Abstract
Background: Although Clostridium perfringens (C. perfringens) is well known as the causative agent of several forms of enteric disease, precise epidemiological and pathobiological aspects are still unknown. Methods: We retrospectively reviewed the culture results of samples collected in our hospital from 2001 through 2013. In addition, for the detection and toxinogenic typing of C. perfringens, polymerase-chain-reaction amplification (PCR)-based rapid analysis was performed in 6 cases using DNA extracted from paraffin-embedded tissues. Results: A total of 35 samples from 33 cases were positive for C. perfringens, representing an incidence of 0.017% (35/205, 114). Among 33 patients, 21 patients manifested sepsis and 7 patients had bacteremia. One of the septic cases was complicated by fatal intravascular hemolysis and thus, the prevalence was estimated at 3.0% among C. perfringens infections (1/33). The direct causative disease or state for C. perfringens infection was identified in 18 patients: surgery or intervention for cancers, 8 patients; chemotherapy for cancer, 2 patients; surgery or intervention for non-neoplastic disease, 6 patients; liver cirrhosis, 3 patients, etc. PCR-based toxinogenic typing of C. perfringens detected the alpha-toxin gene only in tissue from a patient who died of massive hemolysis; none of the toxin genes could be amplified in the other 5 cases examined. Conclusions: The prevalence of overt C. perfringens infection is low, but upon detection, infected patients should be carefully monitored for fatal acute hemolysis caused by type A C. perfringens. Furthermore, PCR-based rapid detection of C. perfringens and toxinogenic typing by archival pathological material is applicable as a diagnostic tool.
Keywords: Clostridium perfringens, fatal acute hemolysis, alpha-toxin, polymerase-chain-reaction amplification
Introduction
Clostridium perfringens (C. perfringens), an anaerobic, gram-positive bacillus, is a normal inhabitant of the human bowel and genitourinary tract [1]. This bacterium is well known as a causative agent of several forms of enteric disease, including food poisoning and fatal enterotoxemia [2,3]. There are five types of C. perfringens, A, B, C, D and E, classified according to the production of four major exo-toxins (alpha, beta, epsilon and iota). The most commonly encountered type A (alpha-toxin-producing type) strain causes gas gangrene (myonecrosis) and food-borne illness in addition to enterotoxemia in humans [4]. Type B (alpha-, beta-, and epsilon-toxin positive) and type D (alpha- and epsilon positive) strains are the causative agents of fatal enterotoxemia in animals and occasionally in humans [4]. Type C (alpha- and beta-toxin positive) also causes fatal enterotoxemia in humans [2,5]. Type E (alpha- and iota-toxin positive) has rarely been isolated in humans and thus, its pathogenicity remains unclear [2]. These species are sporadically isolated from samples, but such results are often interpreted as false positives due to environmental contamination.
The diagnosis of C. perfringens can be routinely made by microbiological isolation and characterization methods, including bacterial culture and biochemical analysis [2,6]. However, isolation from intestinal contents does not necessarily lead to the diagnosis of enterotoxemia, as this micro-organism is a normal inhabitant of the bowel even in healthy humans [7]. The further toxinogenic typing of C. perfringens is performed by the mouse neutralization test (MNT) after the isolation of the micro-organism [6-8]. However, since classic culture procedures detect only live microorganisms, those conventional techniques are not applicable for the retrospective detection and further toxinogenic genotyping of nonviable bacteria, such as those in formalin-fixed, paraffin-embedded (FFPE) tissues. In this context, during the past few decades, polymerasechain-reaction amplification (PCR) techniques have been applied to detect and to type C. perfringens [2,7]. PCR is a well-accepted, rapid and sensitive technique for the detection of microbial pathogens, and moreover, is efficient under circumstances in which bacteria are present in low numbers.
In the current study, we retrospectively examined the epidemiological, clinical, and pathological aspects of the patients from whose cultures C. perfringens was isolated, including a case manifesting fatal acute hemolysis.
Materials and methods
Samples and patients’ profiles
We retrospectively reviewed the bacteriological records of the patients’ samples, including blood, bile, cystic content and pleural/peritoneal fluid submitted and cultured in the Division of Bacteriology, Central Clinical Laboratory, Saitama Medical Center, Jichi Medical University, between 2001 and 2013. The identification of C. perfringens had been performed by Rap ID kit (ANA II System, Remel Inc. KS, U.S.A.) after culture in HK semi-solid medium and on Brucella HK agar plates (Kyokuto Pharmaceutical Industrial. Ltd., Tokyo, Japan) [6-8]. This retrospective review detected 33 patients positive for C. perfringens (Table 1). Next, the medical records of these 33 patients were reviewed for age, gender, clinical profile, underlying disease, past medical events, radiographic records, and laboratory data. Each of the patients was classified into one of three categories: i) sepsis, defined as systemic inflammatory response syndrome (SIRS) caused by infection; ii) bacteremia, without SIRS; and iii) other, defined as no SIRS or bacteremia, according to the guidelines of the ACCP/SCCM Consensus Conference Committee (Table 1) [9]. This study was approved by the Institutional Tissue Committees and written informed consent from each patient was obtained prior to the molecular analysis described below.
Table 1.
Overall clinical profiles of 33 patients
| Clinical factors | Categories | Summary measure |
|---|---|---|
| Age | < 55 years | 1 (3.0%) |
| [mean, 75.6 years; 41-98 years] | 55 ≤, < 65 years | 4 (12.1%) |
| 65 ≤, < 75 years | 8 (24.2%) | |
| 75 ≤, < 85 years | 15 (45.5%) | |
| 85 years ≤ | 5 (15.2%) | |
| Sex | Male | 19 (57.6%) |
| Female | 14 (42.4%) | |
| Samples (35 samples) | arterial blood | 6 (18.2%) |
| venous blood | 19 (57.6%) | |
| bile | 6 (18.2%) | |
| peritoneal fluid | 3 (9.1%) | |
| surgical wound (pus) | 1 (3.0%) | |
| Clinical Diagnosis | cholangitis/cholecystitis | 6 (18.2%) |
| urinary tract infection | 5 (15.2%) | |
| peritonitis | 5 (15.2%) | |
| pneumonia | 5 (15.2%) | |
| sepsis | 4 (12.1%) | |
| liver abscess | 2 (6.1%) | |
| enterocolitis | 2 (6.1%) | |
| infectious endocarditis | 1 (3.0%) | |
| surgical site infection | 1 (3.0%) | |
| myonecrosis | 1 (3.0%) | |
| unknown | 1 (3.0%) | |
| Entry site | respiratory tract | 5 (15.2%) |
| urinary tract | 5 (15.2%) | |
| bowel | 6 (18.2%) | |
| biliary tract | 8 (24.2%) | |
| surgical wound | 1 (3.0%) | |
| unknown | 8 (24.2%) | |
| Clinical condition | Sepsis (*SIRS) | 21 (63.6%) |
| Bacteremia (without SIRS) | 7 (21.2%) | |
| Others | 5 (15.2%) | |
| Trigger | operation or intervention | 15 (45.5%) |
| cancer | 9 (27.3%) | |
| non-neoplastic disease | 6 (18.2%) | |
| Immunocompromised factor | diabetes | 4 (12.1%) |
| liver cirrhosis | 3 (9.1%) | |
| chemo (radiation) therapy | 2 (6.1%) | |
| interstitial pneumonia | 2 (6.1%) | |
| hemodialysis | 2 (6.1%) |
SIRS, systemic inflammatory response syndrome.
Samples used for PCR
Out of 33 cases, FFPE tissues were available in 9 cases, and informed consent for the molecular analysis was obtained from 6 patients (Cases 5, 10-12, 23, and 26). These six cases included resected specimens for cancers of the cecum (Case 5), the pancreas (Case 23), the stomach (Case 26), cholecystitis (Case 11) and liver abscesses obtained by autopsy (Cases 10, 12). All the samples had been fixed in 15% buffered formalin (pH 7.4) for 24 to 48 hrs prior to embedding in paraffin. The paraffin blocks had been stored for periods between 6 months and 11 years. Microscopic examination of the tissue sections from all, but one case (case 12) revealed no identifiable gram-positive bacilli morphologically suggestive of C. perfringens.
Preparation of samples for PCR
Three 10-μm-thick sections were prepared from each paraffin block, using a new microtome blade for each block, and transferred into a microcentrifuge tube. The sections were deparaffinized by two 30-min incubations in 1 mL xylene at 60°C followed by centrifugation at 1.5 × 104 g for 5 min. The resulting pellets were washed 3 times in 1 mL of absolute ethanol. In parallel, pure cultures of C. perfringens type A (NCTC8798) carrying alpha- and enterotoxin gene, type B (GTC15078) carrying alpha-, beta-, and epsilon-toxin gene and type E (GTC15081) carrying alpha- and iota-toxin, were used as positive controls for PCR [2,7].
Total DNA was extracted using the DNeasy blood and tissue kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions. In brief, each sample was placed in 340 μL of buffer ATL with 25 μl of proteinase K (50 mg/mL), and reaction mixtures were incubated at 56°C overnight. The supernatants were obtained by a brief centrifugation, mixed with 400 μL buffer AL, and incubated at 70°C for an additional 20 min. After incubation, 400 μL of ethanol was added, and the mixture was passed through a QIAamp spin column. DNA was eluted from the column with 100 μL of buffer AE.
Primers for PCR
The oligonucleotide primers specific for C. perfringens loci were synthesized as previously described [10]. Sequences and locations of the primers for genes encoding alpha-, beta-, epsilon-, iota-toxin or enterotoxin are shown in Table 3, and the predicted product sizes were 324, 196, 655, 446 and 233 bp, respectively [10].
Table 3.
PCR primers used in this study
| Gene | Primer sequences 5’ to 3’ | Primer position | Primer concentration (μM) | Product size | Reference |
|---|---|---|---|---|---|
| cpa/alpha | GCTAATGTTACTGCCGTTGACC | 1438-1457 | 0.5 | 324 | 26 |
| TCTGATACATCGTGTAAG | 1762-1743 | ||||
| cpb/beta | GCGAATATGCTGAATCATCTA | 871-891 | 0.36 | 196 | 27 |
| GCAGGAACATTAGTATATCTTC | 1067-1046 | ||||
| etx/ipsilon | GCGGTGATATCCATCTATTC | 227-246 | 0.46 | 655 | 28 |
| CCACTTACTTGTCCTACTAAC | 882-862 | ||||
| iA/iota | ACTACTCTCAGACAAGACAG | 275-294 | 0.52 | 446 | 29 |
| CTTTCCTTCTATTACTATACG | 721-701 | ||||
| cpe/enterotoxin | GGAGATGGTTGGATATTAGG | 439-458 | 0.34 | 233 | 30 |
| GGACCAGCAGTTGTAGATA | 672-650 |
PCR procedures
PCR was performed in a 50-μL scale composed of 2 mM Tris-HCl (pH 8.0), 2 mM MgCl2, 10 mM KCl, 0.2 mM of each dNTPs, 0.2 μM of each primer, 20 ng of DNA, and 1.25 U TaKaRa Ex Taq Hot Start Version (TAKARA BIO INC. Otsu, Shiga, Japan). The reaction was performed in a thermal cycler (GeneAmp PCR system 9700; Applied Biosystems, CA, USA) for 35 cycles, with each cycle consisting of 1 min at 94°C, 1 min at 53°C, and 1 min at 72°C, and cycle was followed by 10 minutes at 72°C. PCR products and molecular size markers (Tracklt 100 bp DNA Ladder, Invitirogen CA, USA) were gel-electrophoresed in a 2.0% agarose gel and visualized after staining with 0.5 μg/mL ethidium bromide.
Results
Culture samples and patients’ profiles
All culture samples collected since 2001 have been monitored in our hospital, which is a tertiary care center with 608 beds. Among the total of 205, 114 samples, C. perfringens were isolated from 33 patients (0.017%), of whom 21 were categorized with “sepsis” (SIRS caused by infection), 7 with “bacteremia” (infection that was positive for C. perfringens in blood culture, but was not complicated by SIRS), and 5 with “other” (Table 1). Clinicopathological details of each patient are presented in Table 2. The number of patients presenting with isolates of C. perfringens generally increased with the patient’s age (younger than 55 years, 1 patient; 55-64 years, 4 patients; 65-74 years, 8 patients; 75-84 years, 15 patients; 85-97 years, 5 patients). Twenty eight patients (84.8%) were > 65 years old; mean age and standard deviation was 75 ± 12 years (range, 41-98 years). There were 19 male and 14 female patients. The samples were submitted from various wards: emergency room (8 patients), gastrointestinal medicine (8 patients), gastrointestinal surgery (8 patients), cardiovascular medicine (2 patients), cardiovascular surgery (2 patients), gynecology (2 patients), urology (2 patients) and neurosurgery (1 patient).
Table 2.
Detail clinical profiles of 33 patients in the current series
| Age | Gender | diagnosis | clinical condition | entry site | sample | diabetes | compromised state | cancer | operation | DNA available | prognosis | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 41 | M | Peritonitis | sepsis | bowel | peritoneal fluid | - | P.O.4 3 weeks | rectum | + | alive | |
| 2 | 55 | F | UTI1 | bacteremia | urinary tract | V2 | - | chemotherapy | uterine body | - | alive | |
| 3 | 57 | M | Enterocolitis | sepsis | bowel | V | - | chemoradiation therapy | lung | - | alive | |
| 4 | 59 | M | Peritonitis | sepsis | unknown | V | + | LC5 | - | - | alive | |
| 5 | 61 | F | Peritonitis | no bacteremia | bowel | peritoneal fluid | - | P.O. 1 day | cecum | + | + | alive |
| 6 | 67 | M | Cholangitis | sepsis | biliary tract | bile | - | IP6, CHF7 | - | + | alive | |
| 7 | 69 | F | UTI | sepsis | urinary tract | V | - | - | - | + | alive | |
| 8 | 70 | M | Cholangitis | no bacteremia | biliary tract | bile | + | - | - | + | alive | |
| 9 | 71 | M | Pneumonia | bacteremia | respiratory tract | V | - | - | lung | - | alive | |
| 10 | 71 | M | Liver abscess | sepsis | biliary tract | V | + | LC | liver | + | + | dead |
| 11 | 73 | M | Cholecystitis | sepsis | biliary tract | bile | - | P.O. 3 weeks | - | + | + | alive |
| 12 | 73 | F | Liver abscess | sepsis | biliary tract | bile | - | - | bile duct | + | + | dead |
| 13 | 73 | F | Unknown | bacteremia | unknown | V | - | - | uterine body | - | unknown | |
| 14 | 75 | M | Sepsis | sepsis | unknown | V | - | - | rectum | + | alive | |
| 15 | 75 | F | Cholangitis | sepsis | biliary tract | bile | - | - | - | - | alive | |
| 16 | 76 | F | Peritonitis | sepsis | unknown | A3, V | - | LC | liver | + | dead | |
| 17 | 76 | F | Pneumonia | bacteremia | respiratory tract | V | - | - | - | - | alive | |
| 18 | 76 | M | Peritonitis | no bacteremia | bowel | peritoneal fluid | - | HD8 | - | - | dead | |
| 19 | 76 | F | Myonecrosis | sepsis | bowel | A | - | - | rectum | - | alive | |
| 20 | 77 | F | Cholangitis | sepsis | biliary tract | V | - | P.O. 3 weeks | gallbladder | + | alive | |
| 21 | 78 | M | SSI9 | no bacteremia | wound (surgcal site) | pus | - | IP | - | - | alive | |
| 22 | 78 | F | IE10 | bacteremia | unknown | A | - | IE | - | + | alive | |
| 23 | 79 | M | Cholangitis | no bacteremia | biliary tract | bile | - | P.O. 5 days | pancreas | + | + | alive |
| 24 | 81 | M | Sepsis | sepsis | unknown | A | - | CPA11 on arrival | - | - | dead | |
| 25 | 82 | M | Sepsis | sepsis | unknown | V | - | P.O. 2 months | pancreas | + | alive | |
| 26 | 83 | F | Sepsis | sepsis | unknown | V | - | P.O. 7 days | stomach | + | + | alive |
| 27 | 84 | M | UTI | bacteremia | urinary tract | A | - | - | - | - | alive | |
| 28 | 84 | M | UTI | bacteremia | urinary tract | V | - | - | - | - | alive | |
| 29 | 90 | F | enterocolitis | sepsis | bowel | V | - | - | - | - | alive | |
| 30 | 93 | M | UTI | sepsis | urinary tract | V | - | - | colon, bladder | - | alive | |
| 31 | 96 | M | Pneumonia | sepsis | respiratory tract | V | - | HD | melanoma | + | alive | |
| 32 | 97 | F | Pneumonia | sepsis | respiratory tract | V | - | - | - | - | alive | |
| 33 | 98 | F | Pneumonia | sepsis | respiratory tract | A, V | + | - | - | - | alive |
UTI, urinary tract infection;
V, verous blood;
A, arterial blood;
P.O., post operative state;
LC, liver cirrhosis;
IP, Interstitial pneumonia;
CHF, congestive heart failure;
HD, Hemodialysis;
SSI, surgical site infection;
IE, infectious endocarditis;
CPA, cardiopulomary arrest.
As the most common underlying disease or state, surgery or intervention for cancer of the liver, of the hepatobiliary tract, or of the pancreas was present in 6 patients: operation or intervention for pancreatic cancer, 2 patients; bile duct cancer, 1 patient; gallbladder cancer, 1 patient; transarterial infusion for hepatocellular carcinoma, 2 patients (both died). Other surgeries included those for cancer of the gastrointestinal tract in 3 patients. Chemo (-radiation) therapy was performed in 2 patients: one case each for uterine and lung cancer. Surgery or intervention for non-neoplastic disease was performed in 6 patients: cholecystectomy for cholelithiasis (Case 6), nephrectomy for pyelonephritis (Case 7), partial hepatectomy for benign cyst (Case 8), ileocecal resection for ischemic enteritis (Case 11), colectomy for perforation (Case 18), and mitral valve replacement (Case 22). Five patients were conservatively treated: lung cancer (Case 9), uterine cancer (Case 13), brain abscess (Case 17), rectal cancer (Case 19), and colon cancer (Case 30). Viewed from the entry site, infections were classified as follows: urinary tract, 5 patients; bowel, including bacterial translocation, 6 patients; upper respiratory tract, 5 patients; biliary tract, 8 patients; surgical site, 1 patient; unknown sites, 8 patients.
In addition to the 15 patients who presented with infections after surgical intervention, 13 patients were classified with an immunocompromised or related condition: diabetes mellitus, 4 patients (Cases 4, 8, 10, 38); chemo (radiation) therapy for cancer, 2 patients (Cases 2, 3); liver cirrhosis, 3 patients (Cases 4, 10, 16); interstitial pneumonia, 2 patients (Cases 6, 21); and hemodialysis, 2 patients (Cases 18, 31). A total of five patients (Cases 8, 10, 12, 13, 16) died of sepsis. The other 28 patients recovered from the infection. Among 21 patients with sepsis, one patient (Case 12) was a typical case complicated by fatal acute hemolysis whose profile is presented below.
Report of a typical case
A 73-year-old female, who had a past history of pancreatoduodenectomy for bile duct carcinoma at 59 years old, was admitted to our hospital with chief complaint of general malaise and jaundice. She had suffered from fracture of the right 6th and 7th ribs by traffic accident 7 days prior to admission and had been managed conservatively.
The patient appeared confused at the time of admission, presenting with a temperature of 36.0°C, blood pressure 120/80 mmHg, and pulse rate of 110 bpm. Abdominal examination showed tenderness in the upper abdomen.
Laboratory findings showed severe anemia, massive hemolysis, liver dysfunction, inflammatory reaction, and renal dysfunction: Clinical laboratory assays yielded the following: white blood cell count, 8500/mm3; red blood cell count, 210000/mm3; hemoglobin, 4.8 g/dL; hematocrit, 1.8%; platelet count, 440000/mm3; albumin, 2.4 g/dL; total bilirubin, 10.34 mg/dL; direct bilirubin, 5.84 mg/dL; NH3, 1420 μg/dL (normal range; 30-86); cholinesterase, 118 U/L (normal range; 170-445); aspartate aminotransferase, 706 U/L; alanine aminotransferase, 168 U/L; lactate dehydrogenase, 6138 U/L (normal range; 110-220); creatine kinase, 998 U/L (normal range; 6-142); alkaline phosphatase, 740 U/L; γ-glutamyltransferase, 12 U/L; C-reactive protein, 9.21 mg/dL; urea, 77 mg/dL; creatinine, 1.44 mg/dL.
An abdominal enhanced computed tomographic (CT) scan revealed a low-density area with gas formation in the right lobe of the liver (Figure 1). A diagnosis of liver abscess with possible rupture into the abdominal cavity was made.
Figure 1.
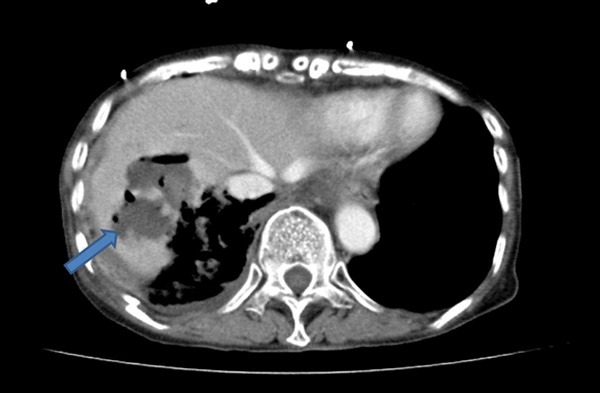
Radiographical imaging of the liver showing gas-containing (pseudo)cystic lesion in the liver found in Case 12.
Despite the fluid replacement therapy, the patient’s hemodynamic condition was unstable. A CT-guided percutaneous transhepatic drainage from the liver abscess was performed. After the drainage in spite of intensive treatment with massive blood transfusion, the patient expired twelve hours after admission. A culture of the liver abscess obtained through drainage tube revealed the presence of C. perfringens and Klebsiella oxytoca. Autopsy was performed and the main finding was an 80-mm liver abscess with perforation to the abdominal cavity (Figure 2A). Cavity was filled with blood clot. Microscopically, inner surface was covered by inflammatory exudate and necrotic material, and surrounded by viable hepatic tissue (Figure 2B). In the necrotic material, colonies of gram-positive bacillus were observed (Figure 2C). Moreover, microscopic aggregates of red blood cells with fibrin were remarkable in renal tubuli, and scattered fresh thrombi also were found in the glomeruli, suggesting disseminated intravascular coagulation.
Figure 2.
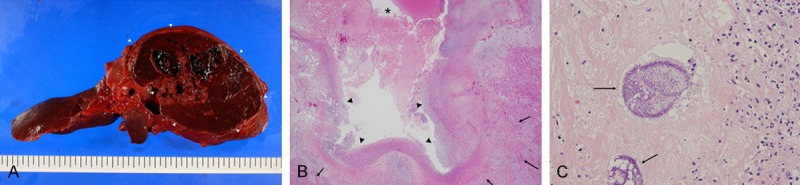
A. Gross appearance of the liver obtained from the autopsy in Case 12. Cut surface revealed pseudocystic and partially necrotic lesion of 8 cm in the maximum dimension (arrow heads). B. Histological features of pseudocyst wall of the liver in Case 12. Lumen was filled with blood clot and fibrin (*). Inner surface was covered by inflammatory exudate and necrotic material (arrow heads), and surrounded by viable hepatic tissue (arrows). C. Around the inner necrotic area, colonies of gram-positive bacillus were observed (arrows).
PCR amplification yielded the products of expected sizes for cpa (alpha-toxin, 324 bp), cpb (beta-toxin, 196 bp), etx (epsilon-toxin, 655 bp), iA (iota-toxin, 446 bp) and cpe (enterotoxin, 233 bp) genes in the appropriate positive controls (Figure 3) [10]. Among the FFPE-derived samples obtained from 6 patients, the alpha-toxin gene was amplified only from the Case-12 specimen; none of the other toxin genes were amplified from any of the samples.
Figure 3.
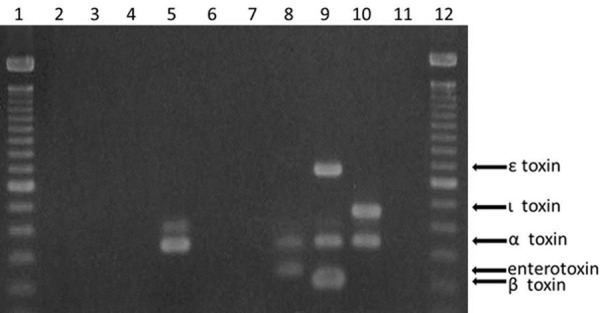
Agarose gel electrophoresis of PCR amplicons obtained using formalin-fixed paraffin-embedded colon tissues from 6 cases (lanes 2-7, Case 5, 10-12, 23, and 25, respectively). Clostridium perfringens type A (NCTC8798) carrying alpha- and entero-toxin gene (lane 8), and type B (GTC15078) carrying alpha-, beta-, and epsilon-toxin gene (lane 9), type E (GTC15081) carrying alpha- and iota-toxin gene (lane 10), negative control (lane 11), and molecular size marker (lanes 1 and 12). Lane 5 (Case 12) showed amplified product of Clostridium perfringens type A-specific alpha-toxin gene.
Discussion
Patients who contract C. perfringens infections exhibit various clinical symptoms including alimentary intoxication, necrotizing enteritis, liver abscess, gas gangrene in the soft tissue, and septic shock [11,12].
Approximately, 0.5-2.0% of all isolates from blood cultures of septic patients are described as clostridial species, and among them, C. perfringens is the most frequently identified micro-organism, accounting for 20-50% [11,13]. Clostridial sepsis often shows very poor prognosis, owing to the life-threatening combination of shock and acute massive hemolysis [14,15]. Massive hemolysis is rare complication of C. perfringens infection, and thus, its prevalence has not been estimated accurately. Among 33 cases from whose samples C. perfringens was cultured in our hospital during the past 13 years, only one case manifested massive intravascular hemolysis, suggesting a prevalence of 3.0%. However, the mortality rate of clostridium-induced massive hemolysis is extremely high, ranging from 70 to 100% [1,16]. Alpha-toxin, mostly in C. perfringens of type A, is a causative toxin with phospholipase C activity, capable of hydrolyzing lecithin into phosphorylcholine and diglyceride. As the cell membrane consists of lipoprotein complexes containing lecithin, alpha-toxin leads to the destruction of cell membrane and subsequently to cell death. The sequelae include necrosis or hemolysis, depending on the tissues involved [16]. The appropriate treatments for C. perfringens sepsis are early diagnosis, extirpation of the focus of infection, prompt initiation of antibiotic treatment, and hyperbaric oxygen therapy [1,16]. Unfortunately, early diagnosis of C. perfringens infection is difficult, because in the early stages, infected patients only manifest non-specific inflammatory symptoms and the slight gas formation as observed in the infected lesions by imaging studies [11].
In Case 12, the diagnosis could not be made when the patient was alive due to the fulminant clinical course. The results of the blood culture and hepatic drainage revealed C. perfringens as the pathogen after the patient died. In addition to this patient, another four patients died, giving an infection-related mortality rate of 15.2%.
The presence of clostridial species in blood cultures are explained by multiple factors, including transient bacteremia (often from an unknown source), trauma, myonecrosis, surgery-related bowel leakage, and occasionally retrograde biliary infections [12,13], consistent with the examples of (emphysematous) cholecystitis, cholangitis, and liver abscess seen in our series. Cancer and immunosuppressive conditions are the main underlying diseases or states for C. perfringens bacteremia or sepsis [11]. In the present study, the most frequent underlying conditions were cancers of the hepatobiliary system and the pancreas (6 cases). Bacteremia caused by C. perfringens often occurs in immunocompromised patients [12], and indeed, 15 of 33 patients (45.5%) in the current series were of this status, including being post-operative, post-intervention, or un-dergoing chemo (radiation) therapy for malignancy. Additional presumed risk factors noted include advanced age, diabetes mellitus, or past history of surgery for biliary and pancreatic cancers (e.g., for placement of choledocho-jejunostomy) as previously described [12].
The identification of bacterial type requires laborious analysis in addition to the routine procedures of bacterial culturing [6-8]. In the current retrospective study, residual samples from patients or bacterial colonies from growth on agar were not available, since most of the samples had been collected more than a year previously. Therefore, we tried to detect the presence of the individual genes encoding each type of exotoxin, as well as the enterotoxin gene, by PCR with total DNA extracted from FFPE tissues. Furthermore, we aimed to find a difference among several categories of pathological lesions comprising gross liver abscesses (2 cases), which were presumed to have locally higher amounts of bacteria, and cancers of the stomach, cecum, and pancreas (one case each) as well as cholecystitis, which were expected to have locally lower levels of bacteria. We detected the presence of the alpha-toxin-encoding gene in only one case (Case 12), obtained from a patient who showed liver abscess and acute hemolysis, confirming that the disease pathogen was type A.
To our knowledge, there is only one previous report that describes the identification of toxinogenic types of C. perfringens from human FFPE tissues. In that work, C. perfringens type C in a patient with emphysematous gastritis, and type B or D in a liver with gas gangrene was detected [2]. Case 12 in our series corresponds to a case complicated by fatal massive hemolysis, which we believe represents the first report of C. perfringens type A detected in FFPE human liver tissue.
Although negative PCR results cannot prove the absence of C. perfringens, the amount of DNA was below the level of detection threshold of this experimental setting. Since the technique presented here is a qualitative rather than quantitative, it was not possible to estimate the amount of C. perfringens in the samples. Moreover, considering the characteristics of the samples, which were FFPE tissues, and the presumed uneven distribution of bacteria within infected tissue, quantification may be impossible. Nonetheless, we speculated that the C. perfringens was locally concentrated and present at higher levels in the liver abscess of Case 12, since that specimen’s DNA was not expected to be well preserved: the tissue was partially necrotic, and the tissues had been left in the paraffin block for a period longer than for the other FFPE specimens. Therefore, the lesion presumably contained locally concentrated C. perfringens, which could have been an inducer of the massive hemolysis observed in this patient. Although the diverse pathobiological mechanisms of C. perfringens remain to be elucidated, this method (PCR-based detection of C. perfringens using DNA extracted from archival FFPE tissues obtained from a pathology laboratory) could be widely utilized for diagnosis and determination of toxinogenic types of C. perfringens in clinical specimens.
In conclusion, C. perfringens was detected in routine bacterial culture of patient samples at a frequency of 0.017%. Among the clostridial-infected patients, fatal massive intravascular hemolysis occurred at 3.0%, possibly due to the presence of a lesion containing localized high-density C. perfringens. In addition, determination of toxigenic gene status by PCR with FFPE tissue may facilitate medical treatment strategies.
Acknowledgements
The authors wish to thank Professor Yoshikazu Hirai, Division of Bacteriology, Department of Infection and Immunity, Jichi Medical University for the helpful support, and the staff in the Department of Pathology, Saitama Medical Center, Jichi Medical University for their excellent technical help. This study was partially supported by grants from the Japan Society for the Promotion of Science C26460438 (Y.D.) and from the Smoking Research Foundation (Y.D).
Disclosure of conflict of interest
None.
References
- 1.van Bunderen CC, Bomers MK, Wesdorp E, Peerbooms P, Veenstra J. Clostridium perfringens septicaemia with massive intravascular haemolysis: a case report and review of the literature. Neth J Med. 2010;68:343–346. [PubMed] [Google Scholar]
- 2.Wu J, Zhang W, Xie B, Wu M, Tong X, Kalpoe J, Zhang D. Detection and toxin typing of Clostridium perfringens in formalin-fixed, paraffin-embedded tissue samples by PCR. J Clin Microbiol. 2009;47:807–810. doi: 10.1128/JCM.01324-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoo HS, Lee SU, Park KY, Park YH. Molecular typing and epidemiological survey of prevalence of Clostridium perfringens types by multiplex PCR. J Clin Microbiol. 1997;35:228–232. doi: 10.1128/jcm.35.1.228-232.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hatheway CL. Toxigenic clostridia. Clin Microbiol Rev. 1990;3:66–98. doi: 10.1128/cmr.3.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petrillo TM, Beck-Sague CM, Songer JG, Abramowsky C, Fortenberry JD, Meacham L, Dean AG, Lee H, Bueschel DM, Nesheim SR. Enteritis necroticans (pigbel) in a diabetic child. N Engl J Med. 2000;342:1250–1253. doi: 10.1056/NEJM200004273421704. [DOI] [PubMed] [Google Scholar]
- 6.Kadra B, Guillou JP, Popoff M, Bourlioux P. Typing of sheep clinical isolates and identification of enterotoxigenic Clostridium perfringens strains by classical methods and by polymerase chain reaction (PCR) FEMS Immunol Med Microbiol. 1999;24:259–266. doi: 10.1111/j.1574-695X.1999.tb01292.x. [DOI] [PubMed] [Google Scholar]
- 7.Warren AL, Uzal FA, Blackall LL, Kelly WR. PCR detection of Clostridium perfringens type D in formalin-fixed, paraffin-embedded tissues of goats and sheep. Lett Appl Microbiol. 1999;29:15–19. doi: 10.1046/j.1365-2672.1999.00567.x. [DOI] [PubMed] [Google Scholar]
- 8.Sterne M, Batty I. Pathogenic Clostridia. London: Butherworth and Co.Ltd; 1975. Diagnostic criteria for clostridial infections; pp. 79–84. [Google Scholar]
- 9.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 10.Meer RR, Songer JG. Multiplex polymerase chain reaction assay for genotyping Clostridium perfringens. Am J Vet Res. 1997;58:702–705. [PubMed] [Google Scholar]
- 11.Fujita H, Nishimura S, Kurosawa S, Akiya I, Nakamura-Uchiyama F, Ohnishi K. Clinical and epidemiological features of Clostridium perfringens bacteremia: a review of 18 cases over 8 year-period in a tertiary care center in metropolitan Tokyo area in Japan. Intern Med. 2010;49:2433–2437. doi: 10.2169/internalmedicine.49.4041. [DOI] [PubMed] [Google Scholar]
- 12.Rechner PM, Agger WA, Mruz K, Cogbill TH. Clinical features of clostridial bacteremia: a review from a rural area. Clin Infect Dis. 2001;33:349–353. doi: 10.1086/321883. [DOI] [PubMed] [Google Scholar]
- 13.Murray PR, Baron EJ, Pfaller MA, Tenover FC, Yolken RH, editors. Manual of Clinical Microbiology. 6th. ed. Washington, DC: ASM Press; 1995. Onderdonk ABA, S.D. Clostridium . [Google Scholar]
- 14.Juntermanns B, Radunz S, Heuer M, Vernadakis S, Reis H, Gallinat A, Treckmann J, Kaiser G, Paul A, Saner F. Fulminant septic shock due to Clostridium perfringens skin and soft tissue infection eight years after liver transplantation. Ann Transplant. 2011;16:143–146. doi: 10.12659/aot.882009. [DOI] [PubMed] [Google Scholar]
- 15.Law ST, Lee MK. A middle-aged lady with a pyogenic liver abscess caused by Clostridium perfringens. World J Hepatol. 2012;4:252–255. doi: 10.4254/wjh.v4.i8.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bodey GP, Rodriguez S, Fainstein V, Elting LS. Clostridial bacteremia in cancer patients. A 12-year experience. Cancer. 1991;67:1928–1942. doi: 10.1002/1097-0142(19910401)67:7<1928::aid-cncr2820670718>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]


