Abstract
Betulinic acid selectively inhibits the growth of ovarian carcinoma cell lines without affecting the normal cells. In the present study, the effect of 5-fluorouracil (5-FU) and betulinic acid (BA) combination on ovarian carcinoma cells was studied. The results demonstrated that ovarian carcinoma cells on concurrent or 5-FU followed by BA treatment show increased Sub-G1 cell population, increased rate of cell apoptosis and morphological changes in mitochondrial membrane. In OVCAR 432 cells treatment with sequential combination of 5-FU and BA increased the Sub-G1 cell population to 51.3% and growth inhibition rate of > 72%. However, exposure to BA before 5-FU treatment caused a decrease in rate of inhibition to < 35%. Treatment with combination of 5 μM of 5-FU and 1 μM of BA for 48 h, led to an induction of apoptosis in 79.7% and induced morphological changes in OVCAR 432 cells. The Western blot results showed high concentration of cytochrome c in the cell cytosol after 24 h of 5-FU and BA combination treatment. Treatment of BA-responsive RMS-13 cells with 5-FU and BA combination resulted in inhibition of GLI1, GLI2, PTCH1, and IGF2 genes. In addition, we found a significant reduction in hedgehog activity of RMS-13 cells after 5-FU and BA combination treatment by means of a hedgehog-responsive reporter assay. Therefore, 5-FU and BA combination can be a promising regimen for the treatment of ovarian carcinoma.
Keywords: Ovarian carcinoma, inhibition, morphological changes, hedgehog, apoptosis
Introduction
Ovarian carcinoma because of late stage detection is the most lethal gynecological malignancy. It is usually diagnosed after tumor cells are widely metastasized within the peritoneal cavity. In spite of advancement in modern cancer treatments like surgery and combination therapy with paclitaxel and carboplatin the death rate of patients is still high [1,2]. All the treatments for ovarian carcinoma at present are inefficient. Radiation therapy has led to an average survival rate of 5-years in 10% [3-6] while as chemotherapy has led to a response rate from 3 to 30% [7-11]. The patients with less-than-25% and greater-than-75% cytoreduction have an average difference in survival of 11 months [12]. Therefore, the need for new treatment regimens with roles in ovarian carcinoma treatment is highly desired.
The 5-fluorouracil (5-FU) and oral fluoropyrimidines have been shown to be effective in many cancers including gastrointestinal tract cancers [13]. New generations of oral fluoropyrimidine such as S-1, which inhibit the degradation of 5-FU and capecitabine have been developed and put into use in the treatment of several solid tumors [14]. 5-FU and 4-HPR showed positive responses in combination with platinum based drugs or as a single agent in phase II trials respectively in ovary carcinoma [15-17].
Betulinic acid (Figure 1), has been reported to selectively inhibit the growth of lung, colon, prostate, and ovary cancer cells [18] without affecting the normal cell lines [19]. It possesses anti-tumor activity by inducing apoptosis in a variety of human cancers [18]. BA treatment in childhood tumors like Ewing sarcoma [20], neuroblastoma [21], and leukaemia [22] has been significantly effective. BA treatment induces distinct morphological changes in sensitive cells [18] and has been found to be effective on both drug-resistant and drug-sensitive tumour cell lines [23].
Figure 1.
Structure of Betulinic acid.
Materials and methods
Chemicals
Betulinic acid and 5-fluorouracil were purchased from Sigma-Aldrich (St. Louis, MO, USA). L-glutamine from Invitrogen (Carlsbad, CA, USA), 1% penicillin and streptomycin from Gibco, (Grand Island, NY, USA). Enhanced chemiluminescence (ECL), nitrocellulose membrane and ECL prime western blotting detection reagents were obtained from GE Healthcare Life Sciences (Buckinghamshire, UK). Hoechst 33342, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenylterazolium bromide (MTT), 1-methyl-4-phenylpyridinium (MPPþ), dimethyl sulfoxide (DMSO) and b-actin were purchased from Sigma (St Louis, MO, USA).
Cell culture
The human ovarian cancer lines OVCAR 432, RMS-13 cell lines and NIH-3T3 fibroblasts were obtained from American Type of Collection centre (Manassas, VA, USA). The cells were maintained in minimum essential medium (MEM), containing 10% fetal bovine serum and antibiotics. The cell lines were maintained in a humidified incubator at 37°C and 5% CO2.
Cytotoxicity assay
In 96-well culture plates 5 × 103 cells were plated per well in MEM. To each well 200 μl containing different concentrations of BA, 5-FU and combination of 5-FU + BA in DMSO were added and the plates were incubated for 24 h at 37°C. After 24 h the medium was changed to drug free medium and incubation was continued for 5 days more. Alamar blue vital dye indicator assay [24] was used to measure the cell viability. Inhibition in cell growth is denoted as cytotoxicity and represented as concentration-response curve (surviving percentage versus drug concentration). The IC50 is determined from a least squares regression fit to the linear portion of the curve.
Flow cytometry
The cells distributed at a density of 8 × 105 onto 100 mm dishes were incubated at 37°C for 24 h. After 24 h, the cells were treated with 5-FU, BA or 5-FU + BA combination at IC50 concentration. Nuclear staining for DNA content was performed after harvesting the cells at 12, 24 and 48 h from treatment. The untreated cells were also harvested at the same time points and used as control. The cells were treated with two solutions, a salt solution containing SDS and RNase A, second solution containing sucrose and citric acid. Additionally both solutions contained ethidium bromide. Becton Dickinson FACS can (cell Quest software) was used to acquire cell cycle data and Cell Cycle Muticycle (Phoenix Flow Systems, San Diego, CA) was used for analysis.
Assessment of apoptosis
The cells treated with BA, 5-FU and 5-FU + BA combination after harvesting were washed with PBS and fixed in formaldehyde at room temperature for 20 min. Then fixative was removed and cells were suspended in 50 μl of 8 μg/ml bisbenzimide trichloride dye (Hoechst-33258). On to the glass slides, 30 μl cell aliquots were fixed. Five hundred cells were counted and scored for the incidence of apoptosis using fluorescence microscope. All the measurements were performed in triplicates.
Western blot assay
In six well plates 1 × 105 cells were platted per plate for 12 h and then treated with 5-FU (20 μM), BA (10 μM) or 5-FU (5 μM) + BA (1 μM) combination in the presence or absence of 50 μM zVAD. fmk. Vehicle treated cells were used as negative and cycloheximide (Sigma-Aldrich, Taufkirchen, Germany) as positive control. After 48 h, cells were lysed in lysis buffer (50 mM Tris-HCl pH 7.4, 137 mM NaCl, 10% glycerol, 100 mM sodium vanadate, 1 mM PMSF, 10 mg/ml aprotinin, 10 mg/ml leupeptin, 1% NP-40, and 5 mM cocktail) and the lysate was centrifuged to remove cell debris. Protein Assay System (Bio-Rad, Hercules, CA, USA) was used to determine concentration of proteins. The protein were loaded and resolved by electrophoresis on a 10% polyacrylamide gel and transferred to nitrocellulose membranes. The semi-dry method was used to transfer proteins onto a PVDF membrane which was then blocked with 5% non-fat dry milk overnight. Incubation of membranes with mouse anti-human caspase 3, rabbit anti-human cleaved caspase 3, rabbit anti-human β-actin (Cell Signalling Technology, Danvers, MA, USA), mouse anti-human COX4, rabbit anti-human cytochrome c (Clontech, Mountain View, CA, USA) or goat anti-human GLI1 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) antibodies was performed for 2 h. Then the incubation was continued for 1.5 h with horseradish peroxidase-conjugated goat anti-rabbit, goat anti-mouse, or rabbit anti-goat IgG secondary antibodies (DakoCytomation, Hamburg, Germany). ECL chemiluminescence detection system (GE Healthcare) was used for visualising the signal.
Hedgehog reporter and activation assays
5 × 105 RMS-13 cells or NIH-3T3 fibroblasts were transfected with 900 ng of the reporter plasmid p11xGli or the empty control pGL3-TK (25) and 100 ng of the reference plasmid pRL-TK using FuGene 6 transfection reagent (Roche Diagnostics). 1 μg of an expression plasmid containing murine sonic hedgehog gene (Karolinska Institute, Stockholm, Sweden) was transfected into fibroblasts. After 24 h transfection, cells were treated with 5 μg/ml BA, 10 μg/ml 5-FU and BA + 5-FU combination, 10 μM cyclopamine (Toronto Research Chemicals, Toronto, Canada), or vehicle and cultured for 24 h. A Dual-Glo Luciferase Reporter Assay System (Promega, Madison, Wisconsin, USA) was used for determination of reporter gene activity. Firefly luciferase activity was normalised to Renilla luciferase activity. After 24 h of transfection total RNA was isolated using Trizol (Invitrogen) from fibroblasts.
Statistical analyses
The vehicle treated control cells were arbitrarily assigned 100% and other data were expressed in comparison to control. Data were analyzed by Dunnett’s multiple comparison test (Sigma Stat, Jandel, San Rafael, CA, USA). For all comparisons, differences were considered statistically significant at P < 0.05.
Results
Cytotoxicity of BA, 5-FU and their combination against ovarian carcinoma cells
The synergistic effect of sequential combination of 5-FU followed by BA on cell apoptosis in OVCAR 432 cells was determined by cytometric analysis of Sub-G1 cell population (Figure 2A). Treatment of OVCAR 432 cells with 5-FU and BA increased the cell population in sub-G1 to 23.4 and 27.9% respectively whereas in control only 16.2% cell population was in Sub-G1 phase. However, treatment of OVCAR 432 cells with sequential combination of 5-FU and BA increased the Sub-G1 cell population to 51.3%, which exceeded the additive effect due to BA and 5-FU when used separately. The concentration of BA that is effective in enhancing 5-FU cytotoxicity is significantly lower than IC50 for this reagent alone. These results suggest that BA and 5-FU exhibit synergistic effect on induction of apoptosis in OVCAR 432 cells.
Figure 2.
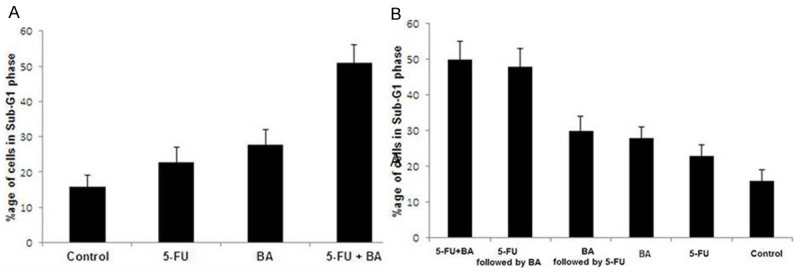
Cytotoxic activity of BA and combination of 5-FU + BA against OVCAR 432 cells. A. Combination of 5-FU + BA increased the population of OVCAR 432 cells in Sub G1 phase compared to that of BA. B. Synergistic effect of BA on 5-FU depends on the schedule of exposure. Exposure of OVCAR 432 cells to 5-FU followed by BA or concurrently leads to synergistic effect. When BA is added before 5-FU treatment there is decrease in rate of inhibition.
We observed that enhancement of 5-FU cytotoxicity by BA depends on exposure schedule (Figure 2B). Exposure to an IC50 concentration of 5-FU for 24 h followed by BA at IC25 for 24 h or both agents concurrently for 48 h resulted in growth inhibition rate of > 72% in OVCAR 432 cells. When BA is added before 5-FU treatment there is decrease in rate of inhibition to < 35% and when each agent is used separately, they produce < 24% inhibition of cell growth. Therefore, the mechanism of action of BA appears to be dependent on 5-FU.
BA treatment increases apoptosis in 5-FU treated cell lines
We used quantitative fluorescence microscopy to observe apoptotic chromatin condensation. The results of the analysis showed that the combination of 5-FU + BA induces a higher rate of apoptosis than either agent used alone (Figure 3). Treatment of OVCAR 432 cells with 10 μM concentration of 5-FU for 24 h induced apoptosis in 5% and with 5 μM concentration of BA for 24 h induced apoptosis in 47.5% of the cells. However when OVCAR 432 cells were treated with a combination of 5 μM of 5-FU and 1 μM of BA for 48 h, there was an induction of apoptosis in 79.7% cells. Higher overall percentages of apoptosis are seen at latter points of time.
Figure 3.
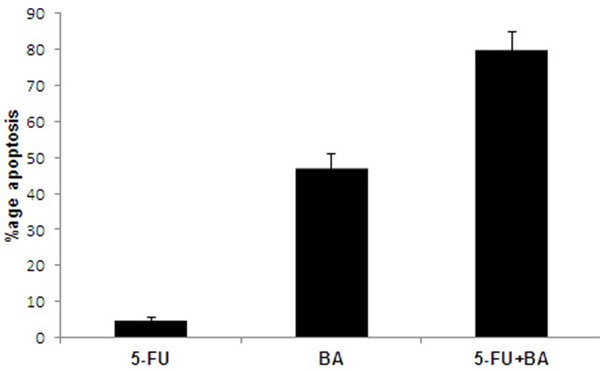
Rate of apoptosis in OVCAR 432 cells using quantitative fluorescence microscopy. The combination of 5-FU + BA enhanced the rate of apoptosis in OVCAR 432 cells compared to that of BA used alone. All the results are mean of triplicate measurements.
Effect of 5-FU and BA combination mitochondria in OVCAR 432 cells
We also analysed the effect of 5-FU + BA combination on morphological changes in OVCAR 432 cells. Compared to BA, the combination of 5-FU + BA induced morphological changes in OVCAR 432 cells at a concentration lower than IC50 of BA when used alone (Figure 4A). The results from flow cytometry showed that combination of 5-FU + BA induced significant rate of apoptosis after 48 h. When OVCAR 432 cells were treated with combination of 5-FU + BA along with caspase inhibitor, zVAD.fmk there was inhibition in rate of apoptosis (Figure 4B, 4C). This indicated involvement of caspases in 5-FU + BA-induced apoptosis. However treatment of OVCAR 432 cells with zVAD.fmk inhibited this effect. Therefore, combination of 5-FU and BA induces apoptosis through caspase dependent pathway.
Figure 4.
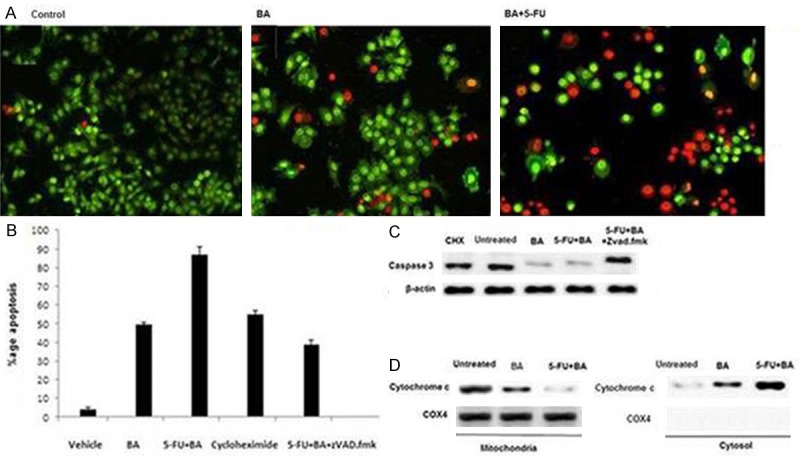
Effect of 5-FU + BA combination on apoptosis in RMS cell lines. A. The RMS cells were treated for 48 h with BA and combination of 5-FU + BA in the presence or absence of 50 μM of the zVAD.fmk. Apoptosis-specific DNA fragmentation was determined by FACS analysis of propidium iodide-stained nuclei; 100 μg/ml cycloheximide (CHX) was used as a positive control for apoptosis induction. B. The RMS-13 cells were treated for 48 h with BA and combination of 5-FU + BA in the absence or presence of zVAD.fmk. Expression of caspase 3 and β-actin was detected by western blot analysis. 100 μg/ml cycloheximide (CHX) was used as a positive control for apoptosis induction. C. The RMS-13 cells were treated for 24 and 48 h with BA and combination of 5-FU + BA. Mitochondria were isolated, lysed, and the expression of cytochrome c and COX4 in the mitochondrial and cytosolic fraction was detected by western blot analysis
The results from Western blot analysis showed high concentration of cytochrome c in the cell cytosol after 48 h of 5-FU + BA combination treatment. On the other hand cytochrome c concentration in the mitochondrial fraction inversely decreased (Figure 4D). The effect was most promising using 5-FU + BA combination compared to that BA alone. Together, these results clearly indicate that 5-FU + BA-induced apoptosis in OVCAR 432 cells is mediated by the intrinsic apoptotic pathway.
5-FU + BA combination specifically targets hedgehog signalling in RMS cells
Treatment of RMS-13 cells with BA and 5-FU + BA combination resulted in inhibition of GLI1, GLI2, PTCH1, and IGF2 genes. However inhibition caused by 5-FU + BA combination was much greater compared to BA alone (Figure 5B-E). We did not observe any significant effect in two GLI1-negative RMS cell lines RH-30 and RD (Figure 5A). Moreover, we found a significant reduction in hedgehog activity of RMS-13 cells after 5-FU + BA combination treatment by means of a hedgehog-responsive reporter assay. To examine whether this inhibition is dependent on hedgehog signalling components upstream of GLI1, RMS-13 cells were treated with 5-FU + BA combination in the presence or absence of 10 μM cyclopamine, a specific hedgehog signalling inhibitor [26]. However, the inhibitory effect of 5-FU + BA combination on GLI1 activity was found to be independent of cyclopamine treatment (Figure 3C), indicating that BA is able to selectively target hedgehog signalling on the level of GLI1.
Figure 5.
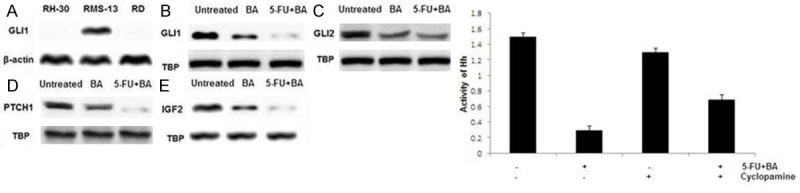
5-FU + BA combination blocks hedgehog signalling A. GLI1 expression of rhabdomyosarcoma cell lines. The RMS cell lines were lysed and the expression of GLI1 and β-actin was detected by western blot analysis. B. Hedgehog signalling dependency. The RMS cells were treated for 48 h with BA and combination of 5-FU + BA. GLI1, GLI2, PTCH1, and IGF2 mRNA expression from untreated and treated RMS cells was measured by quantitative real-time PCR in relation to the house-keeping gene TBP as a calibrator; *P < 0.05 (unpaired Student’s t-test). C. Hedgehog pathway activity. The RMS-13 cells were transiently transfected with 900 ng hedgehog-responsive reporter plasmid (p11xGli) or control plasmid (pGL3-TK) and treated for 24 h with 5 μM 5-FU + (1 Μm) BA and 10 μM cyclopamine or vehicle. Reporter assay experiments were repeated three times and transfections performed in duplicate; *P < 0.05 (unpaired Student’s t-test).
In order to activate hedgehog pathway in untransformed NIH-3T3 cells, we expressed the ligand sonic hedgehog by transient transfection. This resulted in strong expression of the hedgehog target genes Gli1, Gli2, Ptch1, and Igf2 (Figure 6A, 6B). But exposure to hedgehog inhibitor, cyclopamine resulted in inhibition of expression of these hedgehog target genes. The use of 5-FU + BA combination led to inhibition of Gli1 and Gli2 expression without affecting Ptch1 and Igf2. These results led us to conclude that 5-FU + BA combination inhibits hedgehog signalling through GLI family transcription factors selectively.
Figure 6.
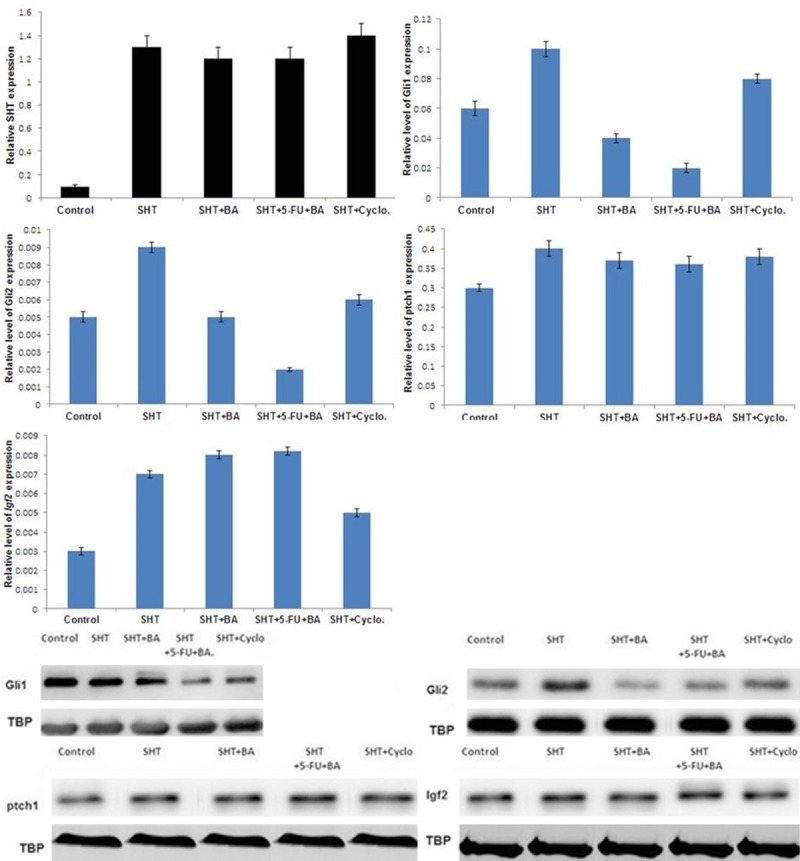
Hedgehog activation assay. The NIH-3T3 cells were transiently transfected with 1 μg sonic hedgehog (Shh) expression plasmid. For the inhibition of hedgehog signalling, cells were additionally exposed to 5-FU + BA combination and 10 μM cyclopamine (Cycl.) after 24 h transfection. Expression of the murine genes SHT, Gli1, Gli2, Ptch1, and Igf2 was determined after an incubation period of 48 h using quantitative real-time PCR in relation to the house-keeping gene TBP as a calibrator; *P < 0.05 (unpaired Student’s t-test).
Discussion
This study was carried out in order to investigate the potential of 5-FU in combination with BA in the treatment of ovary carcinoma. A synergistic effect was observed only when ovary carcinoma cells were treated with 5-FU followed by BA or concurrently in a sequence-dependent manner. Angiogenesis has been reported as a prerequisite for tumour growth beyond certain size as well as for metastatic spread and, therefore, is an attractive target for clinical tumor biologists [27]. There are reports that anti-angiogenic treatments synergise with traditional chemotherapeutic and radiotherapeutic regimens. BA is not only a cytotoxic effect against endothelial cells, but also inhibits tube-like structure formation of aortic endothelial cells [28,29]. In the present study treatment of OVCAR 432 cells with sequential combination of 5-FU and BA increased the Sub-G1 cell population to 51.3%, which exceeded the additive effect due to BA and 5-FU when used separately. The concentration of BA that is effective in enhancing 5-FU cytotoxicity is significantly lower than IC50 for this reagent alone. Obvious synergistic growth inhibition was observed only when OVCAR 432 cells were treated with 5-FU followed by BA. Based on the flow cytometric analysis, this synergism was attributable to the enhancement of apoptotic cell death. Treatment of OVCAR 432 cells with a combination of 10 μM of 5-FU and 5 μM of BA for 24 h, induced apoptosis in 49.7% cells.
The purpose of the present study was to provide experimental evidence in order to help develop chemotherapeutic regimens containing 5-FU and BA for ovary carcinoma. Compared to BA, the combination of 5-FU and BA induced morphological changes in OVCAR 432 cells at a concentration lower than IC50 of BA when used alone. The results from flow cytometry showed that compared to BA-induced apoptosis which was significant after 48 h, the induction of apoptosis by combination of 5-FU and BA was significant after 24 h. When the OVCAR 432 cells were treated with combination of 5-FU and BA along with caspase inhibitor, zVAD.fmk there was inhibition in rate of apoptosis. This led to the conclusion that caspases were involved in 5-FU and BA-induced apoptosis. The Western blot results showed high concentration of cytochrome c in the cell cytosol after 24 h of 5-FU and BA combination treatment. On the other hand cytochrome c concentration in the mitochondrial fraction inversely decreased. The effect was enhanced by 5-FU and BA combination compared to that BA alone.
Treatment of RMS-13 cells with BA and 5-FU + BA combination resulted in inhibition of GLI1, GLI2, PTCH1, and IGF2 genes. However inhibition caused by BA + 5-FU combination was much greater compared to BA alone. Moreover, we found a significant reduction in hedgehog activity of RMS-13 cells after BA + 5-FU combination treatment by means of a hedgehog-responsive reporter assay. To examine whether this inhibition is dependent on hedgehog signalling components upstream of GLI1, RMS-13 cells were treated with BA + 5-FU combination in the presence or absence of 7.5 μM cyclopamine, a specific hedgehog signalling inhibitor [26]. However, the inhibitory effect of BA + 5-FU combination on GLI1 activity was found to be independent of cyclopamine treatment, indicating that BA + 5-FU combination is able to selectively target hedgehog signalling on the level of GLI1.
In conclusion, BA + 5-FU combination has an important role in ovarian carcinoma treatment by inducing apoptosis through mitochondrial pathway and specifically targeting the hedgehog signalling pathway.
Disclosure of conflict of interest
None.
References
- 1.Cannistra SA. Cancer of the ovary. N Engl J Med. 2004;351:2519–29. doi: 10.1056/NEJMra041842. [DOI] [PubMed] [Google Scholar]
- 2.Lengyel E. Ovarian cancer development and metastasis. Am J Pathol. 2010;177:1053–64. doi: 10.2353/ajpath.2010.100105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fuks Z. External radiotherapy of ovarian cancer: Standard approaches and new frontiers. Semin Oncol. 1975;2:253–266. [PubMed] [Google Scholar]
- 4.Dembo AJ. Radiotherapeutic management of ovarian cancer. Semin Oncol. 1984;11:238–250. [PubMed] [Google Scholar]
- 5.Martinez A, Schray MF, Howes AE. Postoperative radiation therapy for epithelial ovarian cancer: the curative role based on a 24-year’s experience. J. Clin. Oncol. 1985;3:901–922. doi: 10.1200/JCO.1985.3.7.901. [DOI] [PubMed] [Google Scholar]
- 6.Fuks Z, Yahalom Y, Brenner H. The treatment of ovarian carcinoma. In: Nori D, Hilaris BS, editors. Radiation Therapy of Gynecological Cancer. New York: Liss; 1987. [Google Scholar]
- 7.Wiltshaw E, Evans B, Rustin G. A prospective randomized trial comparing high-dose cisplatin with low-dose cisplatin and chlorambucil in advanced ovarian carcinoma. J. Clin. Oncol. 1986;4:722–729. doi: 10.1200/JCO.1986.4.5.722. [DOI] [PubMed] [Google Scholar]
- 8.Parker LM, Griffiths CT, Yankee RA. Combination chemotherapy with Adriamycin-cyclophosphamide of advanced ovarian carcinoma. Cancer. 1980;46:669–674. doi: 10.1002/1097-0142(19800815)46:4<669::aid-cncr2820460407>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 9.Wharton JT, Edwards CL, Rutledge FN. Long term survival after chemotherapy for advanced epithelial ovarian carcinoma. Am J Obstet Gynecol. 1984;148:997–1005. doi: 10.1016/0002-9378(84)90543-x. [DOI] [PubMed] [Google Scholar]
- 10.Williams CJ, Mead GM, Macbeth RF. Cisplatin combination chemotherapy versus chlorambucil in advanced ovarian carcinoma: mature results of randomized study. J. Clin. Oncol. 1985;3:1455–146. doi: 10.1200/JCO.1985.3.11.1455. [DOI] [PubMed] [Google Scholar]
- 11.Louie KG, Ozols RF, Myers CE. Long-term results of a cisplatin-containing combination chemotherapy regimen for the treatment of advanced ovarian carcinoma. J. Clin. Oncol. 1986;4:1579–1585. doi: 10.1200/JCO.1986.4.11.1579. [DOI] [PubMed] [Google Scholar]
- 12.Bristow RS, Tomacruz DK, Armstrong EL, Montz FJ. Survival impact of maximum cytoreductive surgery for advanced ovarian carcinoma during the platinum-era: a meta-analysis of 6,848 patients. Proc ASCO. 2001;20:202. doi: 10.1200/JCO.2002.20.5.1248. [DOI] [PubMed] [Google Scholar]
- 13.Takiuchi H, Ajani JA. Uracil-tegafur in gastric carcinoma: a comprehensive review. J. Clin. Oncol. 1998;16:2877–2885. doi: 10.1200/JCO.1998.16.8.2877. [DOI] [PubMed] [Google Scholar]
- 14.Schoffski P. The modulated oral fluoropyrimidine prodrug S-1, and its use in gastrointestinal cancer and other solid tumors. Anticancer Drugs. 2004;15:85–106. doi: 10.1097/00001813-200402000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Rosa DD, Awada A, Mano MS, Selleslags J, Lebrun F, Gil T, Piccart MJ, D’Hondt V. Oxaliplatin/5fluorouracil-based chemotherapy was active and well tolerated in heavily pretreated patients with ovarian carcinoma. Arch Gynecol Obstet. 2008;278:457–462. doi: 10.1007/s00404-008-0592-9. [DOI] [PubMed] [Google Scholar]
- 16.Weiss RB. The anthracyclines: will we ever find a better doxorubicin? Semin Oncol. 1992;19:670–686. [PubMed] [Google Scholar]
- 17.Garcia AA, Morgan R, McNamara M, Scudder S, Tsao-Wei D, Groshen S, Frgala T, Kim Y, Reynolds CP. Phase II trial of fenretinide (4-HPR) in recurrent ovarian and primary peritoneal carcinoma: A California Cancer Consortium trial. Journal of Clinical Oncology, 2004 ASCO Annual Meeting Proceedings (Post-Meeting Edition) 2004;22:5056. [Google Scholar]
- 18.Alakurtti S, Makela T, Koskimies S, Yli-Kauhaluoma J. Pharmacological properties of the ubiquitous natural product betulin. Eur J Pharm Sci. 2006;29:1–13. doi: 10.1016/j.ejps.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 19.Zuco V, Supino R, Righetti SC, Cleris L, Marchesi E, Gambacorti-Passerini C, Formelli F. Selective cytotoxicity of betulinic acid on tumour cell lines, but not on normal cells. Cancer Lett. 2002;175:17–25. doi: 10.1016/s0304-3835(01)00718-2. [DOI] [PubMed] [Google Scholar]
- 20.Fulda S, Friesen C, Los M, Scaffidi C, Mier W, Benedict M, Nunez G, Krammer PH, Peter ME, Debatin KM. Betulinic acid triggers CD95 (APO-1/Fas)- and p53-independent apoptosis via activation of caspases in neuroectodermal tumours. Cancer Res. 1997;57:4956–4964. [PubMed] [Google Scholar]
- 21.Schmidt ML, Kuzmanoff KL, Ling-Indeck L, Pezzuto JM. Betulinic acid induces apoptosis in human neuroblastoma cell lines. Eur J Cancer. 1997;33:2007–2010. doi: 10.1016/s0959-8049(97)00294-3. [DOI] [PubMed] [Google Scholar]
- 22.Ehrhardt H, Fulda S, Fuhrer M, Debatin KM, Jeremias I. Betulinic acid-induced apoptosis in leukemia cells. Leukemia. 2004;18:1406–1412. doi: 10.1038/sj.leu.2403406. [DOI] [PubMed] [Google Scholar]
- 23.Sarek J, Klinot J, Dzubak P, Klinotova E, Noskova V, Krecek V, Korinkova G, Thomson JO, Janost’akova A, Wang S, Parsons S, Fischer PM, Zhelev NZ, Hajduch M. New lupane derived compounds with pro-apoptotic activity in cancer cells: synthesis and structure-activity relationships. J Med Chem. 2003;46:5402–5415. doi: 10.1021/jm020854p. [DOI] [PubMed] [Google Scholar]
- 24.Ahmad SA, Gogal RM Jr, Walsh JE. A new rapid and simple non-radioactive assay to monitor and determine the proliferation of lymphocytes; an alternative to [3H] thymidine incorporation assay. J Immunol Methods. 1994;170:211–224. doi: 10.1016/0022-1759(94)90396-4. [DOI] [PubMed] [Google Scholar]
- 25.Beer C, Buhr P, Hahn H, Laubner D, Wirth M. Gene expression analysis of murine cells producing amphotropic mouse leukaemia virus at a cultivation temperature of 32 and 37 degrees C. J Gen Virol. 2003;84:1677–1686. doi: 10.1099/vir.0.18871-0. [DOI] [PubMed] [Google Scholar]
- 26.Taipale J, Chen JK, Cooper MK, Wang B, Mann RK, Milenkovic L, Scott MP, Beachy PA. Effects of oncogenic mutations in smoothened and patched can be reversed by cyclopamine. Nature. 2000;406:1005–1009. doi: 10.1038/35023008. [DOI] [PubMed] [Google Scholar]
- 27.Folkman J. Role of angiogenesis in tumour growth and metastasis. Semin Oncol. 2002;29:15–18. doi: 10.1053/sonc.2002.37263. [DOI] [PubMed] [Google Scholar]
- 28.Kwon HJ, Shim JS, Kim JH, Cho HY, Yum YN, Kim SH, Yu J. Betulinic acid inhibits growth factor-induced in vitro angiogenesis via the modulation of mitochondrial function in endothelial cells. Jpn J Cancer Res. 2002;93:417–425. doi: 10.1111/j.1349-7006.2002.tb01273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mukherjee R, Jaggi M, Rajendran P, Siddiqui MJ, Srivastava SK, Vardhan A, Burman AC. Betulinic acid and its derivatives as anti-angiogenic agents. Bioorg Med Chem Lett. 2004;14:2181–2184. doi: 10.1016/j.bmcl.2004.02.044. [DOI] [PubMed] [Google Scholar]


