Abstract
Platelet activating factor (PAF), a potent pro-inflammatory phospholipid, has been found to trigger tumor growth and angiogenesis through its G-protein coupled receptor (PAFR). This study was aimed to investigate the potential role of PAF in azoxymethane (AOM)/dextran sulfate sodium (DSS) induced colitis-associated cancer (CAC), using PAFR antagonist Ginkgolide B (GKB). We found GKB up-regulated serum level of PAF-AH activity. As assessed by disease activity index (DAI), histological injury scores, leukocytes infiltration, and expression of pro-inflammatory cytokines, GKB ameliorated colonic inflammation and decreased tumor number and load in mice. GKB also decreased expression of vascular endothelial growth factor (VEGF) and microvessel density (MVD) in tumor. These results suggest that PAFR antagonist might be a potential therapeutic strategy for CAC.
Keywords: PAF, Ginkgolide B, colitis-associated cancer, angiogenesis, VEGF
Introduction
Inflammatory bowel disease (IBD) is a chronic intestinal inflammatory process with two main phenotypes: ulcerative colitis (UC) and Crohn’s disease (CD). IBD is considered as one of the top three risk conditions for colorectal cancer, and this risk increases with the duration of inflammation [1]. To date, the mechanisms by which IBD promotes colorectal cancer have not been fully elucidated. Increasing evidences suggested that inflammation induced angiogenesis contribute to CAC [2,3].
Angiogenesis is one of the essential processes in tumor growth and metastasis. Angiogenesis based targeted therapy is considered as milestone for cancer treatment. Angiogenesis also takes part in inflammatory diseases by increasing influx of inflammatory cells and nutrient supply, activating cytokines, chemokines, and matrix metalloproteinases, et al [4]. Microvessel density and expression of VEGF level and its receptors are increased in IBD mucosa [5]. Human intestinal microvascular endothelial cells (HIMECs) isolated from UC and CD tissue showed enhanced capacity of adhering leukocytes [6]. Serum and mucosa level of VEGF was significantly increased in patients with IBD and correlated with disease activity [7-9]. However, the role of angiogenesis in CAC remains poorly understood.
Platelet activating factor (PAF; 1-O-alkyl-2-acetyl-sn-glycero-3-phosphocholine), a potent pro-inflammatory mediator, can recruit and activate leukocytes, prompt release of cytokines and chemokines and induce angiogenesis [10]. PAF exerts its biological activities in various tissues through a G-protein coupled receptor (PAFR) [11]. In UC and CD, PAF is up-regulated in the inflammatory colonic mucosa, and is considered to be involved in the pathogenesis of IBD [12,13]. PAF level is elevated in colonic tissue in colitis mice model, and PAFR antagonist can significantly alleviate colonic inflammation [14]. On the other hand, PAF could stimulate migration of HIMECs, and promote angiogenesis and vascular permeability by activating VEGF expression [15-18]. In breast, prostate cancer, and Kaposi’s sarcoma, PAFR antagonist could inhibit tumor growth by inhibition of tumor angiogenesis [16,19,20]. Thus, these research suggest that PAF may contribute to CAC by stimulating inflammation related angiogenesis.
To date, the pro-angiogenesis effect of PAF in inflammation and cancer is confirmed, whereas no evidence of PAF taking part in CAC development is found. This study is aimed to investigate the role of PAF in tumorigenesis and angiogenesis of CAC by administration of GKB in mice.
Materials and methods
Animals
Eight-week-old female C57/BL6 mice weighing about 18-20 g were obtained from the Experimental Animal Center of Sun Yat-Sen University, Guangzhou, China and mice were housed in a room with controlled humidity and temperature in collective cages. The animals were supplied a pelleted basal diet and drinking water. This study was approved by the Ethical Committee for Animal Care and Use of Zhongshan School of Medicine, Sun Yat-sun University according to an approved protocol.
Inducement of CAC and drug administration
We used AOM (Sigma Aldrich, St. Louis, MO, USA) and DSS (MP Biomedicals, Santa Ana, CA, USA) establishing CAC model as previously described [21]. The procedure is shown in Figure 1. Animals were sacrificed under anesthesia (1:1, v/v of xylazine 2%-ketamine 10%) 9 weeks after the injection of AOM.
Figure 1.
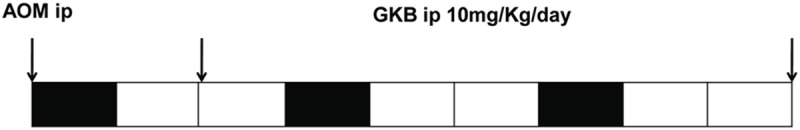
Experimental protocol of CAC model. All mice received i.p. injected AOM on day 1 followed by 3 cycles of 2% DSS in the drinking water for 7 days and then distilled water for 14 days. Mice in control group received no drug treatment; Vehicle treated group received vehicle (4% ethanol + 5% Tween 80 + 5% polyethylene glycol 400) i.p. injection after day 14; GKB treated group received 10mg/kg/day of GKB (Sigma-Aldrich, St Louis, MO, USA) i.p. injection after day 14. (n=8 per group). GKB, Ginkgolide B; PEG, polyethylene glycol; CAC, colitis-associated cancer; AOM, axozymethane; DSS, dextran sulfate sodium.
Evaluation of CAC
Disease activity index (DAI) was determined in accordance with the method described by Murthy et al. [22]. After the mice were killed, colon tissues were removed immediately and the length of colons were measured, and then colons were opened longitudinally, flushed with cold PBS to remove faecal material. The numbers of colon tumors were counted and load per mice (assessed by diameters) were measured with a calliper, and then fixed in 10% buffered formalin, paraffin-embedded, sectioned, and stained with hematoxylin and eosin (H&E). Images were captured by a digital camera (Nikon, Tokyo, Japan) at × 100 magnification. Histological injury scores of H&E-stained specimens were calculated by two investigators who were blinded to the treatment according to previously established criteria [23].
Measurement of PAF-AH activity
Blood samples were collected by orbital bleeding under anesthesia. Serum PAF-AH levels were measured by mouse PAF-AH ELISA kit (Cusabio Biotech, Wuhan, China) according to the manufacturer’s instructions. After color development, the absorbance was measured at 450 nm using a microplate reader (Thermo Scientific, Waltham, MA, USA). The results of PAF-AH activity were expressed by picogram per milliliter.
Myeloperoxidase activity assay
MPO activity was measured by ELISA. Colon samples were weighed and homogenized by a tissue homogeniser in nine volumes of PBS, and then centrifuged at 2,000 ×g for 20 min at 4°C. MPO activity in the resulting supernatant was assayed by mouse MPO ELISA kit (Sigma-Aldrich, St Louis, MO, USA) according to the manufacturer’s instructions. MPO activity was determined by measuring the absorbance at 450 nm using a microplate reader (Thermo Scientific, Waltham, MA, USA). The results were expressed by units of MPO per milligram tissue.
ELISA for cytokines
The colons were harvested and homogenates were made as mentioned above. The concentrations of TNF-α, IL-1β and IL-6 in supernatants of homogenized colon tissue were measured by mouse ELISA kits for TNF-α, IL-1β and IL-6 (BioLegend, SanDiego, CA, USA) according to the manufacturer’s instructions. After color development, the absorbance was measured at 450 nm using a microplate reader (Thermo Scientific, Waltham, MA, USA). The results of TNF-α, IL-1β and IL-6 activity of supernatant were expressed by picogram per milliliter.
Immunohistochemistry for CD31
Immunohistochemistry for CD31 was performed on formalin-fixed, paraffin-embedded tissue sections using the mouse polyclonal antibody (dilution, 1/100; Abcam, Cambridge, UK) and following the protocols. Stained tumor sections were examined under a light microscope, and images were captured by a digital camera (Nikon, Tokyo, Japan) at ×200 magnification. For each tumor section, 5 fields with the greatest density of positively stained vessels were evaluated. MVD was defined as the number of positively stained vessels per high-power field.
Quantitative real time PCR
Total RNA was isolated from tissue samples with Trizol (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. RNA was reverse transcribed by M-MLV Reverse Transcriptase (Promega, Madison, WI, USA) according to the manufacturer’s protocol. Forward (CATCTTCAAGCCGTCCTGTGT) and reverse (CAGGGCTTCATCGTTACAGCA) primers were designed as sequence-specific primers for the detection of VEGF mRNA expression. Real-time PCR was performed with GoTaq qPCR Master Mix (Promega, Madison, WI, USA) on a Mini-Opticon Real-Time PCR detection instrument (Bio-Rad, Hercules, CA, USA) using the SyBr Green detection protocols. Samples were amplified with the following program: initial denaturation at 95°C for 30 sec, followed by 40 cycles of denaturation for 15 s at 95°C and annealing/elongation for 60 s at 60°C. All PCRs were run in triplicate, and control reactions without template were included.
Western blot analysis
Western blot analysis was performed to assay VEGF protein expression in tumor. In short, equal amounts of isolated protein were added to electrophoresis sample buffer and boiled according to the manufacturer’s guidelines. Proteins were separated by SDS-PAGE on precast gels (10% acrylamide; NuSep, AUS) and transferred to a nitrocellulose membrane, which was incubated with specific antibodies against VEGF (dilution, 1/150; Abcam, Cambridge, UK) and GAPDH (dilution, 1/5000; Abcam, Cambridge, UK). Detection of specific bands was performed with the ECL Western blotting analysis system (Abcam, Cambridge, UK).
Statistical analysis
Data were expressed as the mean ± standard error and analyzed using SPSS 17.0 statistical software (SPSS, Chicago, IL, USA). A value of P < 0.05 was considered statistically significant.
Results
Administration of GKB inhibited inflammation in AOM/DSS-induced CAC model
Increased amount of PAF could stimulate synthesis of PAF acetylhydrolase (PAF-AH), which would in turn deactivate PAF to keep a balanced serum level of PAF. Therefore, serum PAF-AH level could act as an indicator for PAF signaling [24]. Meanwhile, PAF-AH plays protective role in inflammatory diseases, such as atherogenesis [25], asthma [26], and CD [27].
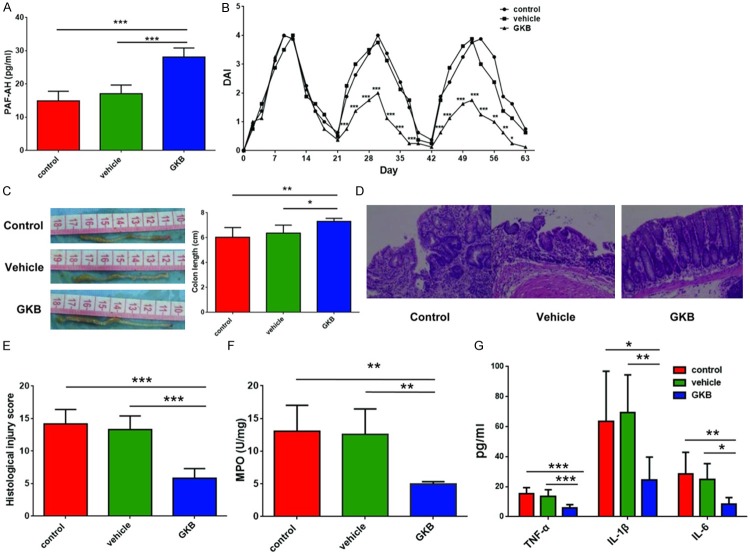
To confirm that GKB could inhibit PAF signaling in vivo, serum PAF-AH activity was tested by ELISA. After 7 weeks of GKB treatment, serum PAF-AH activity was significantly higher in GKB treated group than that in control group and vehicle treated group (P < 0.001 vs. control group, P < 0.001 vs. vehicle treated group) (Figure 2A).
Figure 2.
GKB inhibited inflammation in AOM/DSS-induced CAC model. A. PAF-AH activity. B. DAI. C. Colon length. D. Microscopic (H&E stain, ×100) views of the colon mucosa. E. Histological injury scores. F. MPO activity in colon tissue. G. TNF-α, IL-1β and IL-6 level in colon tissue. *P < 0.05, **P < 0.01, ***P < 0.001. DAI: disease activity index; MPO: Myeloperoxidase.
To analyze the role of PAF in AOM/DSS-induced CAC model, we first examined objective alterations after administration of PAFR antagonist. DAI, assessed by weight loss, stool consistency, hemoccult or gross bleeding, was significant decreased in GKB treated group after day 21 (Figure 2B). Inflammation caused shortening of the colon was markedly ameliorated in GKB treated group than in control group (P = 0.008 vs. control group, P = 0.048 vs. vehicle treated group) (Figure 2C). For microscopic examination, glandular distortion and inflammatory cells infiltration were found in submucosa (Figure 2D), and degrees of mucosal destruction were assessed by histological injury score. We found GKB treated group showed a significant decreased histological injury score compared with the control group and vehicle treated group (P < 0.001 vs. control group, P < 0.001 vs. vehicle treated group) (Figure 2E).
Leukocytes infiltration is one of key events in chronic intestinal inflammation and can serve as an indicator for local inflammation. Assessed by MPO activity, we observed that leukocytes infiltration was significantly decreased in GKB treated group compared with control and vehicle treated group (P = 0.002 vs. control group, P = 0.003 vs. vehicle treated group) (Figure 2F).
We also examined pro-inflammatory cytokines TNF-α, IL-1β and IL-6 in colonic mucosa. As assessed by ELISA, expression of TNF-α, IL-1β and IL-6 were significantly decreased in colon tissue in GKB treated group compared with control (P < 0.001, P = 0.017 and P = 0.003 respectively) and vehicle treated group (P = 0.001, P = 0.006 and P = 0.021 respectively) (Figure 2G). To further prove the influence of PAF signaling on colonic inflammation, we performed correlation analysis between expression of inflammatory cytokines and PAF-AH, the indicator for PAF signaling. We found TNF-α, IL-1β and IL-6 were negatively correlated with activity of PAF-AH by correlation analysis. (P = 0.001, P = 0.048 and P = 0.011 respectively) (Table 1). Taken together, these results suggest that PAFR antagonist could suppress inflammation in AOM/DSS-induced CAC model.
Table 1.
Correlations between PAF-AH and TNF-α, IL-1β, IL-6, tumor number, tumor load, MVD
| P-value | |
|---|---|
| PAF-AH versus TNF-α | 0.001** |
| PAF-AH versus IL-1β | 0.048* |
| PAF-AH versus IL-6 | 0.011* |
| PAF-AH versus tumor number | < 0.001*** |
| PAF-AH versus tumor load | 0.002** |
| PAF-AH versus MVD | < 0.001*** |
P < 0.05;
P < 0.01;
P < 0.001.
GKB inhibited tumorigenesis in AOM/DSS-induced CAC model
Intraperitoneal injection of mutagenic agent AOM with repeated oral administration of pro-inflammatory agent DSS could produce mice tumor model. Most tumors distributed in distal one-third of colon (Figure 3A). After 7 weeks of GKB treatment, tumor number and load (sum of all tumor diameter per mouse) were both significantly reduced in GKB treated group (tumor number: P < 0.001 vs. control group, P < 0.001 vs. vehicle treated group; tumor load: P = 0.004 vs. control group, P = 0.001 vs. vehicle treated group) (Figure 3B, 3C). Correlation analysis showed that tumor number and load were negatively correlated with activity of PAF-AH by correlation analysis. (P < 0.001 and P = 0.002, respectively) (Table 1).
Figure 3.
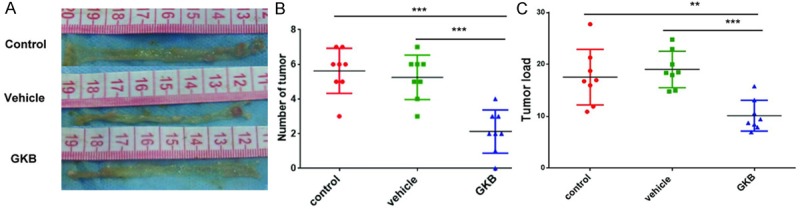
GKB inhibited tumorigenesis in AOM/DSS-induced CAC model. A. Macroscopic views of the colonic tumor. B. Number of colonic tumors per mouse (multiplicity). C. tumor load (sum of tumor diameters) per mouse *P < 0.05, **P < 0.01, ***P < 0.001.
GKB inhibited tumor angiogenesis in AOM/DSS-induced CAC model
Given the pro-angiogenic effect of PAF in some types of human cancer and chronic inflammation [19,28], we wondered whether PAF also exert angiogenic role in CAC models. As assessed by CD31 immunohistochemical staining, MVD were significant decreased in tumors from GKB treated group than in control and vehicle treated group (P < 0.001 vs. control group, P < 0.001 vs. vehicle treated group) (Figure 4A). VEGF is one of the key factors greatly affect angiogenesis. As examined by qPCR and western blot, mRNA and protein levels of VEGF were significantly suppressed by GKB, compared with control group and vehicle treated group (P = 0.003 vs. control group, P = 0.007 vs. vehicle treated group) (Figure 4B, 4C). Correlation analysis showed that MVD (P < 0.001) were negatively correlated with the activity of PAF-AH (Table 1).
Figure 4.
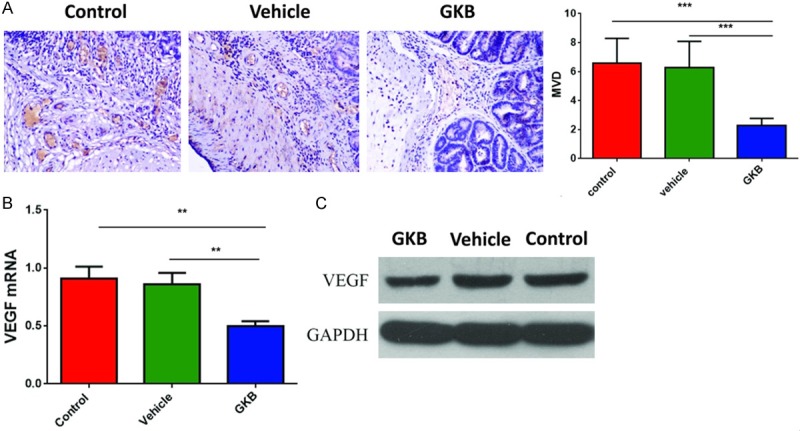
GKB inhibited tumor angiogenesis in AOM/DSS-induced CAC model. A. Microscopic (×200) views of immunohistochemistry for CD31 stained colon tumor and numbers of microvessel density of colon tumor. B. Q-PCR evaluation of VEGF mRNA. C. Western blot analysis evaluation of VEGF protein *P < 0.05, **P < 0.01, ***P < 0.001.
Discussion
The mechanism of how colitis prompting CAC is still not clear. Previous studies showed that PAF is one of the most potent lipid inflammatory mediators in inflammatory diseases and cancers [12,13,16,19,20,29]. However, no research has explored the function of PAF in the development of CAC. In this study, we found that inhibition of PAF by GKB markedly suppressed colitis and reduced tumor number and load in AOM/DSS-induced CAC. In addition, GKB reduced MVD and decreased mRNA and protein level of VEGF in tumor, suggesting that GKB might inhibit CAC by suppressing angiogenesis. Thus, PAFR antagonist may be a novel strategy for the treatment of CAC. To our knowledge, this is the first study characterizing the relationship between PAF and CAC and explaining the pro-angiogenesis role of PAF in CAC model.
Theoretically, PAFR antagonist GKB can increase serum level of PAF by blockade of PAFR and result in increased PAF-AH activity, which could in turn degrade excessive amount of PAF [24]. In this study, GKB could effectively block PAFR in mice, as indicated by higher activity of PAF-AH after administration of GKB. Previous study showed the pro-inflammatory role in several disease [25-27]. Consistent with these findings, activity of PAF-AH was positively correlated with MPO activity and pro-inflammatory cytokines, suggesting the pro-inflammatory role of PAF in AOM/DSS-induced CAC model. Infiltrating leukocytes, over-expressed inflammatory cytokines and enhanced microvascular permeability form a feedback loop and boost the inflammatory response [30]. In the present study, we observed that MPO activity, the index of leukocytes infiltration, was significantly decreased in GKB treated mice, suggesting that inhibition of PAF signaling could suppress the recruitment of leukocytes. Pro-inflammatory cytokines, such as TNF-α, IL-1β, IL-6, and IL-8, are over-expressed in patients with UC and CD and play vital role in development of CAC [31-34]. In our study, administration of GKB could significantly decrease expression of TNF-α, IL-1β and IL-6. Therefore, inhibition of PAF biological activity by PAFR antagonist can effectively ameliorate inflammation in AOM/DSS-induced CAC model.
Inhibit PAF signaling by PAFR antagonist could inhibit breast, prostate cancer, Kaposi’s sarcoma [16,19,20]. We found GKB significantly reduced the tumor number and load in CAC model, which were negatively correlated with the activity of PAF-AH. These results suggest that inhibition of PAF bioactivity might play a preventive role in CAC.
Angiogenesis contributes greatly to inflammation, and is one of the essential processes in the growth and metastasis of solid tumors [4]. PAF was found to regulate angiogenesis in breast, prostate cancer, Kaposi’s sarcoma [16,19] and rheumatoid arthritis [28]. In our study, mice treated with GKB showed significant decreased tumor MVD compared with control group and vehicle treated group, and activity of PAF-AH was significant correlated with MVD. PAF exerts its pro-angiogenic effects mainly through VEGF. PAF could stimulate expression of VEGF and promote angiogenesis in human endometrial epithelial cells, which could be abrogated by PAFR antagonist [35-38]. VEGFR-signaling was found promote growth of tumor cells in CAC, providing a molecular linkage between angiogenesis and CAC [39]. In this study, we found that blockade of PAF signaling was able to inhibit mRNA and protein level of VEGF, which could explain the reduction of MVD and tumor load after administration of GKB. These results suggest PAF may prompt CAC by stimulating angiogenesis.
In this study, PAFR antagonist GKB successfully inhibited inflammation and angiogenesis, and resulted in suppression of CAC in AOM/DSS mice model. Although the detailed mechanism needs further investigation, the current study implicates treatment with PAFR antagonist might serve as a novel therapeutic strategy for CAC.
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (No. 91029702, 81300367, 81402019) and the Guangdong Provincial Natural Science Foundation (No. S2013010014186).
Disclosure of conflict of interest
None.
References
- 1.Mattar MC, Lough D, Pishvaian MJ, Charabaty A. Current management of inflammatory bowel disease and colorectal cancer. Gastrointest Cancer Res. 2011;4:53–61. [PMC free article] [PubMed] [Google Scholar]
- 2.Terzic J, Grivennikov S, Karin E, Karin M. Inflammation and colon cancer. Gastroenterology. 2010;138:2101–2114. e2105. doi: 10.1053/j.gastro.2010.01.058. [DOI] [PubMed] [Google Scholar]
- 3.Azer SA. Overview of molecular pathways in inflammatory bowel disease associated with colorectal cancer development. Eur J Gastroenterol Hepatol. 2013;25:271–281. doi: 10.1097/MEG.0b013e32835b5803. [DOI] [PubMed] [Google Scholar]
- 4.Firestein GS. Starving the synovium: angiogenesis and inflammation in rheumatoid arthritis. J Clin Invest. 1999;103:3–4. doi: 10.1172/JCI5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Danese S, Sans M, de la Motte C, Graziani C, West G, Phillips MH, Pola R, Rutella S, Willis J, Gasbarrini A, Fiocchi C. Angiogenesis as a novel component of inflammatory bowel disease pathogenesis. Gastroenterology. 2006;130:2060–2073. doi: 10.1053/j.gastro.2006.03.054. [DOI] [PubMed] [Google Scholar]
- 6.Hatoum OA, Binion DG. The vasculature and inflammatory bowel disease: contribution to pathogenesis and clinical pathology. Inflamm Bowel Dis. 2005;11:304–313. doi: 10.1097/01.mib.0000160772.78951.61. [DOI] [PubMed] [Google Scholar]
- 7.Kanazawa S, Tsunoda T, Onuma E, Majima T, Kagiyama M, Kikuchi K. VEGF, basic-FGF, and TGF-beta in Crohn’s disease and ulcerative colitis: a novel mechanism of chronic intestinal inflammation. Am J Gastroenterol. 2001;96:822–828. doi: 10.1111/j.1572-0241.2001.03527.x. [DOI] [PubMed] [Google Scholar]
- 8.Bousvaros A, Leichtner A, Zurakowski D, Kwon J, Law T, Keough K, Fishman S. Elevated serum vascular endothelial growth factor in children and young adults with Crohn’s disease. Dig Dis Sci. 1999;44:424–430. doi: 10.1023/a:1026635308127. [DOI] [PubMed] [Google Scholar]
- 9.Griga T, Voigt E, Gretzer B, Brasch F, May B. Increased production of vascular endothelial growth factor by intestinal mucosa of patients with inflammatory bowel disease. Hepatogastroenterology. 1999;46:920–923. [PubMed] [Google Scholar]
- 10.Chao W, Olson MS. Platelet-activating factor: receptors and signal transduction. Biochem J. 1993;292:617–629. doi: 10.1042/bj2920617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Honda Z, Ishii S, Shimizu T. Platelet-activating factor receptor. J Biochem. 2002;131:773–779. doi: 10.1093/oxfordjournals.jbchem.a003164. [DOI] [PubMed] [Google Scholar]
- 12.Eliakim R, Karmeli F, Razin E, Rachmilewitz D. Role of platelet-activating factor in ulcerative colitis. Enhanced production during active disease and inhibition by sulfasalazine and prednisolone. Gastroenterology. 1988;95:1167–1172. doi: 10.1016/0016-5085(88)90346-0. [DOI] [PubMed] [Google Scholar]
- 13.Sobhani I, Hochlaf S, Denizot Y, Vissuzaine C, Rene E, Benveniste J, Lewin MM, Mignon M. Raised concentrations of platelet activating factor in colonic mucosa of Crohn's disease patients. Gut. 1992;33:1220–1225. doi: 10.1136/gut.33.9.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wallace JL. Release of platelet-activating factor (PAF) and accelerated healing induced by a PAF antagonist in an animal model of chronic colitis. Can J Physiol Pharmacol. 1988;66:422–425. doi: 10.1139/y88-071. [DOI] [PubMed] [Google Scholar]
- 15.Camussi G, Montrucchio G, Lupia E, De Martino A, Perona L, Arese M, Vercellone A, Toniolo A, Bussolino F. Platelet-activating factor directly stimulates in vitro migration of endothelial cells and promotes in vivo angiogenesis by a heparin-dependent mechanism. J Immunol. 1995;154:6492–6501. [PubMed] [Google Scholar]
- 16.Bussolino F, Arese M, Montrucchio G, Barra L, Primo L, Benelli R, Sanavio F, Aglietta M, Ghigo D, Rola-Pleszczynski MR. Platelet activating factor produced in vitro by Kaposi’s sarcoma cells induces and sustains in vivo angiogenesis. J Clin Invest. 1995;96:940–952. doi: 10.1172/JCI118142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ko HM, Jung HH, Seo KH, Kang YR, Kim HA, Park SJ, Lee HK, Im SY. Platelet-activating factor-induced NF-kappaB activation enhances VEGF expression through a decrease in p53 activity. FEBS Lett. 2006;580:3006–3012. doi: 10.1016/j.febslet.2006.04.042. [DOI] [PubMed] [Google Scholar]
- 18.Sirois MG, Edelman ER. VEGF effect on vascular permeability is mediated by synthesis of platelet-activating factor. Am J Physiol. 1997;272:H2746–2756. doi: 10.1152/ajpheart.1997.272.6.H2746. [DOI] [PubMed] [Google Scholar]
- 19.Montrucchio G, Sapino A, Bussolati B, Ghisolfi G, Rizea-Savu S, Silvestro L, Lupia E, Camussi G. Potential angiogenic role of platelet-activating factor in human breast cancer. Am J Pathol. 1998;153:1589–1596. doi: 10.1016/S0002-9440(10)65747-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robert EG, Hunt JD. Lipid messengers as targets for antiangiogenic therapy. Curr Pharm Des. 2001;7:1615–1626. doi: 10.2174/1381612013397203. [DOI] [PubMed] [Google Scholar]
- 21.Chumanevich AA, Poudyal D, Cui X, Davis T, Wood PA, Smith CD, Hofseth LJ. Suppression of colitis-driven colon cancer in mice by a novel small molecule inhibitor of sphingosine kinase. Carcinogenesis. 2010;31:1787–1793. doi: 10.1093/carcin/bgq158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murthy SN, Cooper HS, Shim H, Shah RS, Ibrahim SA, Sedergran DJ. Treatment of dextran sulfate sodium-induced murine colitis by intracolonic cyclosporin. Dig Dis Sci. 1993;38:1722–1734. doi: 10.1007/BF01303184. [DOI] [PubMed] [Google Scholar]
- 23.ten Hove T, van den Blink B, Pronk I, Drillenburg P, Peppelenbosch MP, van Deventer SJ. Dichotomal role of inhibition of p38 MAPK with SB 203580 in experimental colitis. Gut. 2002;50:507–512. doi: 10.1136/gut.50.4.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cao Y, Stafforini DM, Zimmerman GA, McIntyre TM, Prescott SM. Expression of plasma platelet-activating factor acetylhydrolase is transcriptionally regulated by mediators of inflammation. J Biol Chem. 1998;273:4012–4020. doi: 10.1074/jbc.273.7.4012. [DOI] [PubMed] [Google Scholar]
- 25.Theilmeier G, De Geest B, Van Veldhoven PP, Stengel D, Michiels C, Lox M, Landeloos M, Chapman MJ, Ninio E, Collen D, Himpens B, Holvoet P. HDL-associated PAF-AH reduces endothelial adhesiveness in apoE-/-mice. FASEB J. 2000;14:2032–2039. doi: 10.1096/fj.99-1029com. [DOI] [PubMed] [Google Scholar]
- 26.Miwa M, Miyake T, Yamanaka T, Sugatani J, Suzuki Y, Sakata S, Araki Y, Matsumoto M. Characterization of serum platelet-activating factor (PAF) acetylhydrolase. Correlation between deficiency of serum PAF acetylhydrolase and respiratory symptoms in asthmatic children. J Clin Invest. 1988;82:1983–1991. doi: 10.1172/JCI113818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kald B, Smedh K, Olaison G, Sjodahl R, Tagesson C. Platelet-activating factor acetylhydrolase activity in intestinal mucosa and plasma of patients with Crohn’s disease. Digestion. 1996;57:472–477. doi: 10.1159/000201376. [DOI] [PubMed] [Google Scholar]
- 28.Lupia E, Montrucchio G, Battaglia E, Modena V, Camussi G. Role of tumor necrosis factor-alpha and platelet-activating factor in neoangiogenesis induced by synovial fluids of patients with rheumatoid arthritis. Eur J Immunol. 1996;26:1690–1694. doi: 10.1002/eji.1830260804. [DOI] [PubMed] [Google Scholar]
- 29.Rubin CE, Haggitt RC, Burmer GC, Brentnall TA, Stevens AC, Levine DS, Dean PJ, Kimmey M, Perera DR, Rabinovitch PS. DNA aneuploidy in colonic biopsies predicts future development of dysplasia in ulcerative colitis. Gastroenterology. 1992;103:1611–1620. doi: 10.1016/0016-5085(92)91185-7. [DOI] [PubMed] [Google Scholar]
- 30.Yost CC, Weyrich AS, Zimmerman GA. The platelet activating factor (PAF) signaling cascade in systemic inflammatory responses. Biochimie. 2010;92:692–697. doi: 10.1016/j.biochi.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Popivanova BK, Kitamura K, Wu Y, Kondo T, Kagaya T, Kaneko S, Oshima M, Fujii C, Mu-kaida N. Blocking TNF-alpha in mice reduces colorectal carcinogenesis associated with ch- ronic colitis. J Clin Invest. 2008;118:560–570. doi: 10.1172/JCI32453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Y, Wang K, Han GC, Wang RX, Xiao H, Hou CM, Guo RF, Dou Y, Shen BF, Li Y, Chen GJ. Neutrophil infiltration favors colitis-associated tumorigenesis by activating the interleukin-1 (IL-1)/IL-6 axis. Mucosal Immunol. 2014;7:1106–1115. doi: 10.1038/mi.2013.126. [DOI] [PubMed] [Google Scholar]
- 33.Grivennikov S, Karin E, Terzic J, Mucida D, Yu GY, Vallabhapurapu S, Scheller J, Rose-John S, Cheroutre H, Eckmann L, Karin M. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15:103–113. doi: 10.1016/j.ccr.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matsumoto S, Hara T, Mitsuyama K, Yamamoto M, Tsuruta O, Sata M, Scheller J, Rose-John S, Kado S, Takada T. Essential roles of IL-6 trans-signaling in colonic epithelial cells, induced by the IL-6/soluble-IL-6 receptor derived from lamina propria macrophages, on the development of colitis-associated premalignant cancer in a murine model. J Immunol. 2010;184:1543–1551. doi: 10.4049/jimmunol.0801217. [DOI] [PubMed] [Google Scholar]
- 35.Ahmed A, Dearn S, Shams M, Li XF, Sangha RK, Rola-Pleszczynski M, Jiang J. Localization, quantification, and activation of platelet-activating factor receptor in human endometrium during the menstrual cycle: PAF stimulates NO, VEGF, and FAKpp125. FASEB J. 1998;12:831–843. doi: 10.1096/fasebj.12.10.831. [DOI] [PubMed] [Google Scholar]
- 36.Brkovic A, Sirois MG. Vascular permeability induced by VEGF family members in vivo: role of endogenous PAF and NO synthesis. J Cell Biochem. 2007;100:727–737. doi: 10.1002/jcb.21124. [DOI] [PubMed] [Google Scholar]
- 37.Kim HA, Seo KH, Kang YR, Ko HM, Kim KJ, Back HK, Lee HK, Im SY. Mechanisms of platelet-activating factor-induced enhancement of VEGF expression. Cell Physiol Biochem. 2011;27:55–62. doi: 10.1159/000325205. [DOI] [PubMed] [Google Scholar]
- 38.Ma X, Ottino P, Bazan HE, Bazan NG. Platelet-activating factor (PAF) induces corneal neovascularization and upregulates VEGF expression in endothelial cells. Invest Ophthalmol Vis Sci. 2004;45:2915–2921. doi: 10.1167/iovs.04-0128. [DOI] [PubMed] [Google Scholar]
- 39.Waldner MJ, Wirtz S, Jefremow A, Warntjen M, Neufert C, Atreya R, Becker C, Weigmann B, Vieth M, Rose-John S, Neurath MF. VEGF receptor signaling links inflammation and tumorigenesis in colitis-associated cancer. J Exp Med. 2010;207:2855–2868. doi: 10.1084/jem.20100438. [DOI] [PMC free article] [PubMed] [Google Scholar]