Abstract
ATP-binding cassette transporter A1 (ABCA1) plays a crucial role in reverse cholesterol transport and anti-atherosclerosis. Liver X receptor alpha (LXRα) can stimulate cholesterol efflux through ABCA1. It has been well known that adiponectin has cardiovascular protection. In this study, we attempted to clarify the effect of adiponectin on expression of ABCA1, and explored the role of LXRα in the regulation of ABCA1 in RAW 264.7 macrophages. Our results showed that adiponectin increased ABCA1 expression at both the mRNA and protein levels in a dose-dependent and time-dependent manner. Consequently, adiponectin promoted cholesterol efflux and decreased cholesterol content in RAW 264.7 macrophages. Moreover, adiponectin up-regulated the expression of LXRα in a dose-dependent and time-dependent manner in RAW 264.7 macrophages. LXRα small interfering RNA completely abolished the promotion effects of adiponectin. In summary, adiponectin up-regulates ABCA1 expression via the LXRα pathway in RAW 264.7 macrophages. This novel insight could prove useful for developing new treatment strategies for cardiovascular diseases.
Keywords: Adiponectin, atherosclerosis, ATP-binding cassette transporter A1, reverse cholesterol transport
Introduction
It has been well known that foam cells formation when monocyte-derived macrophages accumulated atherogenic lipoproteins is one of the hallmarks of early atherosclerosis [1,2]. Thus, it is becoming more and more important in atherosclerosis therapy to find proper ways to prevent the formation of foam cells. There is increasing evidence to suggest that high density lipoprotein (HDL) can protect against atherosclerosis due to reverse cholesterol transport (RCT), a process that carries excess cholesterol from foam cells back to liver to remove from human bodies [3,4]. The RCT pathway is currently considered to be the only mechanism by which the human body clears away excess cholesterol [4,5]. Previous research showed that the RCT could be promoted by various kinds of mechanisms (e.g. targeted receptor-mediated process, passive aqueous diffusion). Among all of them, ATP-binding cassette (ABC) A1 which is member of ATP-binding cassette transporter superfamily was thought to be able to mediate the transfer of cellular cholesterol to lipid-poor apolipoprotein A-I (apoA-I), so it is the rate-limiting step in RCT [6,7].
Liver X receptors (LXRs) are ligand-activated transcription factors that appear to play an important role as regulators for cholesterol efflux from macrophages. LXRα was reported to potentially regulate the transcription of target genes (e.g., genes encoding ABCA1) by binding to specific elements in their promoters or enhancers [8,9]. Thus, by promoting the expression of ABCA1, LXRα could regulate RCT to maintain the cholesterol level in the human body.
Adiponectin, an adipocyte-derived polypeptide hormone, is well known for its anti-diabetic, insulin-sensitizing, anti-inflammatory and anti-atherogenic properties [10,11]. Recently, we also found that the protective effects of adiponectin during ischemia/reperfusion are mediated by modulating ER Ca2+-ATPase activity and consequently suppressing ER stress via the activation of PI3K/Akt signaling [12]. However, whether adiponectin could affect the ABCA1 expression and ABCA1-dependent cholesterol efflux is still unknown in RAW 264.7 macrophages, and weather LXRα pathway involves in this progress is unclear. Here in this study, we attempted to clarify the effect of adiponectin on expression of ABCA1, and explored the role of LXRα in the regulation of ABCA1 in RAW 264.7 macrophages. Our results showed that adiponectin could increase the ABCA1 expression and cholesterol efflux through LXRα pathway to eventually promote RCT in RAW 264.7 macrophages.
Materials and methods
Materials
Murine RAW 264.7 macrophages line was purchased from Cell Bank of the Chinese Academy of Science. (Shanghai, China). Adiponectin and apoA-I were purchased from Sigma-Aldrich (St Louis, MO, USA); TRIzol Reagent was from Invitrogen (Carlsbad, USA), Rabbit anti-ABCA1, mouse anti-LXRα and GAPDH-specific antibodies were obtained from Abcam (Cambridge, UK). BCA Protein Assay Kit was from Pierce Chemical (Rockford, IL, USA).
Cell culture
The RAW 264.7 macrophages were seeded in six-well plates at 1.0×106 cells per well in RPMI 1640 medium containing 10% fetal bovine serum (FBS), 20 IU/mL penicillin, and 20 µg/mL streptomycin, and maintained at 37°C in a humidified atmosphere of 5% CO2.
RNA isolation and real-time quantitative PCR
Total RNA from cells was extracted using TRIzol reagent according to manufacturer protocol. The targeted genes and primer sequences are as follows: ABCA1: Forward primer: 5’-AACAGTTTGTGGCCCTTTTG-3’, Reverse primer: 5’-AGTTCCAGGCTGGGGTACTT-3’, β-actin: Forward primer: 5’-CCTAGAAGCATTTGCGGTGG-3’, Reverse primer: 5’-GAGCTACGAGCTGCCTGACG-3’. Real-time quantitative PCR, using SYBR Green detection chemistry, was performed on ABI 7300 Real-Time PCR System under the following conditions: 95°C denaturation for 2 minutes, followed by 35 cycles of 95°C for 30 seconds and 60°C for 30 seconds. RT-PCR was performed in a real-time PCR system (Applied Biosystems, USA). Quantitative measurements were Determined using the Ct method and the expression of β-actin was used as the internal control.
Western blot analyses
The macrophages were harvested and protein extracts prepared as previously described [16]. They were then subjected to western blot analyses [10% SDS-polyacryl-amide (SDS-PAGE); 30 µg protein per lane] using mouse anti-ABCA1 and GAPDH -specific antibodies. Immunoreactivity was detected by the ECL test.
Cellular cholesterol efflux experiments
The cellular cholesterol efflux experiments were also performed as previously described [13]. The macrophages were cultured and labeled with 0.2 µCi/mL [3H] cholesterol for 72 h. Cells were washed with phosphate-buffered saline (PBS) and incubated overnight in RPMI 1640 medium containing 0.1% (w/v) bovine serum albumin (BSA). Equilibrated [3H] cholesterol-labeled cells were washed with PBS and incubated in efflux medium containing RPMI 1640 medium and 0.1% BSA with 25 µg/mL apoA-I. Monolayers were washed twice in PBS, and cellular lipids were extracted with isopropanol. Medium and cell-associated [3H] cholesterol was measured by liquid scintillation counting. Percent efflux was calculated by the following equation: [total media counts/(total cellular counts + total media counts)] ×100%.
High performance liquid chromatography (HPLC) assays
HPLC analysis was conducted as previously described [13]. After washed with PBS for three times, cells were removed by scraping with a rubber and sonicated using an ultrasonic processor for 10 min. A 0.1 ml aliquot cell solution (containing 5-20 µg protein) was used to measure the free cholesterol, and another aliquot for total detection. 15% KOH ethanol solution were added and 50°C heated for 2 h for total cholesterol extracting or 300 µl ethanol for free cholesterol extracting. Thereafter, 500 µl of n -hexane: isopropanol (4:1) were added. The mixture was stirred for 1 minute at room temperature (RT). After centrifugation at 3,600×g for 5 min, the supernatants were collected and freeze-dried with liquid nitrogen. The dried samples were reconstituted in mobile phase and 20 µl was injected onto the HPLC system (Waters Co., US). Analysis was performed on C8 reverse phase ODS2 HPLC column with a mobile phase, isopropanol: acetonitrile (50:50) at a flow rate of 1 ml/min and UV detection set at 215 nm. Data were analyzed with Empower software from Waters.
Transfection for LXRα silencing
Pre-designed small interfering RNAs (siRNAs) targeted to LXRα and the negative control siRNAs were purchased from the GenePharma Co. (Shanghai, China). For transfection with siRNA, macrophages were plated in 96-well plates at a density 2×106 cells per well. After 24 h, cells were transfected with LXRα siRNA in Opti-MEM with 5 μg/mL Lipofectamine®RNAiMAX (Invitrogen). The target sequence for LXRα siRNA was AAGTACACAGGAGGCCATCTT, and the target sequence for control non-silencing siRNA was AATTCTCCGAACGTCTCACGT. In comparison to control siRNA, the siRNA of LXRα suppressed the expression of LXRα proteins by 84% according to Western blot analysis.
Statistical analysis
All values are expressed as the mean ± SD. Student’s t test was used for two groups. ANOVA analysis was used to compare means between three or more groups, and Post Hoc multiple comparisons was used for any two groups. All data analyses were conducted using SPSS16.0 software. Statistical significance was determined when P-values were less than 0.05.
Results
Adiponectin up-regulates ABCA1 expression in RAW 264.7 macrophages
We first examined the effect of adiponectin on ABCA1 expression in RAW 264.7 macrophages by real-time quantitative PCR and western blot analyses. The results showed that adiponectin increased ABCA1 expression at both the mRNA and protein levels in a dose-dependent and time-dependent manner (Figure 1A-D).
Figure 1.
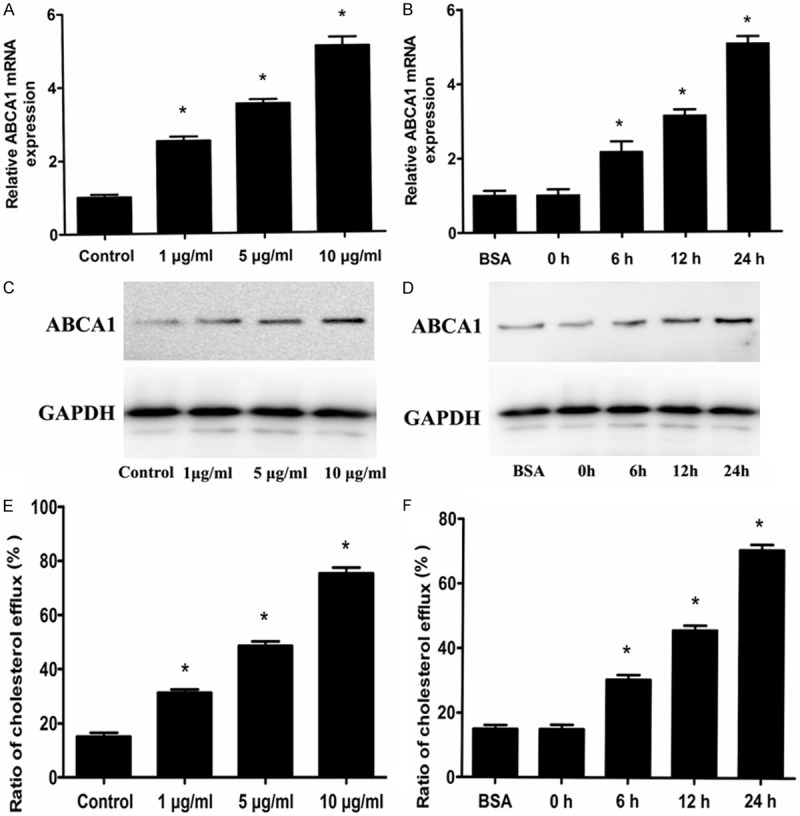
The effects of adiponectin on ABCA1 expression and the cholesterol efflux. A, C and E: The RAW 264.7 macrophages were incubated with 25 µg/mL apoA-I and with 1 µg/mL, 5 µg/mL and 10 µg/mL adiponectin for 24 h respectively. B, D and F: The RAW 264.7 macrophages were incubated with 25 µg/mL apoA-I and 5 mg/mL BSA for 24 h or incubated with 25 µg/mL apoA-I and 10 µg/mL adiponectin for 0 h, 6 h, 12 h, 24 h, respectively. A and B: ABCA1 gene was measured by real-time quantitative PCR. C and D: ABCA1 protein expression was measured by western blot analyses. E and F: Cellular cholesterol efflux from RAW 264.7 macrophages was analyzed using liquid scintillation counting assays. Data are the mean ± SD of three independent experiments. *P < 0.05 compared with the control group.
Adiponectin increases cholesterol efflux and decreases cellular cholesterol content in RAW 264.7 macrophages
ABCA1 is a key player in RCT and is important for regulating cellular cholesterol homeostasis. Because ABCA1 was up-regulated by adiponectin, we next examined the effects of adiponectin on apoA-I specific cholesterol efflux and cholesterol content by liquid scintillation counting and HPLC in RAW 264.7 macrophages. As shown in Figure 1E, 1F; Tables 1, 2, cholesterol efflux was increased while cellular cholesterol content was decreased when cells were treated with adiponectin, suggesting that adiponectin increased ABCA1-mediated cholesterol efflux in RAW 264.7 macrophages.
Table 1.
Effect of adiponectin on cellular cholesterol content at different concentrations in RAW 264.7 macrophages
| Adiponectin | Control | 1 µg/mL | 5 µg/mL | 10 µg/mL |
|---|---|---|---|---|
| TC (mg/dL) | 430 ± 42 | 406 ± 42* | 286 ± 26* | 200 ± 24* |
| FC (mg/dL) | 169 ± 18 | 160 ± 16* | 117 ± 15* | 84 ± 16* |
| CE (mg/dL) | 261 ± 24 | 246 ± 26* | 169 ± 16* | 116 ± 18* |
| CE/TC (%) | 60.6 | 60.5 | 59.1 | 58 |
The RAW 264.7 macrophages were incubated with 25 µg/mL apoA-I and with 1 µg/mL, 5 µg/mL and 10 µg/mL adiponectin for 24 h respectively. HPLC was performed to determine the cellular total cholesterol and free cholesterol. Data are the mean ± SD of three independent experiments.
P < 0.05 compared with the control group.
Table 2.
Effect of adiponectin on cellular cholesterol content at different time in RAW 264.7 macrophages
| Time | BSA (24 h) | 0 h | 6 h | 12 h | 24 h |
|---|---|---|---|---|---|
| TC (mg/dL) | 442 ± 36 | 436 ± 34 | 396 ± 43* | 292 ± 24* | 216 ± 23* |
| FC (mg/dL) | 173 ± 16 | 170 ± 20 | 155 ± 18* | 115 ± 16* | 85 ± 13* |
| CE (mg/dL) | 269 ± 22 | 266 ± 28 | 241 ± 24* | 177 ± 16* | 131 ± 19* |
| CE/TC (%) | 60.9 | 61 | 60.8 | 60.6 | 60.5 |
The RAW 264.7 macrophages were incubated with 25 µg/mL apoA-I and 5 mg/mL BSA for 24 h or incubated with 25 µg/mL apoA-I and 10 µg/mL adiponectin for 0 h, 6 h, 12 h, 24 h, respectively. HPLC was performed to determine the cellular total cholesterol and free cholesterol. Data are the mean ± SD of three independent experiments.
P < 0.05 compared with the control group.
Adiponectin up-regulates LXRα expression in RAW 264.7 macrophages
The above results showed that Adiponectin up-regulates ABCA1 expression in RAW 264.7 macrophages. To confirm whether the LXRα expression can be affected by adiponectin, real-time quantitative PCR and Western blot analyses were performed. As illustrated in Figure 2, adiponectin up-regulated mRNA and protein expression of LXRα in a concentration-dependent and time-dependent manner in RAW 264.7 macrophages.
Figure 2.
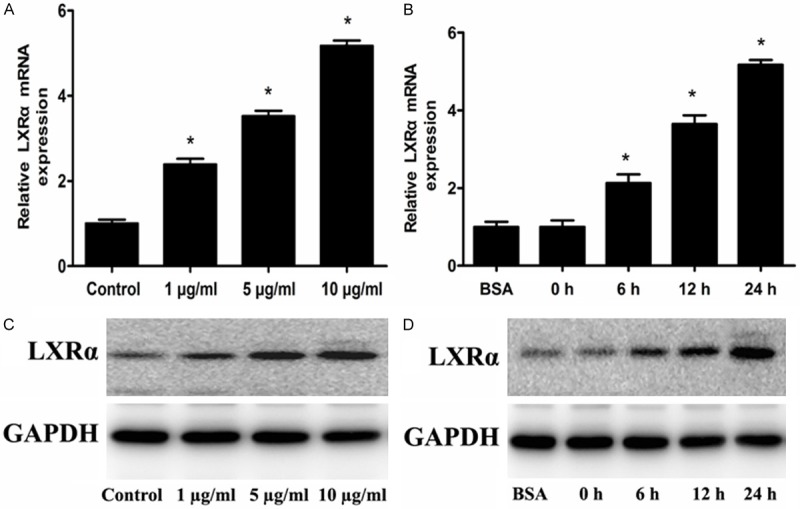
The effect of adiponectin on LXRα expression. The RAW 264.7 macro1phages were incubated with 1 µg/mL, 5 µg/mL and 10 µg/mL adiponectin for 24 h respectively, and macrophages were incubated with 5 mg/mL BSA for 24 h or with 10 µg/mL adiponectin for 0 h, 6 h, 12 h, 24 h, respectively. A and B: LXRα gene expression was measured by real-time quantitative PCR. C and D: LXRα protein expression was measured by Western blot analyses. Data are the means ± SD of three independent experiments. *P < 0.05 compared with the control group.
LXRα is involved in adiponectin up-regulation ABCA1 expression in RAW 264.7 macrophages
In order to study whether adiponectin increases ABCA1expression through LXRα pathway, we finally examined the effect of LXRα siRNA on up-regulation ABCA1 expression by adiponectin. When macrophages were transfected with LXRα siRNA, the expression of LXRα was decreased 86% measured by Western blot analysis (Figure 3A). Treatment with LXRα siRNA completely abolished the upregulation of ABCA1 expression by adiponectin (Figure 3B, 3C). At the same time, the cholesterol efflux to apoA-I and cholesterol content in cells treated by the combination of LXRα siRNA and adiponectin were completely abolished compared with those treated by adiponectin alone (Figure 3D; Table 3). All of these results indicated that the function of adiponectin was through LXRα pathway.
Figure 3.
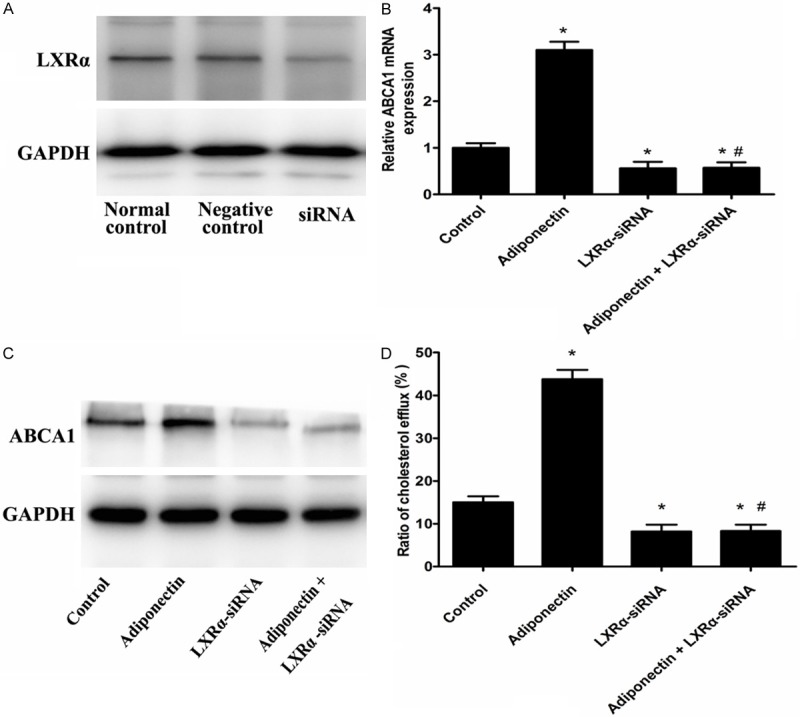
LXRα is involved in adiponectin up-regulation ABCA1 expression in RAW 264.7 macrophages. A: The RAW 264.7 macro1phages were transfected with control or LXRα siRNA, LXRα protein expression was measured by Western blot analyses. B-D: The RAW 264.7 macrophages were tranfected with control or LXRα siRNA, and then incubated with 25 µg/mL apoA-I and 10 µg/mL adiponectin for 24 h. B: ABCA1 gene was measured by real-time quantitative PCR. C: ABCA1 protein expression was measured by western blot analyses. D: cellular cholesterol efflux from RAW 264.7 macrophages was analyzed using liquid scintillation counting assays. Data are the mean ± SD of three independent experiments. *P < 0.05 compared with the control group. #P < 0.05 compared with the adiponectin group.
Table 3.
Effect of LXRα siRNA on cellular cholesterol content in RAW 264.7 macrophages
| Control | Adiponectin | LXRα siRNA | Adiponectin + LXRα siRNA | |
|---|---|---|---|---|
| TC (mg/dL) | 406 ± 43 | 198 ± 34* | 624 ± 23* | 636 ± 24*,# |
| FC (mg/dL) | 158 ± 17 | 78 ± 15* | 244 ± 13* | 249 ± 13*,# |
| CE (mg/dL) | 248 ± 24 | 120 ± 26* | 380 ± 16* | 387 ± 16*,# |
| CE/TC (%) | 61 | 60.5 | 60.9 | 60.9 |
The RAW 264.7 macrophages were tranfected with control or LXRα siRNA, and then incubated with 25 µg/mL apoA-I and 10 µg/mL adiponectin for 24 h. HPLC was performed to determine the cellular total cholesterol and free cholesterol. Data are the mean ± SD of three independent experiments.
P < 0.05 compared with the control group;
P < 0.05 compared with the adiponectin group.
Discussion
In the progression of atherosclerosis, a major factor is the differentiation of monocytes to macrophages that accumulate cholesterol to form foam cells in the vessel wall. HDL plays a key role in protecting against atherosclerosis mainly by RCT, a process that carries excess cholesterol from the arterial wall back to the liver for removal from the body. It has been demonstrated that ABCA1 plays key regulatory roles in RCT by mediating foam cells and other peripheral cellular free cholesterol efflux to apoA-I. So ABCA1 is an important target for the prevention and treatment of atherosclerosis.
Adiponectin is one of several important metabolically active cytokines and plays an important role in metabolic and cardiovascular homeostasis. Low circulating levels of adiponectin have been associated epidemiologically with obesity, metabolic syndrome, type II diabetes, and atherosclerosis [14,15]. Adiponectin has anti-inflammatory, insulin-sensitizing, and antioxidant effects [10,11]. These properties may help understand the negative associations between circulating adiponectin levels and above diseases. It was reported that adiponectin reduced atherosclerotic lesion in apolipoprotein E (apoE) knock-out mice [16]. In addition, adiponectin is an important player in the development of atherosclerotic plaques [17]. In the present study, we found that adiponectin treated cells exhibited a significant increase in ABCA1 expression at both mRNA and protein levels. Consequently, the increase in apoA-I-mediated cholesterol efflux was consistent with an ABCA1 expression increase in adiponectin treated cells. These finding suggest that adiponectin may have atheroprotective properties by increasing cholesterol efflux and reducing macrophage foam cell formation.
As a member of the nuclear receptor superfamily, LXRα is associated with genes related to glucose metabolism, lipid metabolism, cellular cholesterol efflux, and other pathophysiological processes [8,9,18]. LXR agonists were found to upregulate the expression of macrophage ABCA1, which is LXR-target gene, resulting in cholesterol efflux [19]. In this study, adiponectin increased LXRα expression at the mRNA and protein levels in RAW 264.7 macrophages in a concentration-dependent and time-dependent manner. LXRα siRNA treatment resulted in decreased expression of ABCA1 and reduced cholesterol efflux and increased cholesterol content compared with adiponectin macrophages without LXRα siRNA. These results confirmed that the regulation of Adiponectin on ABCA1 is mediated via the LXRα pathway.
Taken together, our data indicate that adiponectin could increase ABCA1 expression and cholesterol efflux of RAW 264.7 macrophages due to the LXRα pathway (Figure 4). These findings provide a theoretical basis for the prevention and treatment of atherosclerosis.
Figure 4.
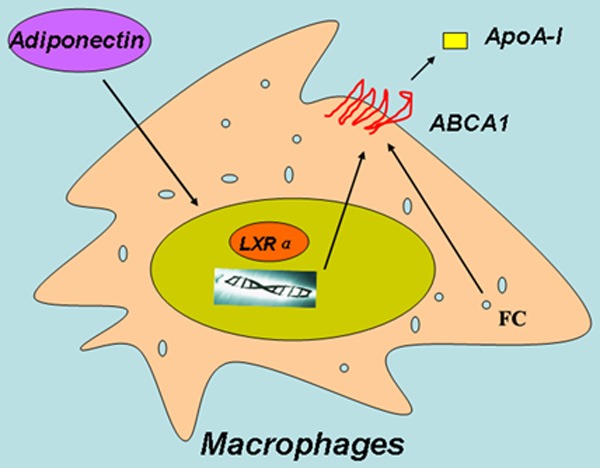
Model of Adiponectin up-regulation of ABCA1 and cellular cholesterol efflux. Adiponectin treatment in RAW 264.7 macrophages increases the expression of LXRα which then upregulates ABCA1 expression, leading to increased cellular cholesterol efflux. FC is free cholesterol.
Acknowledgements
This project was supported by a grant from the National Natural Science Foundation of China (81341025), and Youth Foundation of the Second Hospital of Shanxi Medical University (No.20130104).
Disclosure of conflict of interest
None.
References
- 1.Choudhury RP, Lee JM, Greaves DR. Mechanisms of disease: macrophage-derived foam cells emerging as therapeutic targets in atherosclerosis. Nat Clin Pract Cardiovasc Med. 2005;2:309–315. doi: 10.1038/ncpcardio0195. [DOI] [PubMed] [Google Scholar]
- 2.Yuan Y, Li P, Ye J. Lipid homeostasis and the formation of macrophage-derived foam cells in atherosclerosis. Protein Cell. 2012;3:173–181. doi: 10.1007/s13238-012-2025-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Escola-Gil JC, Rotllan N, Julve J, Blanco-Vaca F. In vivo macrophage-specific RCT and antioxidant and antiinflammatory HDL activity measurements: New tools for predicting HDL atheroprotection. Atherosclerosis. 2009;206:321–327. doi: 10.1016/j.atherosclerosis.2008.12.044. [DOI] [PubMed] [Google Scholar]
- 4.Lee CH, Tai BC, Lim GH, Chan MY, Low AF, Tan KC, Chia BL, Tan HC. Correlation between high density lipoprotein-cholesterol and remodeling index in patients with coronary artery disease: IDEAS (IVUS diagnostic evaluation of atherosclerosis in Singapore)-HDL study. Int J Cardiovasc Imaging. 2012;28:33–41. doi: 10.1007/s10554-010-9777-y. [DOI] [PubMed] [Google Scholar]
- 5.Rosenson RS, Brewer HB Jr, Davidson WS, Fayad ZA, Fuster V, Goldstein J, Hellerstein M, Jiang XC, Phillips MC, Rader DJ. Cholesterol efflux and atheroprotection: advancing the concept of reverse cholesterol transport. Circulation. 2012;125:1905–1919. doi: 10.1161/CIRCULATIONAHA.111.066589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fitzgerald ML, Mujawar Z, Tamehiro N. ABC transporters, atherosclerosis and inflammation. Atherosclerosis. 2010;211:361–370. doi: 10.1016/j.atherosclerosis.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmitz G, Langmann T. Structure, function and regulation of the ABC1 gene product. Curr Opin Lipidol. 2001;12:129–140. doi: 10.1097/00041433-200104000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Delvecchio CJ, Bilan P, Nair P, Capone JP. LXR-induced reverse cholesterol transport in human airway smooth muscle is mediated exclusively by ABCA1. Am J Physiol Lung Cell Mol Physiol. 2008;295:L949–957. doi: 10.1152/ajplung.90394.2008. [DOI] [PubMed] [Google Scholar]
- 9.Xu X, Li Q, Pang L, Huang G, Huang J, Shi M, Sun X, Wang Y. Arctigenin promotes cholesterol efflux from THP-1 macrophages through PPAR-gamma/LXR-alpha signaling pathway. Biochem Biophys Res Commun. 2013;441:321–326. doi: 10.1016/j.bbrc.2013.10.050. [DOI] [PubMed] [Google Scholar]
- 10.Ntaios G, Gatselis NK, Makaritsis K, Dalekos GN. Adipokines as mediators of endothelial function and atherosclerosis. Atherosclerosis. 2013;227:216–21. doi: 10.1016/j.atherosclerosis.2012.12.029. [DOI] [PubMed] [Google Scholar]
- 11.Lee S, Kwak HB. Role of adiponectin in metabolic and cardiovascular disease. J Exerc Rehabil. 2014;10:54–59. doi: 10.12965/jer.140100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo J, Bian Y, Bai R, Li H, Fu M, Xiao C. Globular adiponectin attenuates myocardial ischemia/reperfusion injury by upregulating endoplasmic reticulum Ca2+-ATPase activity and inhibiting endoplasmic reticulum stress. J Cardiovasc Pharmacol. 2013;62:143–53. doi: 10.1097/FJC.0b013e31829521af. [DOI] [PubMed] [Google Scholar]
- 13.Liang B, Wang X, Bian YF, Yang H, Liu M, Bai R, Yang Z, Xiao C. Angiotensin-(1-7) upregulates expression of ABCA1 and ABCG1 through the Mas receptor via the liver X receptor alpha signaling pathway in THP-1 macrophages treated with Angiotensin II. Clin Exp Pharmacol Physiol. 2014;41:1023–1030. doi: 10.1111/1440-1681.12312. [DOI] [PubMed] [Google Scholar]
- 14.Li S, Shin HJ, Ding EL, van Dam RM. Adiponectin levels and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2009;302:179e88. doi: 10.1001/jama.2009.976. [DOI] [PubMed] [Google Scholar]
- 15.Ayao Pischon T, Girman CJ, Hotamisligil GS, Rifai N, Hu FB, Rimm EB. Plasma adiponectinlevels and risk of myocardial infarction in men. JAMA. 2004;291 doi: 10.1001/jama.291.14.1730. [DOI] [PubMed] [Google Scholar]
- 16.Okamoto Y, Kihara S, Ouchi N, Nishida M, Arita Y, Kumada M, Ohashi K, Sakai N, Shimomura I, Kobayashi H, Terasaka N, Inaba T, Funahashi T, Matsuzawa Y. Adiponectin reduces atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2002;106:2767–2770. doi: 10.1161/01.cir.0000042707.50032.19. [DOI] [PubMed] [Google Scholar]
- 17.Zhu W, Cheng KK, Vanhoutte PM, Lam KS, Xu A. Vascular effects of adiponectin: molecular mechanisms and potential therapeutic intervenetion. Clin Sci. 2008;114:361–374. doi: 10.1042/CS20070347. [DOI] [PubMed] [Google Scholar]
- 18.Pettersson AM, Stenson BM, Lorente-Cebrian S, Andersson DP, Mejhert N, Kratzel J, Astrom G, Dahlman I, Chibalin AV, Arner P, Laurencikiene J. LXR is a negative regulator of glucose uptake in human adipocytes. Diabetologia. 2013;56:2044–2054. doi: 10.1007/s00125-013-2954-5. [DOI] [PubMed] [Google Scholar]
- 19.Aravindhan K, Webb CL, Jaye M, Ghosh A, Willette RN, DiNardo NJ, Jucker BM. Assessing the effects of LXR agonists on cellular cholesterol handling: a stable isotope tracer study. J Lipid Res. 2006;47:1250–1260. doi: 10.1194/jlr.M500512-JLR200. [DOI] [PubMed] [Google Scholar]


