Abstract
Chordoma is a rare and low-malignant neoplasm which is considered to arise from notochord remnants. Due to its large resistance to chemotherapy and radiotherapy, surgical resection so far is the prior treatment for chordoma. However, the recurrence rate is high even after complete surgical resection. Recently, targeted cancer therapy has been demonstrated to be effective in several other tumors, while the related research on chordoma is rare. Mitogen-activated protein kinase signaling pathway is acknowledged to participate in tumor development, in which Raf-1 and extracellular regulated protein kinase 1/2 (ERK1/2) play vital roles. In this study, we evaluated the expression of Raf-1 and ERK1/2 by immunohistochemical staining in 42 chordoma tissue and 16 distant normal tissue. Moreover, we also investigated the correlations of Raf-1 and ERK1/2 expression with clinical features in sacral chordoma. Expression of Raf-1 and ERK1/2 was both significantly higher in sacral chordoma tissue than distant normal tissue (P = 0.008, P = 0.019). Raf-1 positive expression was related to surrounding muscle invasion (P = 0.032) and chordoma recurrence (P = 0.002), but the results did not indicate any association with patients’ age, gender, tumor size and location. ERK1/2 was associated with tumor size (P = 0.044) instead of other clinical factors (P > 0.05). Spearman correlation test showed close relation between ERK1/2 and Raf-1 (P = 0.001, r = 0.518). Kaplan–Meier survival Curve and log-rank test showed that Raf-1 positive expression was associated with shorter continuous disease-free survival time (CDFS) (P = 0.001), while ERK1/2 had no relation to CDFS (P = 0.961). Conclusively, Raf-1 may be an important biomarker in predicting the prognosis of chordoma patients.
Keywords: Raf-1, sacral chordoma, ERK1/2, recurrence, prognosis
Introduction
Chordoma is a rare, slow-growing and aggressive neoplasm which accounts for 1%-4% of all primary bone tumors [1]. It is considered to stem from embryonic remnants of the notochord and occurs most commonly within the sacrum (50-60%) [2-4]. The overall 5 and 10-year survival rates following sacrectomy are 45-77% and 28-50% respectively [5]. As chordoma is largely resistant to conventional chemotherapy and radiotherapy, surgical resection is the mainstay of chordoma treatment [6]. However, the high recurrence rate does occur even after complete resection, which leads to poor prognosis [7]. Therefore, assessing risk factors for chordoma clinical behaviors is of great value.
Previous studies have shown that activation of the Raf/mitogen-activated extracellular signal-regulated kinase (MEK)/extracellular regulated protein kinase (ERK) signaling cascade promotes an autocrine growth loop critical for tumor genesis, cell proliferation, apoptosis and survival [8,9]. Raf-1 and ERK1/2, which are important members of this pathway, have been reported to be up-regulated in several cancers and correlated with tumor progression [10-16]. Several studies reported that overexpression of Raf-1 in cancer had close relationship with patients’ poor prognosis [10,17]. However, to our knowledge, whether the expression of Raf-1 and ERK1/2 is involved in the development and recurrence of sacral chordoma remains unclear now. Thus, the purpose of this study is to evaluate the expression of Raf-1 and ERK1/2 in sacral chordoma and investigate their association with clinical factors and patients’ prognosis.
Materials and methods
Patients and tissue samples
In this study, 42 patients (24 males and 18 females) were collected and they received the first tumor resection at the First Affiliated Hospital of Soochow University (Suzhou, china) from 1996 to 2012. All of them were histopathologically diagnosed to be chordoma and the average age at the time of surgery was 50.3 years (18-77 years). Meanwhile, 16 distant normal tissue specimens, which were used as control, were obtained at least 3 cm away from surgical margins. All the specimens were fixed in 10% formalin and embedded in paraffin. The medical record was reviewed to obtain clinical information for each case, including patients’ age, gender, tumor location, tumor size, surrounding muscle invasion and pathological results. Surrounding muscle invasion, which means the tumor invasion into surrounding muscle, was evaluated by magnetic resonance images before surgery. Our study was approved by the Institutional Research Ethics Committee and all the patients gave informed consent.
Immunohistochemistry
EnVision two-step staining method was used to perform immunohistochemical staining on 4-um-thick tissue sections. The sections were dewaxed in xylene and rehydrated in ethanol before the antigen retrieval. The primary antibodies used were rabbit monoclonal anti-Raf-1 (ab32025, Abcam, Cambridge, UK, dilution at 1/50) and mouse monoclonal anti-ERK1/2 (#4696, Cell Signaling Technology, Boston, US, dilution at 1/100). Secondary antibody used was ChemMateTM EnVisionTM Detection Kit (GK500710, Gene Tech, Shanghai, China). For positive controls, tissue sections of colon carcinoma with known positivity were used in each batch of staining. Negative controls were prepared by substituting Phosphate Buffered Solution (PBS) for primary antibody.
The evaluation of immunohistochemistry was assessed and scored independently by two experienced pathologists who were blinded to the patients’ clinicopathological information and outcome. We evaluated the staining semi-quantitatively based on the percentage of positive staining and color intensity. The percentage of positive staining tumor cells was scored as 0 (0%), 1 (< 20%), 2 (20-50%), 3 (> 50%). The color intensity was scored as 0 (no staining), 1 (weak staining), 2 (moderate staining), 3 (strong staining). The product of both scores was used as the final score. We divided the specimens into two groups according to the final score: 0-2, negative; 3-9, positive.
Follow-up
In the first two years after operation, patients took plain radiographs, computed tomography scans and MR imaging test every three months, and after three years they would take those imaging tests at six-month intervals. Continuous disease-free survival time (CDFS) was defined as the time interval from primary surgery to tumor recurrence.
Statistical analysis
Statistical analysis was performed by using SPSS 18.0 statistical software (SPSS Inc, Chicago, IL). Student’s t test and Chi-Square analysis method were used appropriately to evaluate the association of Raf-1 or ERK1/2 expression with clinical data of sacral chordoma patients. The correlation of Raf-1 with ERK1/2 was assessed by Spearman correlation test. The effect of Raf-1 or ERK1/2 up-regulation on CDFS was estimated by Kaplan-Meier survival curve and log-rank test. P < 0.05 was considered statistically significant.
Results
In this study, positive expression of Raf-1 was mainly in the cytoplasm of chordoma cell, while ERK1/2 positive staining was mainly in the nucleus and cytoplasm of chordoma cell (Figure 1). The average expression level of Raf-1 or ERK1/2 in sacral chordoma tissue specimens was significantly higher than distant normal muscle tissue samples (P = 0.008, P = 0.019) (Table 1). The positive expression of Raf-1 in sacral chordoma was 43% (18/42), while it was only 6% (1/16) in 16 distant normal tissue (Table 1). ERK1/2 had positive expression in 59.5% (25/42) chordoma tissue and 25% (4/16) normal tissue. Chi-Square analysis showed high expression of Raf-1 was related to surrounding muscle invasion (P = 0.032, Table 2). No significant association was discovered between Raf-1 expression and patients’ age, gender, tumor size and location (P > 0.05, Table 2). In addition, the results indicated ERK1/2 was correlated with tumor size (P = 0.044) but not other clinical factors of chordoma (P > 0.05, Table 2). Spearman correlation test showed close relationship between Raf-1 and ERK1/2 (P = 0.001, r = 0.518).
Figure 1.
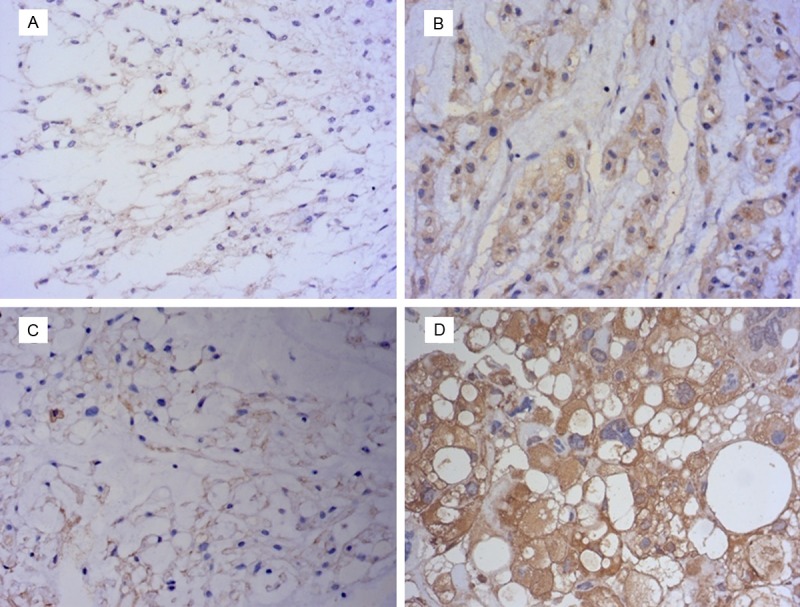
A. Negative expression of Raf-1 in sacral chordoma. B. Positive expression of Raf-1 in sacral chordoma. C. Negative expression of ERK1/2 in sacral chordoma. D. Positive expression of ERK1/2 in sacral chordoma (magnification, ×400).
Table 1.
Expression of Raf-1 and ERK1/2 in sacral chordoma and distant normal tissue
| Tissue sample | N | Raf-1 expression | P value | ERK1/2 expression | P value |
|---|---|---|---|---|---|
|
|
|
||||
| Positive (%) | Positive (%) | ||||
| Sacral chordoma | 42 | 18 (43%) | 0.008 | 25 (60%) | 0.019 |
| Distant normal tissue | 16 | 1 (6%) | 4 (25%) |
Table 2.
Association of Raf-1 and ERK1/2 expression with clinicopathological factors in sacral chordoma
| Parameters | N | Raf-1 | ERK1/2 | ||
|---|---|---|---|---|---|
|
|
|||||
| Positive | P value | Positive | P value | ||
| Age (years) | 0.929 | 0.845 | |||
| < 50 | 19 | 8 | 14 | ||
| ≥ 50 | 23 | 10 | 11 | ||
| Gender | 0.418 | 0.276 | |||
| Male | 24 | 9 | 16 | ||
| Female | 18 | 9 | 9 | ||
| Tumor size (mm) | 0.710 | 0.044 | |||
| < 90 | 23 | 11 | 13 | ||
| ≥ 90 | 19 | 7 | 12 | ||
| Tumor location | 0.179 | 0.145 | |||
| Above S3 | 27 | 12 | 16 | ||
| S3 and below | 15 | 6 | 9 | ||
| Surrounding muscle invasion | 0.032 | 0.568 | |||
| Yes | 20 | 12 | 11 | ||
| No | 22 | 6 | 14 | ||
| Recurrence | 0.002 | 0.753 | |||
| Yes | 21 | 14 | 12 | ||
| No | 21 | 4 | 13 | ||
Moreover, follow-up data was obtained for all cases. The average duration of follow-up was 123.6 months (range 23-216 months). The local recurrence rate was 50% (21/42) and median recurrence time was 52.5 months. Of all the patients with Raf-1 positive expression, 78% (14/18) recurred, while in patients with low expression of Raf-1, 29% (7/24) relapsed (P = 0.002, Table 2). Kaplan-Meier survival curve and log-rank test showed patients with positive expression of Raf-1 had a shorter median CDFS (23.5 versus 78.0 months, P = 0.001, Figure 2). Furthermore, the results showed ERK1/2 positive expression was not related to tumor recurrence (P = 0.753) and the difference of CDFS in positive and negative group was not statistically significant (P = 0.961, Figure 3).
Figure 2.
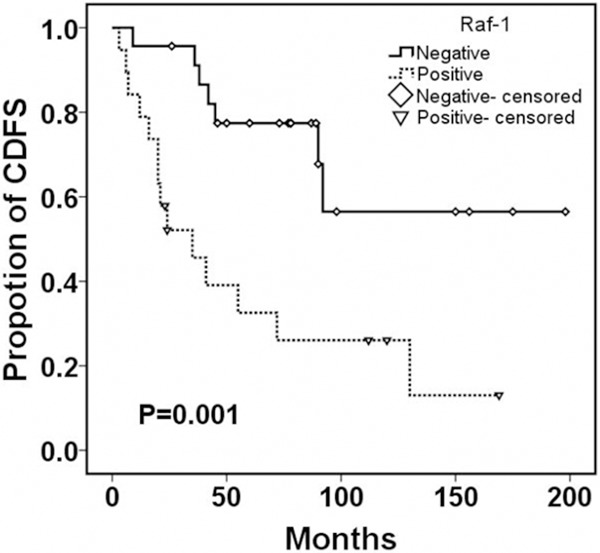
Continuous disease-free survival according to the expression of Raf-1 in sacral chordoma.
Figure 3.
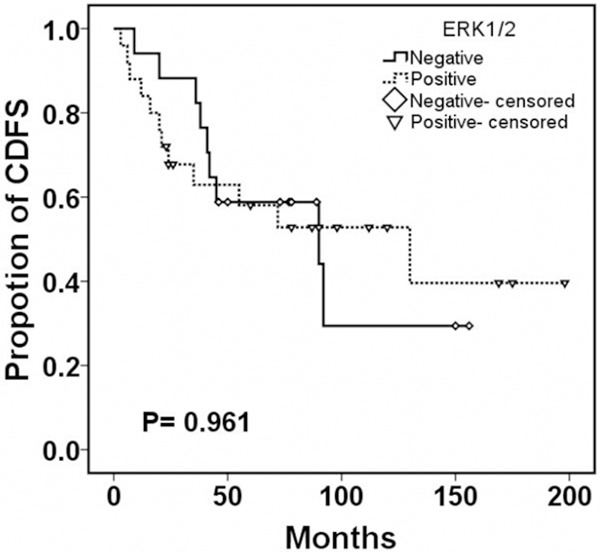
Continuous disease-free survival according to the expression of ERK1/2 in sacral chordoma.
Discussion
Sacral chordoma is a primary malignant bone tumor which has a high local recurrence rate. The incidence rate is 0.8/1000000/year, with about 1.8 times higher in males than females [18,19]. Frequent recurrence, even after wide en-bloc resection, is the problem confuses us most [7]. It suggests that there may be existed several unknown risk factors besides the resection range. Our previous studies have shown that tumor location and surrounding muscle invasion may be the risk factors for chordoma recurrence [20,21]. To the best of our knowledge, the molecular mechanism for the clinical behaviors of sacral chordoma is field with insufficient investigation. Accumulating evidence showed that up-regulation of Raf-1 and ERK1/2 was observed in several other cancer types and their over-expression was closely related to cell proliferation and tumor progression [11,15,22,23]. Based on these studies, we speculated that Raf-1 and ERK1/2 were elevated in sacral chordoma and might have reasonable prognostic roles for chordoma patients.
Over-expression of Raf-1 in transgenic mice made them prone to develop lung cancer and it was regarded as an early tumor marker for human lung adenocarcinoma [11,13]. Dai et al. [10] also detected that Raf-1was highly expressed in 49% hepatocellular carcinoma tissue specimens. In this study, about 43% tumor samples presented positive expression of Raf-1. The expression of Raf-1 was obviously elevated in chordoma tissue samples compared to surrounding normal muscle tissue specimens (P < 0.05). ERK1/2 was reported to be highly expressed in colon adenocarcinoma and endometrioid adenocarcinoma [16,24]. Consistent with these studies, we also found the level of ERK1/2 in sacral chordoma tissue was significantly higher than surrounding normal tissue (P = 0.019). In addition, we also found Raf-1 expression was associated with ERK1/2 (P = 0.001). These studies demonstrated that Raf-1 and ERK1/2 may be key factors in the development of sacral chordoma.
Previous studies have shown that Raf-1 was significantly related to promoting tumor cell proliferation and invasion [25,26]. Over-expression of Raf-1 in epithelial cells affected the expression of genes which were involved in promoting cell proliferation, invasion and angiogenesis [27]. The expression of Raf-1 was correlated with surrounding normal muscle invasion (P < 0.05), but had no association with patients’ age, gender, tumor location and size. Interestingly, we found ERK1/2 expression was related to tumor size but not other clinical features of chordoma (P = 0.044). ERK1/2 has been shown to regulate cell survival and proliferation, which plays a pivotal role in tumor growth [28]. Activation of Raf-1 signaling pathway was reported to promote an invasive phenotype in breast cancer cells [26]. Taken together, Raf-1 and ERK1/2 may be of great value in enhancing cell proliferation and invasion of chordoma.
In present study, we revealed that expression of Raf-1 has close relationship with chordoma recurrence (P < 0.05). Of all the patients with positive Raf-1 expression, 78% developed local recurrence, while similar phenomenon was observed in hepatocellular carcinoma, in which the elevation of Raf-1 was involved in tumor recurrence [10]. The continuous disease-free survival time was significantly shorter in Raf-1 positive group than negative group. Similarly, patients with Raf-1 up-regulation had significantly shorter time to relapse in androgen insensitive prostate cancer [17]. Raf-1 was likely to induce proliferation of tumor cells and promote invasive ability in vitro study [11]. Loss of Rictor promoted matrix metalloproteinase-9 activity and invasion through Raf-1/ERK pathway in glioma cells [29]. Even though we found ERK1/2 was highly expressed in chordoma tissue, we did not find any statistically significant correlation between ERK1/2 and CDFS in sacral chordoma (P = 0.961). Krishdeep S et al. [30] showed phosphorylated ERK1/2 (p-ERK) instead of ERK1/2 was correlated with prognosis of Pancreatic Carcinoma. Over-expression of Raf-1 was considered as an independent prognostic biomarker and correlated with shorter disease free survival in hepatocellular carcinoma [10]. In brief, Raf-1 may play a pivotal role in predicting the prognosis of chordoma patients.
In conclusion, we evaluated the expression of Raf-1 and ERK1/2 in sacral chordoma and analyzed their association with tumor prognosis. Over-expression of Raf-1 was significantly correlated with tumor invasion. Although ERK1/2 was over-expressed in chordoma, it was not related to tumor recurrence. Raf-1 might become a valuable biomarker in predicting the tumor recurrence and patients’ prognosis in sacral chordoma. However, further study was required to evaluate the exact role of Raf-1 and ERK1/2 in the development and progression of sacral chordoma.
Acknowledgements
This study was funded by Jiangsu Provincial Special Program of Medical Science (BL2012004) and Suzhou city “Science and Education Guardian” Youth science and technology project (KJXW2014009).
Disclosure of conflict of interest
None.
References
- 1.Tirabosco R, Mangham DC, Rosenberg AE, Vujovic S, Bousdras K, Pizzolitto S, De Maglio G, den Bakker MA, Di Francesco L, Kalil RK, Athanasou NA, O’Donnell P, McCarthy EF, Flanagan AM. Brachyury expression in extra-axial skeletal and soft tissue chordomas: a marker that distinguishes chordoma from mixed tumor/myoepithelioma/parachordoma in soft tissue. Am J Surg Pathol. 2008;32:572–580. doi: 10.1097/PAS.0b013e31815b693a. [DOI] [PubMed] [Google Scholar]
- 2.Scheil-Bertram S, Kappler R, von Baer A, Hartwig E, Sarkar M, Serra M, Bruderlein S, Westhoff B, Melzner I, Bassaly B, Herms J, Hugo HH, Schulte M, Moller P. Molecular profiling of chordoma. Int J Oncol. 2014;44:1041–1055. doi: 10.3892/ijo.2014.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chugh R, Tawbi H, Lucas DR, Biermann JS, Schuetze SM, Baker LH. Chordoma: the nonsarcoma primary bone tumor. Oncologist. 2007;12:1344–1350. doi: 10.1634/theoncologist.12-11-1344. [DOI] [PubMed] [Google Scholar]
- 4.Li X, Ji Z, Ma Y, Qiu X, Fan Q, Ma B. Expression of hypoxia-inducible factor-1alpha, vascular endothelial growth factor and matrix metalloproteinase-2 in sacral chordomas. Oncol Lett. 2012;3:1268–1274. doi: 10.3892/ol.2012.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kayani B, Hanna SA, Sewell MD, Saifuddin A, Molloy S, Briggs TW. A review of the surgical management of sacral chordoma. Eur J Surg Oncol. 2014;40:1412–1420. doi: 10.1016/j.ejso.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 6.Sciubba DM, Cheng JJ, Petteys RJ, Weber KL, Frassica DA, Gokaslan ZL. Chordoma of the sacrum and vertebral bodies. J Am Acad Orthop Surg. 2009;17:708–717. doi: 10.5435/00124635-200911000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Hanna SA, Aston WJ, Briggs TW, Cannon SR, Saifuddin A. Sacral chordoma: can local recurrence after sacrectomy be predicted? Clin Orthop Relat Res. 2008;466:2217–2223. doi: 10.1007/s11999-008-0356-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCubrey JA, Steelman LS, Abrams SL, Lee JT, Chang F, Bertrand FE, Navolanic PM, Terrian DM, Franklin RA, D'Assoro AB, Salisbury JL, Mazzarino MC, Stivala F, Libra M. Roles of the RAF/MEK/ERK and PI3K/PTEN/AKT pathways in malignant transformation and drug resistance. Adv Enzyme Regul. 2006;46:249–279. doi: 10.1016/j.advenzreg.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 9.Sippel RS, Chen H. Activation of the ras/raf-1 signal transduction pathway in carcinoid tumor cells results in morphologic transdifferentiation. Surgery. 2002;132:1035–1039. doi: 10.1067/msy.2002.128877. [DOI] [PubMed] [Google Scholar]
- 10.Chen L, Shi Y, Jiang CY, Wei LX, Wang YL, Dai GH. Expression and prognostic role of pan-Ras, Raf-1, pMEK1 and pERK1/2 in patients with hepatocellular carcinoma. Eur J Surg Oncol. 2011;37:513–520. doi: 10.1016/j.ejso.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 11.Qiu ZX, Wang L, Han J, Liu D, Huang W, Altaf K, Qiu XS, Javed MA, Zheng J, Chen BJ, Li WM. Prognostic impact of Raf-1 and p-Raf-1 expressions for poor survival rate in non-small cell lung cancer. Cancer Sci. 2012;103:1774–1779. doi: 10.1111/j.1349-7006.2012.02375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hagemann C, Gloger J, Anacker J, Said HM, Gerngras S, Kuhnel S, Meyer C, Rapp UR, Kammerer U, Vordermark D, Flentje M, Roosen K, Vince GH. RAF expression in human astrocytic tumors. Int J Mol Med. 2009;23:17–31. [PubMed] [Google Scholar]
- 13.Cekanova M, Majidy M, Masi T, Al-Wadei HA, Schuller HM. Overexpressed Raf-1 and phosphorylated cyclic adenosine 3’-5’-monophosphatate response element-binding protein are early markers for lung adenocarcinoma. Cancer. 2007;109:1164–1173. doi: 10.1002/cncr.22520. [DOI] [PubMed] [Google Scholar]
- 14.Daum G, Eisenmann-Tappe I, Fries HW, Troppmair J, Rapp UR. The ins and outs of Raf kinases. Trends Biochem Sci. 1994;19:474–480. doi: 10.1016/0968-0004(94)90133-3. [DOI] [PubMed] [Google Scholar]
- 15.Bartholomeusz C, Gonzalez-Angulo AM, Liu P, Hayashi N, Lluch A, Ferrer-Lozano J, Hortobagyi GN. High ERK protein expression levels correlate with shorter survival in triple-negative breast cancer patients. Oncologist. 2012;17:766–774. doi: 10.1634/theoncologist.2011-0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levidou G, Saetta AA, Gigelou F, Karlou M, Papanastasiou P, Stamatelli A, Kavantzas N, Michalopoulos NV, Agrogiannis G, Patsouris E, Korkolopoulou P. ERK/pERK expression and B-raf mutations in colon adenocarcinomas: correlation with clinicopathological characteristics. World J Surg Oncol. 2012;10:47. doi: 10.1186/1477-7819-10-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mukherjee R, Bartlett JM, Krishna NS, Underwood MA, Edwards J. Raf-1 expression may influence progression to androgen insensitive prostate cancer. Prostate. 2005;64:101–107. doi: 10.1002/pros.20211. [DOI] [PubMed] [Google Scholar]
- 18.McMaster ML, Goldstein AM, Bromley CM, Ishibe N, Parry DM. Chordoma: incidence and survival patterns in the United States, 1973-1995. Cancer Causes Control. 2001;12:1–11. doi: 10.1023/a:1008947301735. [DOI] [PubMed] [Google Scholar]
- 19.Maclean FM, Soo MY, Ng T. Chordoma: radiological-pathological correlation. Australas Radiol. 2005;49:261–268. doi: 10.1111/j.1440-1673.2005.01433.x. [DOI] [PubMed] [Google Scholar]
- 20.Yang H, Zhu L, Ebraheim NA, Liu X, Castillo S, Tang T, Liu J, Cui H. Analysis of risk factors for recurrence after the resection of sacral chordoma combined with embolization. Spine J. 2009;9:972–980. doi: 10.1016/j.spinee.2009.08.447. [DOI] [PubMed] [Google Scholar]
- 21.Chen KW, Yang HL, Lu J, Liu JY, Chen XQ. Prognostic factors of sacral chordoma after surgical therapy: a study of 36 patients. Spinal Cord. 2010;48:166–171. doi: 10.1038/sc.2009.95. [DOI] [PubMed] [Google Scholar]
- 22.Leicht DT, Balan V, Kaplun A, Singh-Gupta V, Kaplun L, Dobson M, Tzivion G. Raf kinases: function, regulation and role in human cancer. Biochim Biophys Acta. 2007;1773:1196–1212. doi: 10.1016/j.bbamcr.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cui X, Li S, Li T, Pang X, Zhang S, Jin J, Hu J, Liu C, Yang L, Peng H, Jiang J, Liang W, Suo J, Li F, Chen Y. Significance of elevated ERK expression and its positive correlation with EGFR in Kazakh patients with esophageal squamous cell carcinoma. Int J Clin Exp Pathol. 2014;7:2382–2391. [PMC free article] [PubMed] [Google Scholar]
- 24.Gungorduk K, Ertas IE, Sahbaz A, Ozvural S, Sarica Y, Ozdemir A, Sayhan S, Gokcu M, Yilmaz B, Sanci M, Inan S, Harma M, Yildirim Y. Immunolocalization of ERK1/2 and p-AKT in normal endometrium, endometrial hyperplasia, and early and advanced stage endometrioid endometrial adenocancer and their prognostic significance in malignant group. Eur J Obstet Gynecol Reprod Biol. 2014;179:147–152. doi: 10.1016/j.ejogrb.2014.05.040. [DOI] [PubMed] [Google Scholar]
- 25.McPhillips F, Mullen P, MacLeod KG, Sewell JM, Monia BP, Cameron DA, Smyth JF, Langdon SP. Raf-1 is the predominant Raf isoform that mediates growth factor-stimulated growth in ovarian cancer cells. Carcinogenesis. 2006;27:729–739. doi: 10.1093/carcin/bgi289. [DOI] [PubMed] [Google Scholar]
- 26.Leontovich AA, Zhang S, Quatraro C, Iankov I, Veroux PF, Gambino MW, Degnim A, McCubrey J, Ingle J, Galanis E, D’Assoro AB. Raf-1 oncogenic signaling is linked to activation of mesenchymal to epithelial transition pathway in metastatic breast cancer cells. Int J Oncol. 2012;40:1858–1864. doi: 10.3892/ijo.2012.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schulze A, Lehmann K, Jefferies HB, McMahon M, Downward J. Analysis of the transcriptional program induced by Raf in epithelial cells. Genes Dev. 2001;15:981–994. doi: 10.1101/gad.191101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lefloch R, Pouyssegur J, Lenormand P. Single and combined silencing of ERK1 and ERK2 reveals their positive contribution to growth signaling depending on their expression levels. Mol Cell Biol. 2008;28:511–527. doi: 10.1128/MCB.00800-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Das G, Shiras A, Shanmuganandam K, Shastry P. Rictor regulates MMP-9 activity and invasion through Raf-1-MEK-ERK signaling pathway in glioma cells. Mol Carcinog. 2011;50:412–423. doi: 10.1002/mc.20723. [DOI] [PubMed] [Google Scholar]
- 30.Chadha KS, Khoury T, Yu J, Black JD, Gibbs JF, Kuvshinoff BW, Tan D, Brattain MG, Javle MM. Activated Akt and Erk expression and survival after surgery in pancreatic carcinoma. Ann Surg Oncol. 2006;13:933–939. doi: 10.1245/ASO.2006.07.011. [DOI] [PubMed] [Google Scholar]


