Abstract
High-grade non-muscle-invasive bladder cancer (Non-MIBC) has a high risk of stage progression to muscle-invasive bladder cancer (MIBC) and could be managed either conservatively by transurethral resection of bladder tumor (TURBT) or more aggressively by radical cystectomy. The selection of patients who may benefit from early radical intervention is a challenge. To define useful prognostic markers for progression, we analyzed clinicopathological features and immunohistochemical expression patterns of E2F1, p27, survivin, p53, EZH2, IMP3, TSC1/hamartin, fatty acid synthase, androgen receptor, 14-3-3σ, MAGEA4, and NY-ESO-1 on 118 cases of high-grade Non-MIBC. During the mean follow-up period of 64.3 months, progression occurred in 18 patients (15.3%). Histologically, large amount of invasive component (> 50%) was noted in 35 cases (29.7%) and was strongly associated with progression. Among the 12 biomarkers, high expressions of E2F1 and nuclear p27 were noted in 46 cases (40.0%) and 14 cases (12.7%), respectively, and were associated with frequent progression. Using multivariate analysis, the proportion of invasive component and high E2F1 expression were independent prognostic factors for the prediction of progression. Our results indicated that large amount of invasive carcinoma component and high expressions of p27 and E2F1 were predictive markers for progression in Non-MIBC. Therefore, we suggest that these parameters, especially proportion of invasive carcinoma component and E2F1 expression, should be evaluated during pathologic examination and considered during selection of the appropriate management strategy for high grade Non-MIBC patients.
Keywords: Bladder cancer, non-muscle-invasive bladder cancer, progression, urothelial carcinoma, high-grade
Introduction
Bladder cancer is the most common malignancy of the urinary tract and the sixth most common cancer in men worldwide [1]. Approximately 75% of patients with bladder cancer initially present with non-muscle-invasive bladder cancer (Non-MIBC) that is either confined to the mucosa as a papillary tumor (stage Ta) or carcinoma in situ (CIS, stage Tis) without stromal invasion, or invasion limited to the submucosa (stage T1) [2]. Non-MIBC is a heterogeneous group of tumors with different rates of progression to MIBC, ranging from 0.8% to 45% in 5 years [3]. Previous studies suggest that important risk factors for the progression are the presence of concomitant CIS, higher grade, and T1 stage [3,4]. In addition, multiplicity, large tumor size (≥ 3 cm), and a history of recurrence are considered as risk factors [3,4].
Non-MIBC is generally managed by bladder-conserving transurethral resection of bladder tumor (TURBT), while in MIBC, the urinary bladder is removed by radical cystectomy. Because of the high risk of progression, the management of high-grade Non-MIBC is challenging. Some patients are treated with a combination of TURBT with or without intravesical instillation of Bacillus Calmette-Guérin (BCG), whereas others have been treated more aggressively with cystectomy [5,6]. Therefore, discrimination of high-grade Non-MIBC cases with progressive potential to MIBC is crucial, considering the benefit of early radical intervention, but should be cautious given the cost of removing a patient’s urinary bladder. Nevertheless, few tools are available to predict progression in Non-MIBC patients.
There are recent studies describing the molecular mechanisms behind the progression of bladder cancer [7-14]. They have suggested various proliferation- and progression-related proteins as prognostic markers, including E2F1, p27, p53, EZH2, IMP3, and survivin [7-10]. High expressions of fatty acid synthase (FASN) and the androgen receptor have been related to poor disease-specific survival in bladder cancer [11,12]. TSC1/hamartin, a tumor suppressor involved in the development of various malignancies, controls cell proliferation partly by up-regulating p27 and 14-3-3σ. Low expression of TSC1/hamartin tends to lead to a high risk of progression in Non-MIBC [13]. MAGEA4 and NY-ESO-1 are cancer/testis (CT) antigens, normally expressed only in human germ cells in the testis, but their expression is increased in various types of human cancers including urinary bladder tumors [14].
In this study, we tried to identify clinicopathological features and immunohistochemical (IHC) markers from a panel of biomarkers for the prediction of tumor progression in high-grade Non-MIBC. Several of these were found to be significant predictive markers for progression. Because immunohistochemistry is widely used in pathology laboratories worldwide, the results from our study can be easily applied in the clinic for patient management.
Materials and methods
Study samples
This retrospective study was approved by the Asan Medical Center Institutional Review Board. A total of 403 patients who underwent TURBT between January 1996 and December 2006 at the Asan Medical Center and whose tumor tissues were available for tissue microarray (TMA) construction were included. Cases were reviewed for various pathological features and graded according to the 2004 World Health Organization Tumor Classification and assigned tumor, node, metastasis stages according to the American Joint Committee on Cancer Staging System, 7th edition [15,16]. Patients’ clinical information including age, sex, tumor recurrence and progression was obtained from electronic medical records or hospital charts.
After microscopic examination for diagnostic reassessment and histological tumor grading, 167 patients with high-grade Non-MIBC were selected. Among them, 49 patients were excluded because of residual tumor detection, immediate radical cystectomy within a month after initial TURBT, or a short follow-up period of less than a month. No cases with isolated Tis were included in this study. A total of 118 cases were finally included in this study. Tumor progression was defined as an increase of the T stage from Non-MIBC (Ta or T1) to MIBC (T2) on follow-up TURBT or radical cystectomy, which was performed more than 1 month after initial TURBT. The Cutoff value for the proportion of invasive carcinoma component was determined by ROC curve analysis.
Tissue microarray block construction and immunohistochemistry
TMA blocks with 0.6-mm diameter cores were constructed from formalin-fixed, paraffin-embedded bladder cancer tissue blocks of TURBT specimens using a tissue microarrayer (Beecher Instruments, Silver Spring, MD). Three representative cores were obtained for each case to be included in TMA blocks. One core of normal tonsil was included in each TMA block as a positive control.
The primary antibodies used in this study and subcellular location of corresponding antigens are summarized in Table 1. IHC staining was performed using an automated staining system (BenchMark XT; Ventana Medical Systems, Tucson, AZ) and an ultraView Universal DAB detection kit (Ventana Medical Systems). The results of IHC staining for p53, p27, EZH2, E2F1, IMP3, survivin, and FASN obtained in our previous study (“Kim K.” et. al, 2014, submitted) were utilized to examine their clinical significance as predictive markers of progression in high-grade Non-MIBC. Representative expression patterns of those markers are presented in Figure 1. Nuclei were counterstained with hematoxylin.
Table 1.
Primary antibodies and subcellular location of antigen
| Antibody | Dilution | Company | Subcellular location |
|---|---|---|---|
| p53 | 1:1500 | Dako Corp., Carpentaria, CA | Nucleus |
| p27 | 1:100 | Santa Cruz Biotechnology, Inc., CA | Nucleus, cytoplasm |
| Androgen receptor | 1:100 | Epitomics, CA | Nucleus |
| E2F transcription factor 1 (E2F1) | 1:200 | Invitrogen, Carlsbad, CA | Nucleus |
| Enhancer of zeste homolog 2 (EZH2) | 1:25 | BD Biosciences Pharmingen, San Diego, CA | Nucleus |
| Survivin | 1:50 | Santa Cruz Biotechnology, Inc., CA | Nucleus, cytoplasm |
| Insulin-like growth factor 2 mRNA binding protein 3 (IMP3) | 1:500 | Dako Corp., Carpentaria, CA | Cytoplasm |
| Fatty acid synthase (FASN) | 1:500 | Novus Biologicals, Littleton, CO | Cytoplasm |
| Tuberous sclerosis gene 1 (TSC1)/Hamartin | 1:150 | Abcam, CA | Cytoplasm |
| MAGEA4 | 1:200 | Abcam, CA | Cytoplasm |
| NY-ESO-1 | 1:500 | Invitrogen, Carlsbad, CA | Cytoplasm |
| 14-3-3σ | 1:50 | Santa Cruz Biotechnology, Inc., CA | Cytoplasm |
Figure 1.
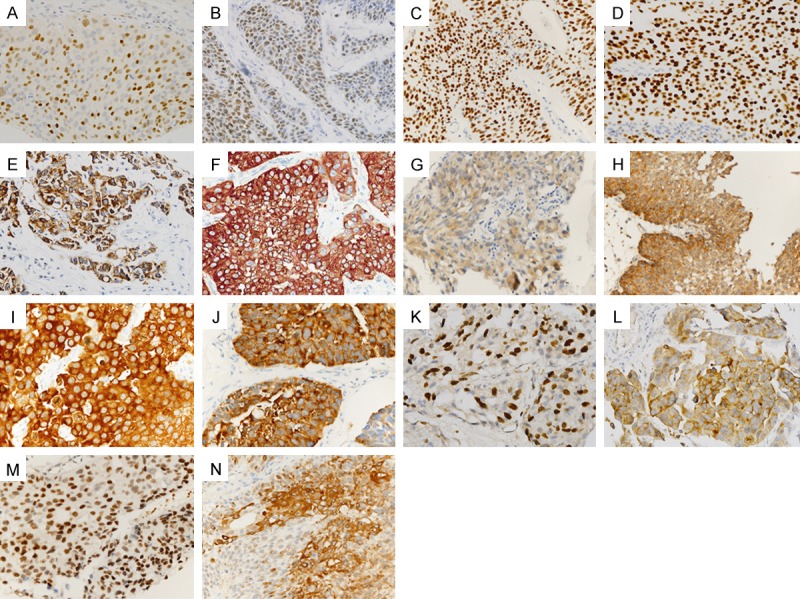
Representative immunohistochemical staining results in urothelial carcinoma: E2F1 (A), EZH2 (B), androgen receptor (C), p53 (D), IMP3 (E), FASN (F), tuberous sclerosis gene 1 (TSC1)/Hamartin (G), MAGEA4 (H), NY-ESO-1 (I), 14-3-3σ (J), nuclear survivin (K), cytoplasmic survivin (L), nuclear p27 (M) and cytoplasmic p27 (N).
Assessment of immunohistochemical results
The TMA slides were evaluated by three independent pathologists (KEK, HJG, and YMC) who were blinded to the associated clinical and pathological information. The percentage of stained tumor cells in the entire area of three cores was recorded. Cutoff values for high expression of each protein were determined by ROC curve analysis as follows: E2F1 (5%), p53 (30%), nuclear p27 (30%), cytoplasmic p27 (30%), EZH2 (30%), IMP3 (30%), cytoplasmic survivin (5%), nuclear survivin (30%), TSC1/hamartin (30%), FASN (30%), androgen receptor (30%), 14-3-3σ (30%), MAGEA4 (30%), and NY-ESO-1 (30%).
Statistical analysis
The relationships between protein expression or clinicopathological parameters and progression were evaluated by cross-correlation analysis, a Cox proportional hazards model and Kaplan-Meier analysis. The hazard ratio, along with the 95% confidence interval, was assessed for each factor. All tests were two-sided, and P-values less than 0.05 were considered statistically significant.
Results
Clinicopathological features of high grade non-MIBC cases
The clinicopathological features of 118 cases are summarized in Table 2. The median age was 70 years (range, 32-93) at the initial TURBT with a 7.4:1 male-to-female ratio. The cases showed the following high risk features: multiplicity (67 cases, 56.8%), tumor size of ≥ 3 cm (40 cases, 33.9%), stage T1 (101 cases, 85.6%), and concurrent CIS (39 cases, 33.1%). The majority of cases (83 cases, 70.9%) included the muscularis propria in the TURBT specimens. The mean proportion of invasive component of the tumor was 34.2% (0-100%). During the mean follow-up period of 64.3 months (range, 1.8-188.5 months), 46 cases (39.0%) experienced tumor recurrence in the urinary bladder at a mean of 11.4 months (range, 1.2-38.5 months). Stage progression occurred in 18 patients (15.3%) at a mean of 17.7 months (range, 2.8-65.3 months) after the initial TURBT.
Table 2.
Clinicopathological features of 118 cases of high-grade non-muscle invasive bladder cancer
| Variables | Number of cases (%) | |
|---|---|---|
| Sex | Male | 104 (88.1) |
| Female | 14 (11.9) | |
| Multiplicity | Absence | 51 (43.2) |
| Presence | 67 (56.8) | |
| Tumor size | Not recorded | 14 (11.9) |
| < 1.5 cm | 16 (13.6) | |
| 1.5 cm-3 cm | 48 (40.7) | |
| > 3 cm | 40 (33.9) | |
| pT stage | Ta | 17 (14.4) |
| T1 | 101 (85.6) | |
| Proportion of invasive component | ≤ 50 | 84 (71.2) |
| > 50 | 34 (28.8) | |
| Carcinoma in situ | Absence | 79 (66.9) |
| Presence | 39 (33.1) | |
| Lymphovascular invasion | Absence | 116 (98.3) |
| Presence | 2 (1.7) | |
| Muscularis propria | Not included | 34 (28.8) |
| Included | 83 (70.3) | |
| Not assessablea | 1 (0.8) | |
| Stage progression | Absence | 100 (84.7) |
| Presence | 18 (15.3) |
Not assessable: due to cautery artifact, fragmentation, or incorrect orientation of tumor tissues.
Clinicopathological factors for the prediction of stage progression in high grade non-MIBC cases
The cases with progression showed more invasive component (> 50%), which was also strongly associated with progression in a Cox regression analysis (Tables 3 and 5). Gender, tumor size, initial T stage of TURBT specimens, concurrent CIS were not associated with progression (Tables 3 and 5). Histological variants, including micropapillary carcinoma, did not meet statistical significance (data not shown).
Table 3.
Correlation between clinicopathological features and stage progression in 118 cases of high grade non-muscle invasive bladder cancer
| Variables | Cases without progression | Cases with progression | P-value | |
|---|---|---|---|---|
| Sex | Male | 88 (88.0) | 16 (88.9) | > 0.999 |
| Female | 12 (12.0) | 2 (11.1) | ||
| Multiplicity | Absence | 47 (47.0) | 4 (22.2) | 0.070 |
| Presence | 53 (53.0) | 14 (77.8) | ||
| Tumor size (cm) | < 1.5 | 14 (15.7) | 2 (13.3) | 0.494 |
| 1.5-3 | 39 (43.8) | 9 (60.0) | ||
| > 3 | 36 (40.5) | 4 (26.7) | ||
| pT stage | Ta | 14 (14.0) | 3 (16.7) | 0.723 |
| T1 | 86 (86.0) | 15 (83.3) | ||
| Invasive component (%) | ≤ 50 | 75 (75.0) | 9 (50.0) | 0.046 |
| > 50 | 25 (25.0) | 9 (50.0) | ||
| Carcinoma in situ | Absence | 67 (67.0) | 12 (66.7) | > 0.999 |
| Presence | 33 (33.0) | 6 (33.3) | ||
| Lymphovascular invasion | Absence | 99 (98.3) | 17 (94.4) | 0.283 |
| Presence | 1 (1.0) | 1 (5.6) |
Table 5.
Prognostic parameters to predict stage progression in 118 cases of high grade non-muscle invasive urothelial carcinomas
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Variables | P value | HR | 95% CI | P value | HR | 95% CI |
| Sex | 0.840 | 1.163 | 0.267-5.061 | |||
| Multiplicity | 0.141 | 2.308 | 0.759-7.019 | |||
| Tumor size (cm) | 0.795 | 1.218 | 0.275-5.400 | |||
| pT stage | 0.902 | 1.081 | 0.312-3.739 | |||
| CIS | 0.868 | 0.920 | 0.345-2.453 | |||
| Invasive component (> 50%) | 0.004 | 3.908 | 1.544-9.888 | 0.004 | 4.306 | 1.577-11.758 |
| E2F1 | 0.009 | 4.050 | 1.424-11.521 | 0.038 | 3.204 | 1.066-9.623 |
| p27 (nucleus) | 0.028 | 3.230 | 1.137-9.172 | 0.120 | 2.401 | 0.796-7.241 |
Abbreviations: HR, hazard ratio; CI, confidence interval; LVI, lymphovascular invasion; CIS, carcinoma in situ.
Protein expression for prediction of stage progression in high grade non-MIBC cases
E2F1, EZH2, androgen receptor, and p53 localized to the nucleus, whereas IMP-3, FASN, hamartin, MAGEA4, NY-ESO-1 and 14-3-3σ localized to the cytoplasm (Figure 1A-J). Survivin and p27 were found in both the nucleus and the cytoplasm (Figure 1K-N).
Cases with progression had more frequent and high expression of E2F1 and nuclear p27 than cases without progression (Table 4). In a Cox regression analysis, cases with high expression of E2F1 and nuclear p27 were associated with frequent progression; this was supported by a Kaplan-Meier analysis (Table 5; Figure 2C, 2D). In a multivariate analysis, the proportion of the invasive component and E2F1 expression were independent prognostic factors for the prediction of progression (Table 5). The expressions of other markers (cytoplasmic p27, nuclear and cytoplasmic survivin, p53, androgen receptor, EZH2, IMP-3, FASN, MAGEA4, TSC1/hamartin, NY-ESO-1, and 14-3-3σ) were not associated with progression in high grade (Tables 4 and 5).
Table 4.
Correlation between protein expression and stage progression in 118 cases of high grade non-muscle invasive bladder cancer
| Variables | Cases without progression | Cases with progression | P-value | |
|---|---|---|---|---|
| P53 | Low | 74 (76.3) | 12 (70.6) | 0.760 |
| High | 23 (23.7) | 5 (29.4) | ||
| Nuclear p27 | Low | 84 (90.3) | 12 (70.6) | 0.040 |
| High | 9 (9.7) | 5 (29.4) | ||
| Cytoplasmic p27 | Low | 85 (91.4) | 15 (88.2) | 0.651 |
| High | 8 (8.6) | 2 (11.8) | ||
| Androgen receptor | Low | 86 (89.6) | 15 (88.2) | > 0.999 |
| High | 10 (10.4) | 2 (11.8) | ||
| E2F1 | Low | 64 (65.3) | 5 (29.4) | 0.007 |
| High | 34 (34.7) | 12 (70.6) | ||
| EZH2 | Low | 87 (90.6) | 15 (88.2) | 0.670 |
| High | 9 (9.4) | 2 (11.8) | ||
| Nuclear survivin | Low | 55 (57.9) | 10 (55.6) | > 0.999 |
| High | 40 (42.1) | 8 (44.4) | ||
| Cytoplasmic survivin | Low | 85 (89.5) | 14 (77.8) | 0.234 |
| High | 10 (10.5) | 4 (22.2) | ||
| IMP3 | Low | 93 (94.9) | 15 (88.2) | 0.276 |
| High | 5 (5.1) | 2 (11.8) | ||
| FASN | Low | 46 (46.0) | 5 (27.8) | 0.199 |
| High | 54 (54.0) | 13 (72.2) | ||
| TSC1/Hamartin | Low | 86 (87.8) | 12 (70.6) | 0.130 |
| High | 12 (12.2) | 5 (29.4) | ||
| MAGEA4 | Low | 29 (30.9) | 2 (11.8) | 0.145 |
| High | 65 (69.1) | 15 (88.2) | ||
| NY-ESO-1 | Low | 96 (97.0) | 100 (100) | > 0.999 |
| High | 3 (3.0) | 0 (0) | ||
| 14-3-3σ | Low | 41 (42.7) | 6 (35.3) | 0.606 |
| High | 55 (57.3) | 11 (64.7) |
Figure 2.
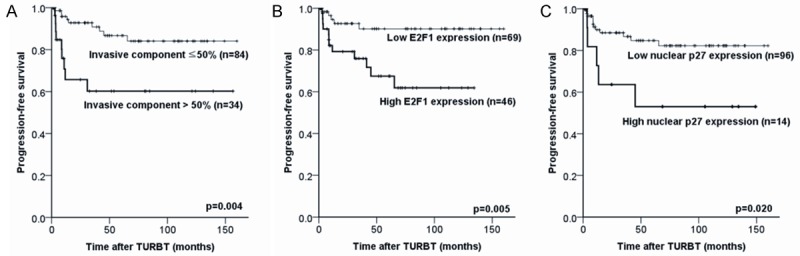
Kaplan-Meier analysis for predicting progression. The invasive tumor component (A), E2F1 expression (B), and nuclear p27 expression (C) are associated with frequent progression.
Discussion
Here, we showed that proportion of invasive carcinoma component is a histological feature independently predictive of progression from Non-MIBC to MIBC. Among the 12 IHC markers, high expressions of nuclear p27 and, especially, E2F1 are strongly associated with tumor progression.
To the best of our knowledge, our study is the first to analyze the prognostic significance of the proportion of the invasive component in Non-MIBC. By definition, all Ta tumors are composed of non-invasive papillary urothelial carcinoma [16]. During TURBT specimen review, we noticed that many T1 tumors were also composed of mostly non-invasive papillary urothelial carcinoma. Since clinical behavior of malignant tumors is usually determined by the invasive component, we decided to evaluate whether the proportion of invasive component in T1 tumors is important for the tumor progression or not. Although the proportion of the invasive component was not measured directly, previous studies had suggested that the presence of a non-papillary solid tumor as a prognostic factor of progression and disease-specific survival in Non-MIBC, in which non-papillary solid pattern was usually the growth pattern of the invasive component [17,18].
E2F1 is a tumor suppressor that plays a critical role in cell-cycle progression and induction of apoptosis in response to DNA damage under normal conditions, but increased levels of E2F1 promote cellular proliferation [19]. Dysregulated E2F1 also mediates tumor progression through the upregulation of epidermal growth factor receptor (EGFR) and activation of the cytoplasmic Ras/mitogen-activated protein kinases (MAPK)/extracellular signal-regulated kinase (ERK) and phosphoinositide-3-kinase (PI3K)/AKT signaling cascades [19]. In fact, high expression of E2F1 and its associated target genes predicted progression from Non-MIBC to MIBC [7].
p27, a cyclin-dependent kinase inhibitor, inhibits cyclin E-CDK2 in the nucleus and regulates the G1-S transition of the cell cycle. Low protein levels and cytoplasmic mislocalization of p27 result in loss of its antiproliferative role and lead to increased cell proliferation and cell migration. Accordingly, low p27 expression was suggested as a poor prognostic factor in various malignant tumors including bladder cancer [5,8,20]. In contrast, this study suggests that the nuclear localization of p27 is directly correlated with progression. Lopez-Beltran et al. also failed to find the correlation between p27 expression and survival for NMIBC [21]. Therefore, a further study is required to confirm the prognostic significance of nuclear p27 expression in high grade Non-MIBC.
Against our expectations, the T stage, CIS, and multiplicity did not predict stage progression in this study. It can be partly explained by the fact that this study included only high-grade cases of Non-MIBC and a relative small number of Ta cases (17 cases, 14.4%). One previous study analyzing all grades (grades 1, 2, and 3) of Non-MIBC reported that the T stage was a statistically significant predictor for recurrence but not progression, where they suggested that this discrepancy stems from an incomplete resection of tumors during TURBT [22].
Although this study showed prognostic significance of histological features (proportion of invasive component) and IHC markers (E2F1 and nuclear p27) in Non-MIBC, its limitations include the retrospective design and reliance on experience from a single institution. Furthermore, remarkable advances in molecular technologies and elucidation of the mechanisms of carcinogenesis and tumor progression have led to the discovery of new molecular markers, including mutant forms of FGFR3 as well as USP18 and DGCR2 expressions [23-25]. Therefore, further multi-institutional studies with newly discovered molecular markers will help validate and strengthen the clinical utility of these results and improve the accuracy of predicting progression in Non-MIBC.
Disclosure of conflict of interest
None.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman DD, Bray F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Lamm D, Persad R, Brausi M, Buckley R, Witjes JA, Palou J, Böhle A, Kamat AM, Colombel M, Soloway M. Defining progression in nonmuscle invasive bladder cancer: it is time for a new, standard definition. J Urol. 2014;191:20–27. doi: 10.1016/j.juro.2013.07.102. [DOI] [PubMed] [Google Scholar]
- 3.Sylvester RJ, van der Meijden AP, Oosterlinck W, Witjes JA, Bouffioux C, Denis L, Newling DW, Kurth K. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol. 2006;49:466–75. doi: 10.1016/j.eururo.2005.12.031. discussion 475-7. [DOI] [PubMed] [Google Scholar]
- 4.van Rhijn BW, Burger M, Lotan Y, Solsona E, Stief CG, Sylvester RJ, Witjes JA, Zlotta AR. Recurrence and progression of disease in non-muscle-invasive bladder cancer: from epidemiology to treatment strategy. Eur Urol. 2009;56:430–42. doi: 10.1016/j.eururo.2009.06.028. [DOI] [PubMed] [Google Scholar]
- 5.Mitra AP, Hansel DE, Cote RJ. Prognostic value of cell-cycle regulation biomarkers in bladder cancer. Semin Oncol. 2012;39:524–33. doi: 10.1053/j.seminoncol.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alkarain A, Slingerland J. Deregulation of p27 by oncogenic signaling and its prognostic significance in breast cancer. Breast Cancer Res. 2004;6:13–21. doi: 10.1186/bcr722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee JS, Leem SH, Lee SY, Kim SC, Park ES, Kim SB, Kim SK, Kim YJ, Kim WJ, Chu IS. Expression signature of E2F1 and its associated genes predict superficial to invasive progression of bladder tumors. J. Clin. Oncol. 2010;28:2660–7. doi: 10.1200/JCO.2009.25.0977. [DOI] [PubMed] [Google Scholar]
- 8.Rabbani F, Koppie TM, Charytonowicz E, Drobnjak M, Bochner BH, Cordon-Cardo C. Prognostic significance of p27Kip1 expression in bladder cancer. BJU Int. 2007;100:259–63. doi: 10.1111/j.1464-410X.2007.06927.x. [DOI] [PubMed] [Google Scholar]
- 9.Wang H, Albadine R, Magheli A, Guzzo TJ, Ball MW, Hinz S, Schoenberg MP, Netto GJ, Gonzalgo ML. Increased EZH2 protein expression is associated with invasive urothelial carcinoma of the bladder. Urol Oncol. 2012;30:428–33. doi: 10.1016/j.urolonc.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 10.Sitnikova L, Mendese G, Liu Q, Woda BA, Lu D, Dresser K, Mohanty S, Rock KL, Jiang Z. IMP3 predicts aggressive superficial urothelial carcinoma of the bladder. Clin Cancer Res. 2008;14:1701–6. doi: 10.1158/1078-0432.CCR-07-2039. [DOI] [PubMed] [Google Scholar]
- 11.Sugino T, Baba K, Hoshi N, Aikawa K, Yamaguchi O, Suzuki T. Overexpression of fatty acid synthase in human urinary bladder cancer and combined expression of the synthase and Ki-67 as a predictor of prognosis of cancer patients. Med Mol Morphol. 2011;44:146–50. doi: 10.1007/s00795-010-0517-0. [DOI] [PubMed] [Google Scholar]
- 12.Miyamoto H, Yang Z, Chen YT, Ishiguro H, Uemura H, Kubota Y, Nagashima Y, Chang YJ, Hu YC, Tsai MY, Yeh S, Messing EM, Chang C. Promotion of bladder cancer development and progression by androgen receptor signals. J Natl Cancer Inst. 2007;99:558–68. doi: 10.1093/jnci/djk113. [DOI] [PubMed] [Google Scholar]
- 13.Mhawech-Fauceglia P, Alvarez V, Fischer G, Beck A, Herrmann FR. Association of TSC1/hamartin, 14-3-3sigma, and p27 expression with tumor outcomes in patients with pTa/pT1 urothelial bladder carcinoma. Am J Clin Pathol. 2008;129:918–23. doi: 10.1309/D81QMXPMC3QHT57Y. [DOI] [PubMed] [Google Scholar]
- 14.Simpson AJ, Caballero OL, Jungbluth A, Chen YT, Old LJ. Cancer/testis antigens, gametogenesis and cancer. Nat Rev Cancer. 2005;5:615–25. doi: 10.1038/nrc1669. [DOI] [PubMed] [Google Scholar]
- 15.Eble JN, Sauter G, Epstein JI, Sesterhenn IA. World Health Organization classification of tumors. Lyon: IARC Press; 2004. [Google Scholar]
- 16.Edge SB, Fritz AG, Byrd DR, Greene FL, Compton CC, Trotti A. Cancer Staging Handbook from the AJCC Cancer Staging Manual. 7th edition. New York: Springer-Verlag; 2010. [Google Scholar]
- 17.Andius P, Johansson SL, Holmang S. Prognostic factors in stage T1 bladder cancer: tumor pattern (solid or papillary) and vascular invasion more important than depth of invasion. Urology. 2007;70:758–62. doi: 10.1016/j.urology.2007.06.638. [DOI] [PubMed] [Google Scholar]
- 18.Park J, Song C, Hong JH, Park BH, Cho YM, Kim CS, Ahn H. Prognostic significance of non-papillary tumor morphology as a predictor of cancer progression and survival in patients with primary T1G3 bladder cancer. World J Urol. 2009;27:277–83. doi: 10.1007/s00345-008-0350-4. [DOI] [PubMed] [Google Scholar]
- 19.Engelmann D, Putzer BM. The dark side of E2F1: in transit beyond apoptosis. Cancer Res. 2012;72:571–5. doi: 10.1158/0008-5472.CAN-11-2575. [DOI] [PubMed] [Google Scholar]
- 20.Wander SA, Zhao D, Slingerland JM. p27: a barometer of signaling deregulation and potential predictor of response to targeted therapies. Clin Cancer Res. 2011;17:12–8. doi: 10.1158/1078-0432.CCR-10-0752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lopez-Beltran A, Luque RJ, Alvarez-Kindelan J, Quintero A, Merlo F, Requena MJ, Montironi Rl. Prognostic factors in survival of patients with stage Ta and T1 bladder urothelial tumors: the role of G1-S modulators (p53, p21Waf1, p27Kip1, cyclin D1, and cyclin D3), proliferation index, and clinicopathologic parameters. Am J Clin Pathol. 2004;122:444–52. doi: 10.1309/LTFU-3UUM-BY09-5HUM. [DOI] [PubMed] [Google Scholar]
- 22.Kim JH, Park J. Prognostic significance of heme oxygenase-1, S100 calcium-binding protein A4, and syndecan-1 expression in primary non-muscle-invasive bladder cancer. Human Pathology. 2014;45:1830–8. doi: 10.1016/j.humpath.2014.04.020. [DOI] [PubMed] [Google Scholar]
- 23.Sung JY, Sun JM, Chang Jeong B, Il Seo S, Soo Jeon S, Moo Lee H, Yong Choi H, Young Kang S, Choi YL, Young Kwon G. FGFR3 overexpression is prognostic of adverse outcome for muscle-invasive bladder carcinoma treated with adjuvant chemotherapy. Urol Oncol. 2014;32:49, e23–31. doi: 10.1016/j.urolonc.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 24.Burger M, van der Aa MN, van Oers JM, Brinkmann A, van der Kwast TH, Steyerberg EC, Stoehr R, Kirkels WJ, Denzinger S, Wild PJ, Wieland WF, Hofstaedter F, Hartmann A, Zwarthoff EC. Prediction of progression of non-muscle-invasive bladder cancer by WHO 1973 and 2004 grading and by FGFR3 mutation status: a prospective study. Eur Urol. 2008;54:835–43. doi: 10.1016/j.eururo.2007.12.026. [DOI] [PubMed] [Google Scholar]
- 25.Hernández S, López-Knowles E, Lloreta J, Kogevinas M, Amorós A, Tardón A, Carrato A, Serra C, Malats N, Real FX l. Prospective study of FGFR3 mutations as a prognostic factor in nonmuscle invasive urothelial bladder carcinomas. J. Clin. Oncol. 2006;24:3664–71. doi: 10.1200/JCO.2005.05.1771. [DOI] [PubMed] [Google Scholar]


