjc.6605174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mei H, Lian S, Zhang S, Wang W, Mao Q, Wang H. High expression of ROR2 in cancer cell correlates with unfavorable prognosis in colorectal cancer. Biochem Biophys Res Commun. 2014;453:703–9. doi: 10.1016/j.bbrc.2014.09.141. [DOI] [PubMed] [Google Scholar]
- 15.Ford CE, Qian Ma SS, Quadir A, Ward RL. The dual role of the novel Wnt receptor tyrosine kinase, ROR2, in human carcinogenesis. Int J Cancer. 2013;133:779–787. doi: 10.1002/ijc.27984. [DOI] [PubMed] [Google Scholar]
- 16.Geng M, Cao YC, Chen YJ, Jiang H, Bi LQ, Liu XH. Loss of Wnt5a, Ror2 protein in hepatocellular carcinoma associated with poor prognosis. World J Gastroenterol. 2012;18:1328–1338. doi: 10.3748/wjg.v18.i12.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li C, Chen H, Hu L, Xing Y, Sasaki T, Villosis MF, Li J, Nishita M, Minami Y, Minoo P. Ror2 modulates the canonical Wnt signaling in lung epithelial cells through cooperation with Fzd2. BMC Mol Biol. 2008;9:11. doi: 10.1186/1471-2199-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O’Connell MP, Fiori JL, Xu M, Carter AD, Frank BP, Camilli TC, French AD, Dissanayake SK, Indig FE, Bernier M, Taub DD, Hewitt SM, Weeraratna AT. The orphan tyrosine kinase receptor, ROR2, mediates Wnt5A signaling in metastatic melanoma. Oncogene. 2010;29:34–44. doi: 10.1038/onc.2009.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu BJ, Wang YQ, Wei XJ, Rong LQ, Wei D, Yan CM, Wang DJ, Sun JY. Expression of WNT-5a and ROR2 correlates with disease severity in osteosarcoma. Mol Med Rep. 2012;5:1033–1036. doi: 10.3892/mmr.2012.772. [DOI] [PMC free article] [PubMed] [Google Scholar]


