Abstract
Background: Anaplastic thyroid carcinoma (ATC) is an undifferentiated tumor of the thyroid that has poor prognosis owing to its aggressive behavior and resistance to current treatments. We hypothesized that the stem cell properties induced by the epithelial-mesenchymal transition (EMT) was one of reasons for the dismal outcome of ATC. Materials and methods: Paraffin blocks and slides of 17 ATC cases were retrieved. We also collected 60 cases of papillary thyroid carcinoma (PTC) for comparison. We used immunohistochemistry to examine the expression of multiple markers of cancer stem cells and EMT-activating transcriptional factors. Results: Majority of ATC cases showed loss of epithelial (E)-cadherin expression (15/17); however, all PTC cases (60/60) retained E-cadherin expression. EMT-activating transcription factors, such as snail and slug, were more frequently expressed in ATC than PTC cases (35.3% versus 6.7%, 76.5% versus 5%, respectively). Cancer stem cell markers such as CD133 and nestin were more highly expressed in ATC than PTC (52.9% versus 5%, 52.9% versus 0%, respectively). Conclusion: We found that the expression of EMT-related factors and stem cell markers was higher in ATC than PTC. We therefore conclude that stemness induced by EMT plays an important role in the pathogenesis of ATC.
Keywords: Epithelial-mesenchymal transition (EMT), cancer stem cell, anaplastic thyroid carcinoma (ATC), immunohistochemistry
Introduction
Anaplastic thyroid carcinoma (ATC) is an undifferentiated tumor of the thyroid that has poor prognosis owing to its aggressive behavior and resistance to treatment [1]. The overall median survival is only 2.5-6 months [2]. Recently, new targeted therapies have improved life expectancy in cancer patients. However, this has not been the case for ATC patients. Therefore, it is necessary to identify the specific molecular alterations or mechanisms to target in order to overcome the dismal outcome of ATC.
There have been several reports revealing the molecules and mechanisms that are closely associated with the poor clinical outcome of ATC [3]. Among of them, we focused on the epithelial-mesenchymal transition (EMT) and the cancer stem cell (CSC) properties that are induced by EMT as one of the potential causes of poor clinical outcome [4].
EMT is a complicated mechanism that is involved in pathologic conditions such as cancer cell progression as well as physiological development [5-7]. Several studies suggest a close correlation between poor prognosis and genetic changes on the EMT-related genes of many epithelial cancers [8-10]. Indeed, as with other epithelial tumors, the role of EMT-activating transcription factors in thyroid carcinogenesis is well documented [11-13]. According to these studies, the expression of EMT-related factors such as snail, slug, and twist were observed in ATC and papillary thyroid carcinoma (PTC). Recently, interesting results suggested that EMT is related with tumor recurrence and drug resistance, processes that are tightly linked to the biology of CSCs [14-17]. Based on these results, we hypothesized that EMT induction and CSC properties strongly affect the pathogenesis of ATC. Therefore, we evaluated the expression of CSC markers and EMT regulators simultaneously in ATC cases. We also studied mutations on the B-RAF serine/threonine kinase proto-oncogene (BRAF), a well-known oncogenic gene in PTC. BRAF mutations are the most prevalent and important oncogenic alterations in thyroid tumors [18]. The association between BRAF mutations and the tumorigenesis of PTC has been consistently revealed in many studies, regardless of the diverse geographical and ethnic backgrounds of study subjects [19]. In addition to the pivotal role of these mutations in the initiation of PTC tumorigenesis, they may also be involved in the progression from PTC to ATC [18,20]. Therefore, we thought that BRAF mutations would have an important role in the anaplastic transformation of thyroid tumors. Therefore, we hypothesized that BRAF mutations affect the expression of CSC and EMT-related markers. To study this, we evaluated the association between BRAF mutations and the expression of CSC or EMT-related markers in ATC as well as PTC.
Although the EMT process and CSC properties have been widely studied in relation to the poor clinical outcomes of many epithelial cancers, there are limited studies in thyroid tumors [11-13,21-27]. In addition, the association between BRAF mutations and EMT-induced stemness is less well known in thyroid tumors. Therefore, we used immunohistochemistry to evaluate the expression of these markers and to determine their effects on the pathogenesis of ATC compared to PTC. We also performed additional BRAF V600E mutation analysis to evaluate the effect of BRAF mutations on the expression of EMT and CSC markers between ATC and PTC.
Materials and methods
Case selection
Paraffin blocks and slides from 17 cases of ATC and 60 cases of PTC were retrieved from the archives of the Department of Pathology at the Korea Cancer Center Hospital from 1998 to 2013. The electrical medical records of the patients corresponding to these cases were reviewed. This study was approved by the Institutional Review Board of the Korea Cancer Center Hospital.
Tissue microarray
Tissue microarray (TMA) was generated by obtaining a 4 mm diameter core from each donor block and transferring them to a recipient block using a trephine apparatus (SuperBioChips laboratories, Seoul, Republic of Korea). The TMA consisted of 17 cases of ATC and 60 cases of PTC.
Immunohistochemistry
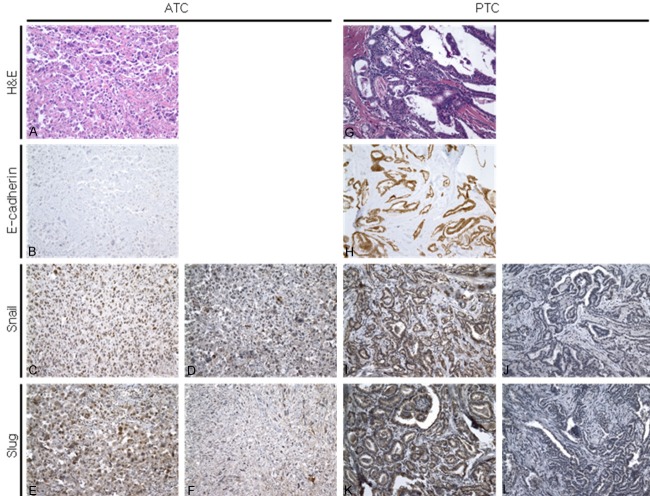
Four-micrometer thick sections were obtained from the TMA blocks and were stained with the primary antibody. Immunohistochemical staining was carried out using the standard avidin-biotin-peroxidase complex (ABC) method. Primary antibodies used in the immunohistochemical study are summarized in Table 1. Results from epithelial (E)-cadherin, snail, slug, CD133, and nestin immunostaining were divided into two groups: negative and positive. The presence of immunoreactivity for cytoplasmic and membrane E-cadherin regardless of the intensity was regarded as positive. In the absence of immunoreactivity, staining was defined as a negative. For snail and slug, the nuclear staining of more than 50% of the specimen regardless of staining intensity was considered positive. Cytoplasmic or nuclear staining of less than 50% of the specimen was defined as a negative. Positive staining for CD133 and nestin was defined as membrane or cytoplasmic staining with strong intensity in more than 50% of the specimen. Nuclear staining, cytoplasmic staining with weak intensity, and cytoplasmic staining with strong intensity in less than 50% of the specimen were regarded as negative. Figure 1 shows representative images of immunohistochemistry for E-cadherin, snail, slug, CD133 and nestin staining in ATC and PTC cases.
Table 1.
Summary of the antibodies used in this study
| Name | Manufacturer | Antigen retrieval | Dilution |
|---|---|---|---|
| E-cadhrein | DAKO, Glostrup, Denmark | Citrate | 1:50 |
| Snail | Abcam, Cambridge, UK | Microwave | 1:100 |
| Slug | Abcam, Cambridge, UK | Microwave | 1:50 |
| CD133 | Abcam, Cambridge, UK | Microwave | 1:100 |
| Nestin | Chemicon, Temecula, USA | Microwave | 1:200 |
Figure 1.
The expression of EMT-related markers in ATC and PTC. ATC cells show mesenchymal features (A) and frequently show loss of E-cadherin expression (B). Snail and slug proteins are expressed in the nucleus of ATC cells and this staining is regarded as positive (C and E). Cytoplasmic staining is defined as negative (D and F). Representative hematoxylin and eosin staining (G) and staining for E-cadherin (H), snail (I, J), and slug (K, L) in PTC cases. All PTC cases show characteristic nuclear features of PTC (G) and retain E-cadherin expression (H). A small percentage of PTC cases are immunoreactive for snail (I) and slug (K), and almost all cases reveal no immunoreactivity for snail (J) and slug (L). Original magnification: ×200.
DNA extraction and polymerase chain reaction amplification
Tumor rich areas (>80%) were extracted from formalin-fixed paraffin-embedded (FFPE) tumor samples using manual microdissection under microscopy. DNA was isolated from tumor tissue using the DNeasy Blood and Tissue kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions. Then, 5 μL of template DNA was added to 50 μL of the polymerase chain reaction (PCR) solution (5 μL of 10x MG Taq-HF buffer, 0.4 μL of 25 mM MG deoxynucleotide triphosphate mixture, 1 μL of 10 pmol Primer (×2), 0.3 μL of MG Taq-HF polymerase, and distilled water). The BRAF-Forward (5’-CTTCATAATGCTTGCTCTGATAGG-3’)/BRAF-Reverse (5’-GGCCAAAAATTTAATCAGTGGAA-3’) primers were used. The PCR cycling conditions were as follows: initial denaturation at 95°C for 10 min, followed by 40 cycles of amplification consisting of denaturation at 95°C for 30 sec, annealing at 55°C for 30 sec, and extension at 72°C for 30 sec.
BRAF pyrosequencing
A 20 μL aliquot of PCR product was bound to streptavidin sepharose HP (GE Healthcare, Uppsala, Sweden), purified, washed, denatured in 0.2 mol/L NaOH solution, and washed again. Then, 10 pmol/L of pyrosequencing primer (5’-CCACTCCATCGAGATT-3’) was annealed to the purified single-stranded PCR product, and pyrosequencing was performed on a PyroMark ID system (Qiagen) following the manufacturer’s instructions.
Statistics
Pearson’s chi-square test was used to assess the relevance of expression of the EMT-activating factors and CSC markers with reference to histological diagnosis, combined histology, and BRAF V600E mutation. All statistical analyses were performed with SPSS version 18 (Chicago, IL, USA); P < 0.05 was considered significant.
Results
Clinicopathological findings
Our ATC patients ranged in age from 44 to 77 years (mean age: 61.1 years) and PTC patients ranged in age from 25 to 66 years (mean age: 66.2). The male-to-female ratio was 1:3 in ATC and PTC. Fifteen of 17 ATC patients showed lymph node metastasis, while 2 patients showed no metastasis to the lymph nodes. Twenty-six PTC patients showed lymph node metastasis, while 34 showed no metastasis. On microscopic examination, the largest mass had a mean size of 5.4 cm in ATC patients (range: 2.5-9.5 cm) and 0.9 cm in PTC patients (range: 0.2-2.5 cm). All ATC and 34 PTC cases showed extra-thyroidal extension of mass on microscopic examination. Eight of 17 ATC patients simultaneously had conventional papillary carcinoma as well as ATC and one patient had a poorly differentiated thyroid carcinoma and ATC. Undifferentiated spindle cells were detected in all ATC cases. These results are summarized in Table 2.
Table 2.
Clinical and histopathological characteristics of 17 cases of anaplastic thyroid carcinoma and 60 cases of papillary thyroid carcinoma
| Characteristics | ATC | PTC |
|---|---|---|
| Age (range) | 61 (44-77) | 66.2 (25-66) |
| Sex (M:F) | 1:3 | 1:3 |
| Mean sizes of largest dimension (range) | 5.4 cm (2.5-9.5 cm) | 0.9 cm (0.2-2.5 cm) |
| Lymph node metastasis | ||
| abscent | 2 | 34 |
| present | 15 | 26 |
| Extrathyroidal extension | ||
| abscent | 0 | 26 |
| present | 17 | 34 |
| Histology | ||
| ATC with PTC | 8 | |
| ATC | 8 | |
| PTC | 60 |
ATC: anaplastic thyroid carcinoma, PTC: papillary thyroid carcinoma.
EMT and CSC markers’s expression in ATC and PTC
EMT-associated markers
We compared the expression of EMT-associated markers between ATC and PTC. Loss of E-cadherin expression was observed in 15 ATC cases (88.2%), while 2 ATC cases (11.8%) retained E-cadherin expression. In contrast to ATC, all PTC cases retained E-cadherin expression. The frequency and staining pattern of EMT-associated markers such as snail and slug were also clearly different between ATC and PTC (Figure 1). In PTC, immunoreactivity for snail and slug expression was only observed in 4 of 60 (6.7%) and 3 of 60 (5%) cases, respectively. Based on these results, ATC cases showed more frequent immunoreactivity for snail and slug protein. Six of 17 (35.3%) ATC cases showed immunoreactivity for snail, and 13 of 17 (76.5%) ATC cases showed expression of slug. The frequency of expression of EMT-associated markers was significantly different between ATC and PTC. These results are summarized in Table 3.
Table 3.
Expression of epithelial mesenchymal transition (EMT)-activating factors and cancer stem cell (CSC) markers in ATC and PTC
| Antibody | ATC | PTC | P-value |
|---|---|---|---|
| E-cadherin | |||
| positive | 2 (11.8%) | 60 (100%) | 0.000 |
| negative | 15 (88.2%) | 0 (0%) | |
| snail | |||
| positive | 6 (35.3%) | 4 (6.7%) | 0.006 |
| negative | 11 (64.7%) | 56 (93.3%) | |
| slug | |||
| positive | 13 (76.5%) | 3 (5.0%) | 0.000 |
| negative | 4 (23.5%) | 57 (95.0%) | |
| CD133 | |||
| positive | 9 (52.9%) | 3 (5.0%) | 0.000 |
| negative | 8 (47.1%) | 57 (95.0%) | |
| nestin | |||
| positive | 9 (52.9%) | 0 (0%) | 0.000 |
| negative | 8 (47.1%) | 30 (100%) |
Cancer stem cell markers
To compare the expression of CSC markers between ATC and PTC, we performed immunohistochemistry for CD133 and nestin (Figure 2). Nine of 17 (52.9%) ATC cases showed immunoreactivity for CD133. On the other hand, only 3 cases of PTC (5%) showed immunoreactivity for CD133. The immunostaining result for nestin was also different between the two groups. Nine of 17 (52.9%) ATC cases showed immunoreactivity for nestin, while none of the PTC cases showed any immunoreactivity. Similar to the EMT-associated markers, the expression of CSC markers was significantly different between ATC and PTC patients. These results are summarized in Table 3.
Figure 2.
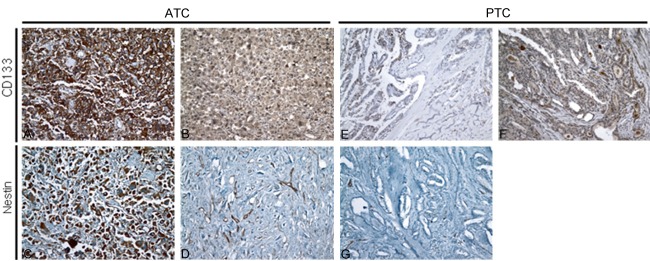
The expression of cancer stem cell markers in ATC and PTC. CD133 and nestin are highly over-expressed in the membrane and cytoplasm of ATC cells and this staining is regarded as positive (A and C). ATC cases showing very weak cytoplasmic staining for CD133 (B) and nestin (D) were regarded as a negative. Representative images of staining for CD133 (E, F) and nestin (G) in PTC cases. A few PTC cases reveal positive staining for CD133 (E) and almost cases show negative staining for CD133 (F). All PTC cases show no immunoreactivity for nestin (G). Original magnification: ×200.
BRAF V600E mutation and the expression of EMT or CSC markers’s expression in ATC and PTC
ATC
Seventeen tumors were analyzed for BRAF mutations. Eight of the 17 cases were suitable for BRAF V600E mutation analysis. BRAF V600E mutations were detected in two cases (2/8, 25%) (Figure 3). One of the 2 cases had both papillary carcinoma and undifferentiated carcinoma in same tumor, while the other case had only homogeneous undifferentiated carcinoma. The expression levels of EMT-associated markers and CSC markers were different in the two BRAF V600E mutant ATC cases. Loss of E-cadherin expression and no immunoreactivity for snail were observed in both cases. Staining results for slug, nestin, and CD133 were different in both cases. One case showed immunoreactivity for slug and nestin but no reactivity for CD133, while the other revealed CD133 immunoreactivity but none for slug and nestin.
Figure 3.
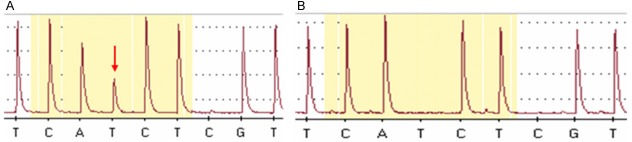
The BRAF mutation study was carried out using pyrosequencing with formalin-fixed paraffin-embedded tissue. Representative images of mutant type (A, arrow indicates the mutation site) and wild type (B). All the BRAF mutated cases had T>A transition change of a single amino acid, from valine to glutamic acid (V600E).
PTC
We divided 60 cases of PTC into 2 groups according to BRAF V600E mutation status, and compared the expression of EMT-related factors and CSC markers between the two groups. Immunoreactivity for snail was observed in 3 cases (3/30, 10%) of PTC with BRAF V600E mutation and 1 case (1/30, 3.3%) of PTC without BRAF V600E mutation. Immunoreactions for slug were different between the two groups and in contrast to those of snail immunoreactivity. Only 3 cases (3/30, 10%) of PTC without BRAF V600E mutation revealed immunoreactivity for slug, while no cases of PTC with BRAF V600E mutation showed immunoreactivity for slug. On examining the expression of CSC markers, there was no distinct difference between the two groups. All PTC cases showed no immunoreaction for nestin regardless of their BRAF mutation status. CD133 expression was observed in 1 case (1/30, 3.3%) of PTC with BRAF V600E mutation and 2 cases (2/30, 6.7%) of PTC without BRAF V600E mutation. The association between the BRAF V600E mutation and the expression of EMT-associated markers and CSC markers was not statistically significant in the two groups. These results are summarized in Table 4.
Table 4.
Expression of EMT-activating factors and CSC markers according to BRAF V600E mutation status in PTC
| Antibody | PTC with BRAF V600E mutation | PTC without BRAF V600E mutation | P-value |
|---|---|---|---|
| E-cadherin | |||
| Positive | 30 (100%) | 30 (100%) | NA |
| Negative | 0 (0%) | 0 (0%) | |
| snail | |||
| Positive | 3 (10%) | 1 (3.3%) | 0.301 |
| negative | 27 (90%) | 29 (96.7%) | |
| slug | |||
| positive | 0 (0%) | 3 (10%) | 0.076 |
| negative | 30 (100%) | 27 (90%) | |
| CD133 | |||
| positive | 1 (3.3%) | 2 (6.7%) | 0.554 |
| negative | 29 (96.7%) | 28 (93.3%) | |
| nestin | |||
| positive | 0 (52.9%) | 0 (0%) | NA |
| negative | 30 (100%) | 30 (100%) |
NA: Not applicable.
Discussion
EMT is a complicated process through which epithelial cancer cells acquire a reversible change in phenotype. The first step of this process is the loss of E-cadherin expression through the activation of transcriptional repressors such as snail, slug, and sip1. The EMT process results in the loss of cell-cell adhesion and the acquisition of invasive or metastatic abilities. Several studies have revealed that EMT-related factors are closely related with clinicopathological features in various epithelial cancers, including thyroid tumors. Salerno et al. [12] and Buehler et al. [11] described the important role of slug and twist in thyroid tumors, especially in ATC. In addition to the role of EMT-activating transcription factors in ATC, their role in well-differentiated thyroid carcinoma was also suggested by Hardy et al. [13]. They described the over-expression of snail and slug in papillary carcinoma and follicular carcinoma. However, the results suggested by Hardy et al. [13] are not consistent with ours. We analyzed the expression of snail and slug in PTC as well as ATC. By comparing the expression of snail and slug between ATC and PTC cases, we found that significant over-expression of EMT-related markers was observed in ATC, while the over-expression in PTC was restricted. Based on these results, we conclude that EMT markers have a more important role in ATC than in PTC. This finding was similar to the results from Hardin et al. [28] indicating that there was no significant over-expression of EMT-activating transcription factors in PTC.
The concept of EMT has been integrated with CSCs in cancer biology [25]. Indeed, experimental data reveal that the EMT process induces CSC features in many epithelial cancer cell lines [25,29,30]. According to these studies, CSC characteristics are sustained by the induction of EMT. Therefore, there is a correlation between CSC properties and the induction of EMT. Recently, CSCs were described to have a major effect on tumor cell proliferation, invasion, and resistance to conventional treatment. ATC showed more invasive and aggressive characteristics compared to PTC; therefore, ATC was more resistant to treatment than PTC. Based on well-known facts about CSCs and the clinical features of ATC, we suspected that the CSC features induced by EMT had an effect on the aggressiveness and treatment-resistance of ATC. Therefore we thought that it would be meaningful to compare the expression of CSC markers between ATC and PTC. Some studies had already revealed the significant over-expression of CSC markers in ATC cells, but these results were obtained using thyroid cell lines [21,22,31,32]. Only a few studies revealed the expression of CSC markers in ATC with FFPE tissues [22,25]. Zito et al. showed CD133 protein over-expression in 10 ATC cases (80%). Although they were limited by having few cases, Liu et al. also showed the over-expression of CSC markers, such as CD133, CD44, and nestin in ATC. Similarly, CSC markers were over-expressed in the majority of our ATC cases compared to the PTC cases. Based on the aforementioned results, we conclude that CSC features induced by EMT have a more important role in the development of ATC than in PTC.
We performed BRAF mutation analysis using pyrosequencing in 8 ATC cases. Few studies have analyzed BRAF mutations in ATC even though the frequency of these mutations ranges 10-20% [20,33-35]. Our results showed 20% BRAF mutation frequency in ATC cases, which was similar to the results of other studies. Using a mouse model, Knauf et al. [1] found that the thyroid-specific expression of oncogenic BRAF mutations enhanced the development of papillary carcinoma including poorly differentiated thyroid tumors. By performing microarray analysis on these tumors, they also found profound deregulation of EMT-related genes. We therefore hypothesized that the role of BRAF mutations was to induce EMT and CSC properties in ATC and PTC. Thus, we evaluated the relation between the BRAF V600E mutation status and expression of EMT markers or CSC markers in ATC and PTC. Two BRAF mutant ATC cases showed no consistent immunostaining for slug, CD133, and nestin. However, because of the few cases examined, it is difficult to interpret these results. Therefore, we performed additional analysis on 60 cases of PTC divided into two groups according to the presence of the BRAF V600E mutation. In this analysis, 3 cases (10%) of PTC with the BRAF mutation showed an immunoreaction for snail, and this immunoreactivity was higher than in PTC without the BRAF mutation (3.3%). However, this did not reach statistical significance, because the frequency of immunoreactivity for snail in 2 cases was too low. In addition to snail expression, slug immunoreactivity was only observed in a few PTC cases regardless of the BRAF mutation, and there was no statistical difference between the two groups. On examining the CSC markers, only a few cases of PTC without the BRAF mutation and PTC with the BRAF mutation were immunoreactive for CD133. Moreover, there was no immunoreactivity for nestin in any of the PTC cases regardless of the presence of the BRAF mutation. Therefore, we conclude that the BRAF mutation in PTC does not affect the expression of EMT-related markers and CSC markers. In addition, the effect of the BRAF V600E mutation on the expression of EMT-related markers and CSC markers in ATC should be re-evaluated on more ATC cases.
Conclusion
Our study reveals that EMT-related factors and CSC markers are more over-expressed in ATC than PTC. Furthermore, BRAF mutations do not significantly affect the expression of CSC markers and EMT-activating factors in PTC. Based on our results, we conclude that stemness induced by EMT plays an important role in ATC.
Acknowledgements
The authors wish to express their gratitude especially to Sun-Ju Lee, M.T., and Gil-HO Kim, M.T., the Laboratory of Radiation Pathology and the Department of Pathology, Korea Cancer center Hospital, Seoul, Korea, for their excellent technical assistance. This work was supported by a grant from the Korea Cancer Center Hospital (50243-2015).
Disclosure of conflict of interest
None.
References
- 1.Knauf JA, Sartor MA, Medvedovic M, Lundsmith E, Ryder M, Salzano M, Nikiforov YE, Giordano TJ, Ghossein RA, Fagin JA. Progression of BRAF-induced thyroid cancer is associated with epithelial-mesenchymal transition requiring concomitant MAP kinase and TGFbeta signaling. Oncogene. 2011;30:3153–3162. doi: 10.1038/onc.2011.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pasieka JL. Anaplastic thyroid cancer. Curr Opin Oncol. 2003;15:78–83. doi: 10.1097/00001622-200301000-00012. [DOI] [PubMed] [Google Scholar]
- 3.Bernet V, Smallridge R. New therapeutic options for advanced forms of thyroid cancer. Expert Opin Emerg Drugs. 2014;19:225–241. doi: 10.1517/14728214.2014.894017. [DOI] [PubMed] [Google Scholar]
- 4.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL, Polyak K, Brisken C, Yang J, Weinberg RA. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 6.Thiery JP. [Epithelial-mesenchymal transitions in cancer onset and progression] . Bull Acad Natl Med. 2009;193:1969–1978. discussion 1978-1969. [PubMed] [Google Scholar]
- 7.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 8.Bates RC, Mercurio AM. The epithelial-mesenchymal transition (EMT) and colorectal cancer progression. Cancer Biol Ther. 2005;4:365–370. doi: 10.4161/cbt.4.4.1655. [DOI] [PubMed] [Google Scholar]
- 9.Ahmed N, Abubaker K, Findlay J, Quinn M. Epithelial mesenchymal transition and cancer stem cell-like phenotypes facilitate chemoresistance in recurrent ovarian cancer. Curr Cancer Drug Targets. 2010;10:268–278. doi: 10.2174/156800910791190175. [DOI] [PubMed] [Google Scholar]
- 10.Rosivatz E, Becker I, Specht K, Fricke E, Luber B, Busch R, Hofler H, Becker KF. Differential expression of the epithelial-mesenchymal transition regulators snail, SIP1, and twist in gastric cancer. Am J Pathol. 2002;161:1881–1891. doi: 10.1016/S0002-9440(10)64464-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buehler D, Hardin H, Shan W, Montemayor-Garcia C, Rush PS, Asioli S, Chen H, Lloyd RV. Expression of epithelial-mesenchymal transition regulators SNAI2 and TWIST1 in thyroid carcinomas. Mod Pathol. 2013;26:54–61. doi: 10.1038/modpathol.2012.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salerno P, Garcia-Rostan G, Piccinin S, Bencivenga TC, Di Maro G, Doglioni C, Basolo F, Maestro R, Fusco A, Santoro M, Salvatore G. TWIST1 plays a pleiotropic role in determining the anaplastic thyroid cancer phenotype. J Clin Endocrinol Metab. 2011;96:E772–781. doi: 10.1210/jc.2010-1182. [DOI] [PubMed] [Google Scholar]
- 13.Hardy RG, Vicente-Duenas C, Gonzalez-Herrero I, Anderson C, Flores T, Hughes S, Tselepis C, Ross JA, Sanchez-Garcia I. Snail family transcription factors are implicated in thyroid carcinogenesis. Am J Pathol. 2007;171:1037–1046. doi: 10.2353/ajpath.2007.061211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zavadil J. A spotlight on regulatory networks connecting EMT and cancer stem cells. Cell Cycle. 2010;9:2927. doi: 10.4161/cc.9.15.12628. [DOI] [PubMed] [Google Scholar]
- 15.Kong D, Li Y, Wang Z, Sarkar FH. Cancer Stem Cells and Epithelial-to-Mesenchymal Transition (EMT)-Phenotypic Cells: Are They Cousins or Twins? Cancers (Basel) 2011;3:716–729. doi: 10.3390/cancers30100716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh A, Settleman J. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene. 2010;29:4741–4751. doi: 10.1038/onc.2010.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fuxe J, Vincent T, Garcia de Herreros A. Transcriptional crosstalk between TGF-beta and stem cell pathways in tumor cell invasion: role of EMT promoting Smad complexes. Cell Cycle. 2010;9:2363–2374. doi: 10.4161/cc.9.12.12050. [DOI] [PubMed] [Google Scholar]
- 18.Xing M. BRAF mutation in thyroid cancer. Endocr Relat Cancer. 2005;12:245–262. doi: 10.1677/erc.1.0978. [DOI] [PubMed] [Google Scholar]
- 19.Trovisco V, Vieira de Castro I, Soares P, Maximo V, Silva P, Magalhaes J, Abrosimov A, Guiu XM, Sobrinho-Simoes M. BRAF mutations are associated with some histological types of papillary thyroid carcinoma. J Pathol. 2004;202:247–251. doi: 10.1002/path.1511. [DOI] [PubMed] [Google Scholar]
- 20.Nikiforova MN, Kimura ET, Gandhi M, Biddinger PW, Knauf JA, Basolo F, Zhu Z, Giannini R, Salvatore G, Fusco A, Santoro M, Fagin JA, Nikiforov YE. BRAF mutations in thyroid tumors are restricted to papillary carcinomas and anaplastic or poorly differentiated carcinomas arising from papillary carcinomas. J Clin Endocrinol Metab. 2003;88:5399–5404. doi: 10.1210/jc.2003-030838. [DOI] [PubMed] [Google Scholar]
- 21.Mitsutake N, Iwao A, Nagai K, Namba H, Ohtsuru A, Saenko V, Yamashita S. Characterization of side population in thyroid cancer cell lines: cancer stem-like cells are enriched partly but not exclusively. Endocrinology. 2007;148:1797–1803. doi: 10.1210/en.2006-1553. [DOI] [PubMed] [Google Scholar]
- 22.Zito G, Richiusa P, Bommarito A, Carissimi E, Russo L, Coppola A, Zerilli M, Rodolico V, Criscimanna A, Amato M, Pizzolanti G, Galluzzo A, Giordano C. In vitro identification and characterization of CD133(pos) cancer stem-like cells in anaplastic thyroid carcinoma cell lines. PLoS One. 2008;3:e3544. doi: 10.1371/journal.pone.0003544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Friedman S, Lu M, Schultz A, Thomas D, Lin RY. CD133+ anaplastic thyroid cancer cells initiate tumors in immunodeficient mice and are regulated by thyrotropin. PLoS One. 2009;4:e5395. doi: 10.1371/journal.pone.0005395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamada H, Takano T, Ito Y, Matsuzuka F, Miya A, Kobayashi K, Yoshida H, Watanabe M, Iwatani Y, Miyauchi A. Expression of nestin mRNA is a differentiation marker in thyroid tumors. Cancer Lett. 2009;280:61–64. doi: 10.1016/j.canlet.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 25.Liu J, Brown RE. Immunohistochemical detection of epithelialmesenchymal transition associated with stemness phenotype in anaplastic thyroid carcinoma. Int J Clin Exp Pathol. 2010;3:755–762. [PMC free article] [PubMed] [Google Scholar]
- 26.Montemayor-Garcia C, Hardin H, Guo Z, Larrain C, Buehler D, Asioli S, Chen H, Lloyd RV. The role of epithelial mesenchymal transition markers in thyroid carcinoma progression. Endocr Pathol. 2013;24:206–212. doi: 10.1007/s12022-013-9272-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wiseman SM, Masoudi H, Niblock P, Turbin D, Rajput A, Hay J, Filipenko D, Huntsman D, Gilks B. Derangement of the E-cadherin/catenin complex is involved in transformation of differentiated to anaplastic thyroid carcinoma. Am J Surg. 2006;191:581–587. doi: 10.1016/j.amjsurg.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 28.Hardin H, Montemayor-Garcia C, Lloyd RV. Thyroid cancer stem-like cells and epithelial-mesenchymal transition in thyroid cancers. Hum Pathol. 2013;44:1707–1713. doi: 10.1016/j.humpath.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 29.Morel AP, Lievre M, Thomas C, Hinkal G, Ansieau S, Puisieux A. Generation of breast cancer stem cells through epithelial-mesenchymal transition. PLoS One. 2008;3:e2888. doi: 10.1371/journal.pone.0002888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giannoni E, Bianchini F, Masieri L, Serni S, Torre E, Calorini L, Chiarugi P. Reciprocal activation of prostate cancer cells and cancer-associated fibroblasts stimulates epithelial-mesenchymal transition and cancer stemness. Cancer Res. 2010;70:6945–6956. doi: 10.1158/0008-5472.CAN-10-0785. [DOI] [PubMed] [Google Scholar]
- 31.Schweppe RE, Klopper JP, Korch C, Pugazhenthi U, Benezra M, Knauf JA, Fagin JA, Marlow LA, Copland JA, Smallridge RC, Haugen BR. Deoxyribonucleic acid profiling analysis of 40 human thyroid cancer cell lines reveals cross-contamination resulting in cell line redundancy and misidentification. J Clin Endocrinol Metab. 2008;93:4331–4341. doi: 10.1210/jc.2008-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen G, Xu S, Renko K, Derwahl M. Metformin inhibits growth of thyroid carcinoma cells, suppresses self-renewal of derived cancer stem cells, and potentiates the effect of chemotherapeutic agents. J Clin Endocrinol Metab. 2012;97:E510–520. doi: 10.1210/jc.2011-1754. [DOI] [PubMed] [Google Scholar]
- 33.Xing M, Vasko V, Tallini G, Larin A, Wu G, Udelsman R, Ringel MD, Ladenson PW, Sidransky D. BRAF T1796A transversion mutation in various thyroid neoplasms. J Clin Endocrinol Metab. 2004;89:1365–1368. doi: 10.1210/jc.2003-031488. [DOI] [PubMed] [Google Scholar]
- 34.Quiros RM, Ding HG, Gattuso P, Prinz RA, Xu X. Evidence that one subset of anaplastic thyroid carcinomas are derived from papillary carcinomas due to BRAF and p53 mutations. Cancer. 2005;103:2261–2268. doi: 10.1002/cncr.21073. [DOI] [PubMed] [Google Scholar]
- 35.Gauchotte G, Philippe C, Lacomme S, Leotard B, Wissler MP, Allou L, Toussaint B, Klein M, Vignaud JM, Bressenot A. BRAF, p53 and SOX2 in anaplastic thyroid carcinoma: evidence for multistep carcinogenesis. Pathology. 2011;43:447–452. doi: 10.1097/PAT.0b013e3283486178. [DOI] [PubMed] [Google Scholar]