Abstract
Asthma is a serious global health problem characterised by airway inflammation, airway epithelial wall shedding, enhanced mucus production, increased IgE levels and airway hyperresponsiveness. The pathophysiology of asthma is mediated by Th2 cells which produce Th2 cytokines like interleukin-4, interleukin-5, interleukin-13 and interleukin-9. The differentiation of Th2 cells is induced by the transcription factor GATA3 which is activated by pSTAT6 via IL-4 signalling. To investigate the anti-asthmatic potential of Boswellic acid, as well as the underlying mechanism involved, we studied its anti-asthmatic potential in a murine model of asthma. In this study, BALB/c mice were systemically sensitized by ovalbumin (OVA) followed by aerosol allergen challenges. We investigated the effect of Boswellic acid on airway hyperresponsiveness, inflammatory cell infiltration, Th2 cytokine and OVA-specific IgE production in a mouse model of asthma. We found that Boswellic acid treated groups suppressed allergic airway inflammation, AHR, OVA-specific IgE and Th2 cytokines secretion. It also suppressed the expression of pSTAT6 and GATA3 in a dose dependent manner. Our data suggest that the mechanism by which Boswellic acid effectively treats asthma is based on reductions of Th2 cytokines via inhibition of pSTAT6 and GATA-3 expression.
Keywords: Asthma, IgE, airway hyperresponsiveness, Th2, eosinophil, GATA-3, pSTAT6
Introduction
Asthma is a chronic inflammatory airway disease affecting over 300 million people worldwide with an expected increase of a further 100 million by 2025 [1,2]. Asthma is a major cause of disability, health resource utilization and poor quality of life for those who are affected. It is the most common chronic disease among children and young adults, particularly because of its early onset (one out of four individuals in the general population develops asthma before the age of 40 years) [3]. The pathogenesis of asthma is the result of chronic allergic inflammation accompanied by progressive airway dysfunction. The pathophysiology of asthma is mediated by Th2 cells which mainly produce Th2 cytokines like interleukin-4 (IL-4), IL-5 and IL-13 [4]. These cytokines initiate a cascade, which recruits inflammatory cells into the lungs causing airway epithelial wall shedding, airway inflammation, increased mucus production and airway hyperresponsiveness. So, inhibition of Th2 effector cytokines would serve to treat disease [5]. GATA-3 is a member of zinc finger transcription factor family [6]. It plays the most important role in regulating Th2 differentiation and works as a transcription factor for Th2 cytokine genes, particularly for IL-4, IL-5 and IL-13. Activation of signal transducer and activator of transcription-6 (STAT6) through Interleukin-4 signalling causes dimerization and phosphorylation of STAT6 to pSTAT6 which enters the nucleus and induces GATA-binding protein-3 (GATA3) mRNA expression, ultimately resulting in Th2 cell differentiation [7].
Boswellia serrata is a type of deciduous tree grows naturally in Indian subcontinent. For centuries, the gum resin of Boswellia serrata has been used for the treatment of various inflammatory diseases including arthritis [8,9]. The pentacyclic triterpenic acids, named boswellic acids, present in the gum resin of B. serrata are the main constituents responsible for its anti-inflammatory property [10]. Suppression of leukotriene synthesis by inhibiting 5-lipoxygenase (5-LOX) is considered the main mechanism underlying their anti-inflammatory effect. Boswellic acids (BAs) are specific and non-redox inhibitors of 5-lipoxygenase, and they do not affect 12-lipoxygenase and cyclooxygenase (COX) activities [9-11]. Among the known BAs, 3-O-acetyl-11-keto-b-boswellic acid (AKBA) possesses the most potent inhibitory activity on 5-LOX [9,12]. A significant number of studies support that Boswellia extract (BE) is beneficial in patients suffering from various diseases such as bronchial asthma, Crohn’s disease, and arthritis [13-15]. In this study we evaluated the effect of Boswellic acid on airway inflammation in a murine model of asthma.
Materials and methods
Experimental animal
Female BALB/c mice (5 weeks old) were taken from the animal house of the Liaocheng People’s Hospital which was maintained under the controlled conditions, temperature (24 ± 2°C), relative humidity (60 ± 10%) and photoperiod (12 h light/dark cycle) respectively. The room was well ventilated (> 10 air changes/h) with fresh air, as per the CPCSEA (Committee for the Purpose of Control and Supervision on Experiments on Animals) guidelines. Animals were fed on standard pellet diet and sterilized water was provided ad libitum. Seven day acclimatized animals were used for pre-clinical studies. Permission was sought from the ethics committee of the Liaocheng People’s Hospital for animal experimentation.
Reagents
Chicken egg albumin (OVA, grade V), aluminium hydroxide gel (alum) and Dexamethsone, acetyl-β-methylcholine chloride (methacholine) and protease inhibitor cocktail and Boswellic acid were purchased from Sigma-Aldrich (St. Louis, MO). All other chemicals or reagents were commercially obtained and of the highest quality.
Segregation of animals and dosing schedule
Mice were segregated into six groups and after acclimatization; each group was named according to sensitization/challenge/treatment: group 1: SHAM/PBS/Veh (normal controls), group 2: OVA/OVA/Veh (OVA controls, OVA sensitised and OVA challenged), group 3: OVA/OVA/DEXA (OVA sensitized, OVA challenged, and Dexamethasone treated, 0.75 mg/kg), groups 4-6 OVA/OVA/Boswellic acid (OVA sensitized, OVA challenged, and Boswellic acid treated, 0.1, 1 and 10 mg/kg) The test compounds and the Dexamethasone were administered orally, once daily from day 19 to day 23 [16] (Figure 1). PBS was used as a vehicle.
Figure 1.
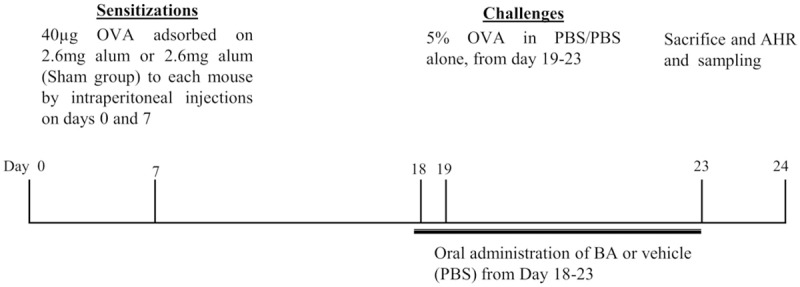
Experimental protocol for the induction of allergic asthma. Five wk-old female BALB/c were grouped, sensitized, and challenged, as described in materials and methods.
Sensitization, airway OVA challenging and treatment
The animals were sensitized intraperitoneally with 40 μg of OVA plus 2.6 mg of aluminum hydroxide in 200 μl of PBS on days 0 and 7. Mice were then challenged from days 19 to 23 (5 min per day) with 5% OVA in PBS (OVA groups) or PBS (Sham/PBS/Veh) as described previously with some modifications [17]. Mice were administered with the test drug and DEXA, once a day from days 19 to 23. Mice were sacrificed on day 24 and bronchoalveolar lavage was performed to evaluate lung eosinophilia.
Evaluation of airway hyper responsiveness
Airway hyperresponsiveness (AHR), in the form of airway resistance was estimated in anesthetized mice by using the FlexiVent system (SYNOL High-Tech, Beijing, China), which uses a computer-controlled mouse ventilator and integrates with respiratory mechanics, as described previously [18]. Final results were expressed as airway resistance with increasing concentrations of methacholine (0, 2, 4, 8, 12, 16 mg/ml).
Bronchoalveolar lavage fluid (BALF) collection
After mice were bled and sacrificed following anesthesia with ether, BALF was collected for differential cell counting and measurement of cytokines. It was carried out by cannulating the upper part of the trachea and lavaging by three times with 0.5 mL of PBS containing 0.05 mM EDTA [7] The BALF was centrifuged at 4000 g at 4°C for 3 min, and the cells were separated from the fluid. The supernatant was stored at -70°C until use. The cells were re-suspended in PBS containing 0.05 mM EDTA, and the total number was determined with the use of a hemocytometer. The differential BAL cells were counted by microscopy after cytospin preparations and Giemsa staining (Giemsa stain modified, Sigma).
Cytokines measurement
Cytokine measurement was done from serum samples of animals. Levels of cytokines IL-5, IL-13 and IL-4 were determined by ELISA (Rand D Systems). The ELISAs were performed as per the manufacturer’s instruction.
Measurement of OVA-specific immunoglobulin E (IgE)
Each well of microtiter plate was coated with 5 μg of OVA in 100 μl volume overnight at 4°C. After three washes, nonspecific sites were blocked with 0.5% Tween 20 in PBS. Mouse sera in duplicate were added to the Ag-coated wells, the plates were incubated, and bound IgE was detected with biotinylated anti-mouse IgE (BD Pharmingen). Streptavidin-peroxidase conjugates were added; the bound enzymes were detected by addition of tetramethylbenzidine substrate system (BD Pharmingen); and absorbances were read at 450 nm. Absorbances were converted to arbitrary units.
Western blot analysis
The lungs were homogenized in a homogenizing buffer (1% NP-40, 150 mM NaCl, 50 mM Hepes, PMSF, and complete protease/phosphatase inhibitor cocktail). Protein estimation of the samples was performed by Bradford method. For western blotting, 40-50 μg of the protein was denatured at 100°C for 5 minutes in Tris-Glycine SDS sample loading buffer. Protein samples were applied to SDS gels and resolved at 70V (300 mA) for 3 hours and then electro-transferred to polyvinylidene difluoride membrane (BioRad, Hercules, USA) in transfer buffer using BioRad Mini Transblot Electrophoretic transfer Cell for 90-120 minutes at 150 V. Membranes were blocked in 5% fat free dry milk/3% BSA dissolved in TBST for 2.5 hours at room temperature. Anti-GATA3, anti-pSTAT6 anti-STAT6 antibodies were used to determine expression of their corresponding proteins [19].
Histological examination
After BALF was obtained, the left lung was removed, fixed in 10% neutral buffered formalin for 24 h, and then the specimens were dehydrated and embedded in paraffin in a standard manner. In order to carry out histological examination, 5 μm sections of fixed embedded tissues were cut and stained with hematoxylin and eosin, according to routine laboratory procedure. Histological analyzes were performed by pathologists blinded to the treatment groups. For each mouse, five airway sections, distributed throughout the left lung, were analyzed use of a light microscope attached to an image-analysis system (Image-Pro Plus; Media Cybernetics, Minneapolis, MN, USA). The images were then cropped and corrected for brightness and contrast, but otherwise were not manipulated [16].
Results
Boswellic acid decreases AHR in experimental asthma
The effect of Boswellic acid on AHR was evaluated by measuring airway resistance in anaesthetized mice by invasive whole-body plethysmography. No significant difference was found in baseline airway resistance among the six groups. The airway resistance generated by administration of Mch at doses from 0 to 16 mg/ml increased significantly in the OVA group. However, control group, DEXA and Boswellic acid (0.1, 1 and 10 mg/kg) treated groups showed a sharp decrease in airway resistance (Figure 2), but the decrease was insignificant in 0.1 mg/kg treated group.
Figure 2.
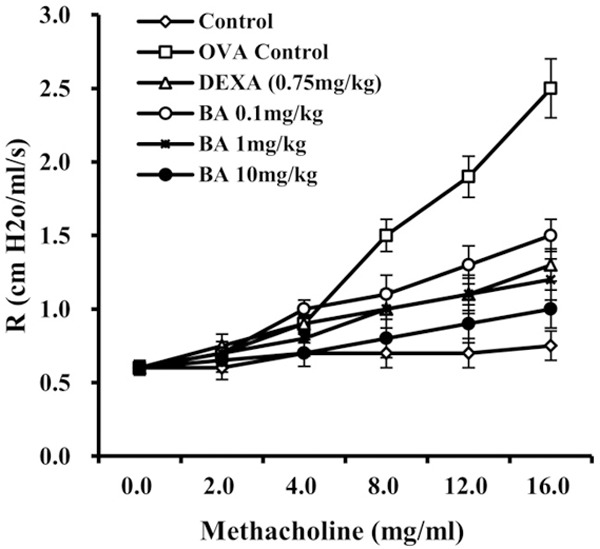
Measurement of Airway hyperresponsiveness (AHR). Boswellic acid administration reduces airway hyperresponsiveness in mice as measured by a methacholine dose responsive cure for airway resistance. Data is represented as mean ± SEM. *denotes < 0.05 vs sham **denote P < 0.01 vs sham (Student’s t test).
Boswellic acid attenuates airway inflammation
Very few inflammatory cells were detected in control group. However, a significant increase in total cell number was exhibited in OVA-sensitized and challenged animals, when compared to the control mice. The effect of Boswellic acid on allergen-induced inflammatory cell infiltration was assessed in animals treated with three different doses of Boswellic acid. As shown in Table 1, Boswellic acid at 0.1, 1 and 10 mg/kg suppressed allergen-induced inflammatory cell infiltration. However, in case of 0.1 mg/kg treated group infiltration of inflammatory cells was more than other treated groups of Boswellic acid. The anti-inflammatory effect of Boswellic acid was further demonstrated in the histological examination of hematoxylin-eosin stained sections (Figure 3). A marked affluence of inflammatory cells into the airway was observed in OVA-sensitized/challenged mice, but not in the PBS treated control mice. Mice treated with different doses of Boswellic acid showed a marked diminution in inflammatory cell infiltration into the airways.
Table 1.
Effect of Boswellic acid on total cell count and differential cell count
| TCC (X 104/ml) | Differential count (In percentage) | ||||
|---|---|---|---|---|---|
|
| |||||
| Macro | Mono | Eosino | Neutro | ||
| SHAM/PBS/VEH | 4.1 ± 1.2 | 39.2 ± 3.1 | 44.3 ± 5.4 | 1.1 ± 0.11 | 1 ± 0.2 |
| OVA/OVA/VEH | 54.2 ± 4.5 | 10.4 ± 2.1 | 12.8 ± 3.4 | 58.3 ± 5.8 | 21.2 ± 3.2 |
| BA-0.1 mg/kg | 38.3 ± 8.7 | 16.1 ± 4.1 | 19.8 ± 4.3 | 29.4 ± 5.3 | 16.1 ± 3.3 |
| BA-1 mg/kg | 13.5 ± 2.3 | 25.3 ± 5.4 | 29.1 ± 5.5 | 8.3 ± 2.2 | 10.9 ± 2.5 |
| BA-10 mg/kg | 10.4 ± 4.5 | 41.3 ± 4.6 | 38.1 ± 5.5 | 2.1 ± 0.1 | 2.3 ± 0.5 |
| OVA/OVA/DEXA | 10.1 ± 1 | 23.4 ± 4.2 | 39.7 ± 3.8 | 11.3 ± 8 | 11.5 ± 3.4 |
Data is represented as mean ± SEM.
Figure 3.
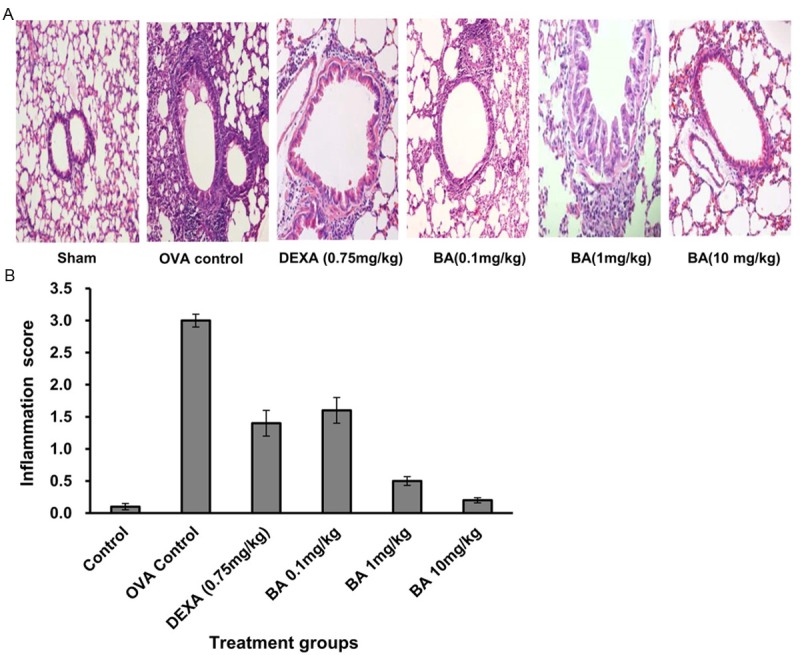
(A) Boswellic acid treatment significantly reduced airway cellular infiltration as detected by Haematoxylin & Eosin staining of lung sections (B), and measurement of inflammation score.
Boswellic acid suppresses Th2 cytokine release
Measurement of Th2 cytokines like IL-4 (Figure 4A), IL-5 (Figure 4B) and IL-13 (Figure 4C) was done from the serum collected from the mice. Mice treated with the test compound Boswellic acid showed no significant change in cytokine release when compared to the control at doses 0.1, 1 and 10 mg/kg.
Figure 4.
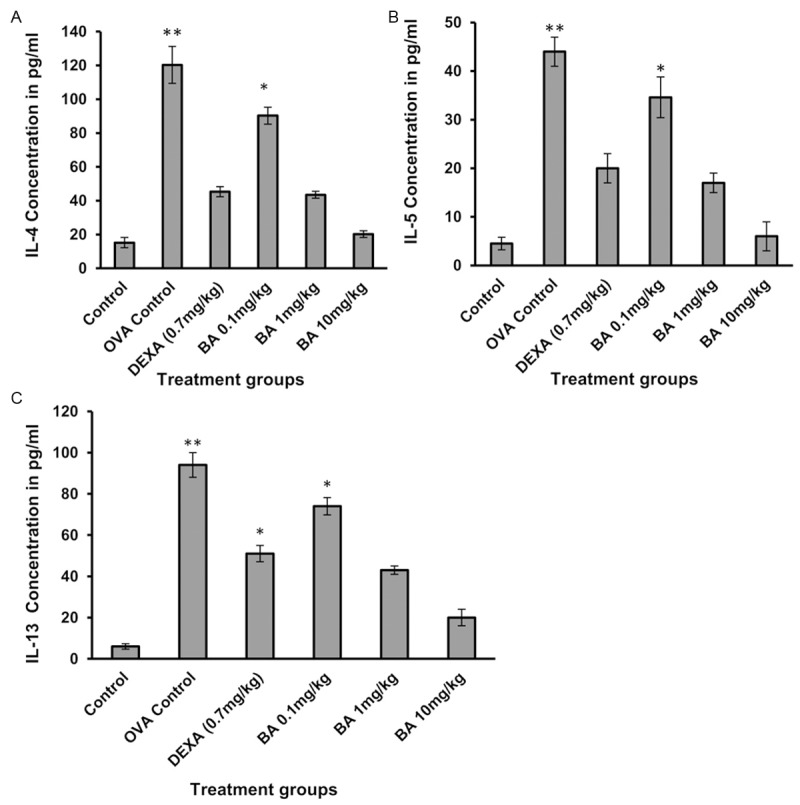
The effect of test compounds on Th2 cytokine release: IL-4 (A), IL-5 (B) and IL-13 (C). The level of IL-4, IL-5, and IL-13 in BAL fluid were quantified by sandwich ELISA and expressed as picogram per milliliter. Data is represented as mean ± SEM. *denotes P < 0.05 vs sham and **denote P < 0.01 vs sham (Student’s t test).
Boswellic acid reduces OVA specific IgE levels
OVA specific IgE levels were elevated in the OVA group as compared to the control group (Figure 5). Treatment with Boswellic acid (0.1, 1.0, 10 mg/kg) showed no significant change in OVA specific IgE levels as compared to control group.
Figure 5.
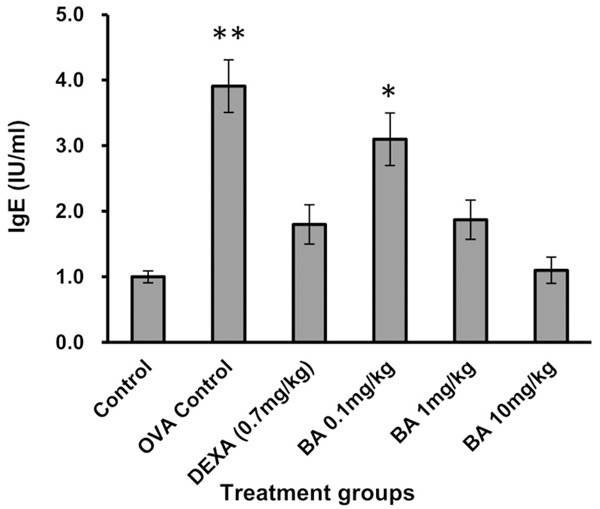
Effect of Boswellic acid on OVA specific IgE release. Data is represented as mean ± SEM. *denotes P < 0.05 vs. sham and **denote P < 0.01 vs. sham (Student’s t test).
Boswellic acid attenuated the expression of pSTAT6 and GATA-3, in lung
Expression of pSTAT6 and GATA-3 in lung homogenates were measured in control, OVA control, Dexa treated and Boswellic acid treated mice. No expression of pSTAT6 and GATA-3 was observed in control mice. However, maximum expression of pSTAT6 and GATA-3 was observed in OVA control mice indicating maximum Th2 cell differentiation in this group. In case of Boswellic acid treated groups decrease expression of pSTAT6 and GATA-3 was observed indicating. The decrease in expression by Boswellic acid was dose dependent with almost no expression in 10 mg/kg treated group (Figure 6).
Figure 6.
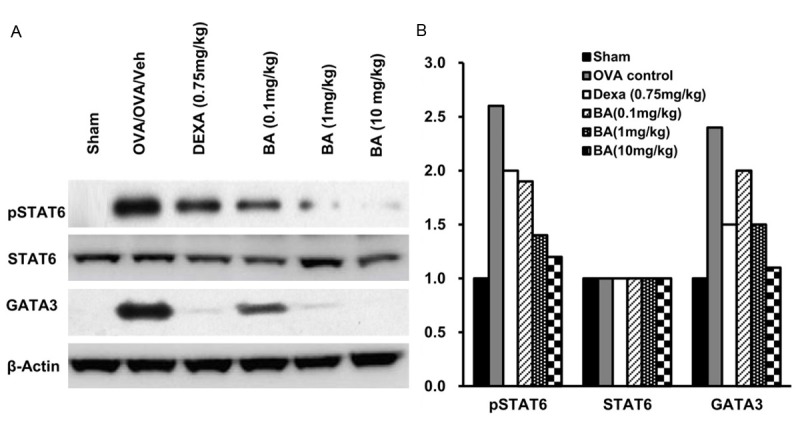
(A) Effect of the test compounds on pSTAT6 and GATA3 expression measured from lung homogenates by western blot (B) Analysis by densitometry.
Statistical analysis
Groups were analysed using one-way analysis of variance followed by Dunnett’s multiple comparison tests to examine differences between OVA-challenged PBS treated and Boswellic acid and DEXA treated groups. Differences were considered significant at P < 0.05. Data are presented as mean ± SEM for each group.
Discussion
In the present study, we have assessed the effects of Boswellic acid on allergen-induced airway inflammation and immune response in acute experimental asthma. We found that administration of Boswellic acid markedly reduced Th2 cytokines, OVA specific IgE, suppressed airway inflammatory cells infiltration induced by allergens, resulting in decreased number of eosinophils and total inflammatory cells in BALF. Lung histology validated Boswellic acid effect on airway inflammation. These findings suggested that Boswellic acid is an anti-asthmatic agent and appears beneficial for the treatment of allergic asthma.
It is widely accepted that T cells play an important role in the injurious lung immune responses associated with asthma. CD4+ Th cells can be divided into Th1 and Th2 groups functionally based on the various kinds of cytokine they produce. The different patterns of T cell differentiation generate the different inflammatory effectors, and those inflammatory effectors are correlated with the extent and type of damage observed in airway [20,21]. Under normal physiologic conditions, the ratio of Th1 to Th2 cells maintains a suitable level. Once the balance between Th1 and Th2 is broken, diseases will occur. The two major Th-specific transcription factors, T-bet and GATA-3, regulating the expression of cytokine genes are characteristics of Th1 or Th2, and play crucial roles in Th cell differentiation [22-24]. The Th2 cell differentiation is induced by Interleukin 4 (IL-4). Binding of IL-4 to its receptor leads to phosphorylation of signal transducer and activator of transcription protein 6 (STAT6), a Th2-cell transcription factor, through the Jak/STAT cascade. The phosphorylated STAT6 forms a homodimer and translocates into the nucleus where it binds to specific DNA sequences and activates transcription of cytokine responsive genes especially GATA3, which is the master regulator of Th2 cell differentiation [25-27]. GATA3 activates the transcription of IL-5 and IL-13 genes by directly binding to their promoters [28]. This transcription factor is also involved in remodeling of chromatin structure and opening of IL-4 locus [29].
In the present study, no significant difference was found in baseline airway resistance among the six groups. The airway resistance generated by administration of Mch at doses from 30 to 270 μg/kg increased significantly in the OVA group and Boswellic acid (0.1 mg/kg) treated group. However, control group, DEXA and Boswellic acid (1 and 10 mg/kg) treated groups showed a sharp decrease in airway resistance. Boswellic acid also showed a significant suppression of Th2 cytokine release and airway eosinophilia. As most of the characteristic features of asthma like AHR, airway eosinophilia, mucus hypersecretion, IgE switching are an outcome of Th2 cells and these cells are differentiated and developed via STAT6 pathway, we evaluated the effect of Boswellic acid on STAT6 phosphorylation and GATA3 expression. It was observed that Boswellic acid upregulate the expression pSTAT6 and GATA3. The decrease in the expression of these two transcription factors corroborated with the decrease in release of cytokines airway eosinophilia or airway inflammation. So, we can postulate that Boswellic acid attenuates the asthma features in a mouse model of asthma via STAT6 inhibition. Overall, Boswellic acid is promising to be a beneficial medication for the treatment of asthma by ameliorating allergic responses.
Disclosure of conflict of interest
None.
References
- 1.GINA Report. “From the Global Strategy for Asthma Management and Prevention, Global Initiative for Asthma (GINA) (updated December 2011; cited; (May 2012)”. Available http://www.ginasthma.org. [Internet]
- 2.Masoli M, Fabian D, Holt S, Beasley R. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy. 2004;59:469–478. doi: 10.1111/j.1398-9995.2004.00526.x. [DOI] [PubMed] [Google Scholar]
- 3.To T, Wang C, Guan J, McLimont S, Gershon AS. What is the lifetime risk of physician-diagnosed asthma in Ontario, Canada? Am J Respir Crit Care Med. 2010;181:337–343. doi: 10.1164/rccm.200907-1035OC. [DOI] [PubMed] [Google Scholar]
- 4.Mojtabavi N, Dekan G, Stingl G, Epstein MM. Longlived Th2 memory in experimental allergic asthma. J Immunol. 2012;169:4788–4796. doi: 10.4049/jimmunol.169.9.4788. [DOI] [PubMed] [Google Scholar]
- 5.Epstein MM. Targeting memory Th2 cells for the treatment of allergic asthma. Pharmacol Ther. 2006;109:107–136. doi: 10.1016/j.pharmthera.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 6.Barnes PJ. Pathophysiology of asthma. Eur Respir Monogr. 2003;8:84–113. [Google Scholar]
- 7.Ho IC, Tai TS, Pai SY. GATA3 and the T-cell lineage: essential functions before and after T-helper-2-cell differentiation. Nat Rev Immunol. 2009;9:125–135. doi: 10.1038/nri2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reddy GK, Chandrasekhar G, Dhar SC. Studies on the metabolism of glycosaminoglycans under the influence of new herbal anti-inflammatory agents. Biochem Pharmacol. 1989;38:3527–3534. doi: 10.1016/0006-2952(89)90124-x. [DOI] [PubMed] [Google Scholar]
- 9.Safayhi H, Mack T, Sabiera J, Anazodo MI, Subramanian LR, Ammon HP. Boswellic acids: novel, specific, nonredox inhibitors of 5-lipoxygenase. J Pharmacol Exp Ther. 1992;261:1143–1146. [PubMed] [Google Scholar]
- 10.Ammon HP, Safayhi H, Mack T, Sabieraj J. Mechanism of anti-inflammatory actions of curcumin and boswellic acids. J Ethnopharmacol. 1993;38:113–119. doi: 10.1016/0378-8741(93)90005-p. [DOI] [PubMed] [Google Scholar]
- 11.Safayhi H, Sailer ER, Ammon HP. Mechanism of 5-lipoxygenase inhibition by acetyl-11-keto-beta-boswellic acid. Mol Pharmacol. 1995;47:1212–1216. [PubMed] [Google Scholar]
- 12.Sailer ER, Subramanian LR, Rall B, Hoernlein RF, Ammon HPT, Safayhi H. Acetyl-11-keto-beta-boswellic acid: structure requirement for binding and 5-lipoxygenase inhibitory activity. Brit J Pharmacol. 1996;117:615–618. doi: 10.1111/j.1476-5381.1996.tb15235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gupta I, Gupta V, Parihar A, Gupta S, Ludtke R, Safayhi H, Ammon HP. Effect of Boswellia serrata gum resin in patients with bronchial asthma: results of double blind, placebo controlled, 6-week clinical study. Eur J Med Res. 1998;3:511–514. [PubMed] [Google Scholar]
- 14.Gupta I, Parihar A, Malhotra P, Singh GB, Safayhi H, Ammon HP. Effect of gum resin of Boswellia serrata in patients with chronic colitis. Planta Medica. 2001;67:391–395. doi: 10.1055/s-2001-15802. [DOI] [PubMed] [Google Scholar]
- 15.Kimmatkar N, Thawani V, Hingorani I, Khiyani R. Efficacy and tolerability of Boswellia serrata extract in treatment of osteoarthritis of knee-A randomized double blind placebo controlled trial. Phytomedicine. 2003;10:3–7. doi: 10.1078/094471103321648593. [DOI] [PubMed] [Google Scholar]
- 16.Mabalirajan U, Dinda AK, Kumar S, Roshan R, Gupta P, Sjarma SK, Ghosh B. Mitochondrial structural changes and dysfunction are associated with experimental allergic asthma. J Immunol. 2008;181:3540–3548. doi: 10.4049/jimmunol.181.5.3540. [DOI] [PubMed] [Google Scholar]
- 17.Pouliot P, Camateros P, Radzioch D, Lambrecht BN, Olivier M. Protein tyrosine phosphatases regulate asthma development in a murine asthma model. J Immunol. 2009;182:1334–1340. doi: 10.4049/jimmunol.182.3.1334. [DOI] [PubMed] [Google Scholar]
- 18.Du Q, Zhou LF, Chen Z, Gu XY, Huang M, Yin KS. Imiquimod, a toll-like receptor 7 ligand, inhibits airway remodelling in a murine model of chronic asthma. Clin Exp Pharmacol Physiol. 2009;36:43–48. doi: 10.1111/j.1440-1681.2008.05027.x. [DOI] [PubMed] [Google Scholar]
- 19.Kim SH, Kim BK, Lee YC. Antiasthmatic effects of hesperidin, a potential Th2 cytokine antagonist, in a mouse model of allergic asthma. Mediators Inflamm. 2011;2011:485402. doi: 10.1155/2011/485402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morrisey EE, Ip HS, Lu MM, Parmacek MS. GATA-6: a zinc finger transcription factor that is expressed in multiple cell lineages derived from lateral mesoderm. Dev Biol. 1996;177:309–322. doi: 10.1006/dbio.1996.0165. [DOI] [PubMed] [Google Scholar]
- 21.Arceci RJ, King AA, Simon MC, Orkin SH, Wilson DB. Mouse GATA-4: a retinoic acid-inducible GATA-binding transcriptionfactor expressed in endodermally derived tissues and heart. Mol Cell Biol. 1993;13:2235–2246. doi: 10.1128/mcb.13.4.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Szabo SJ, Sullivan BM, Peng SL, Glimcher LH. Molecular mechanisms regulating Th1 immune responses. Annu Rev Immunol. 2003;21:713–758. doi: 10.1146/annurev.immunol.21.120601.140942. [DOI] [PubMed] [Google Scholar]
- 23.Szabo SJ, Sullivan BM, Stemmann C, Satoskar AR, Sleckman BP, Glimcher LH. Distinct effects of T-bet in TH1 lineage commitment and IFN-gamma production in CD4 and CD8 T cells. Science. 2002;295:338–342. doi: 10.1126/science.1065543. [DOI] [PubMed] [Google Scholar]
- 24.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 25.Zhang DH, Cohn L, Ray P, Bottomly K, Ray A. Transcription factor GATA-3 is differentially expressed in murine Th1 and Th2 cells and controls Th2-specific expression of the intrleukin-5 gene. J Biol Chem. 1997;272:21597–21603. doi: 10.1074/jbc.272.34.21597. [DOI] [PubMed] [Google Scholar]
- 26.Cohn L, Tepper JS, Bottomly K. IL-4-independent induction of airway hyperresponsiveness by Th2, but not Th1cells. J Immunol. 1998;161:3813–3816. [PubMed] [Google Scholar]
- 27.Zhu J, Guo L, Watson CJ, Hu-Li J, Paul WE. Stat6 is necessary and sufficient for IL-4’s role in Th2 differentiation and cell expansion. J Immunol. 2001;166:7276–7281. doi: 10.4049/jimmunol.166.12.7276. [DOI] [PubMed] [Google Scholar]
- 28.Zhou M, Ouyang W. The function role of GATA-3 in Th1 and Th2 differentiation. Immunol Res. 2003;28:25–37. doi: 10.1385/IR:28:1:25. [DOI] [PubMed] [Google Scholar]
- 29.Ouyang W, Lohning M, Gao Z, Assenmacher M, Ranganath S, Radbruch A, Muphy KM. Stat6-independent GATA-3 autoactivation directs IL-4-independent Th2 development and commitment. Immunity. 2000;12:27–37. doi: 10.1016/s1074-7613(00)80156-9. [DOI] [PubMed] [Google Scholar]


