Abstract
Purpose: To analyze the distribution of Mn2+ in rabbit eyes after topical administration of Mncl2 for manganese-enhanced MRI. Methods: Forty-eight Chinese white rabbits were divided into three groups. In group 1 (n = 4), the baseline concentration of Mn2+ in aqueous, vitreous and serum samples were analyzed. In group 2 and 3, the rabbits received one topical instillation (20 μL) of Mncl2 (1 mol•L-1). In group 2 (n = 40), aqueous, vitreous and serum samples were collected and analyzed at predetermined time points (0.5, 1, 2, 4, 6, 12, 24, 48, 72 and 168 hours postdose). Assays were performed using inductively coupled plasma-mass spectrometer (ICP-MS). In group 3 (n = 4), after topical administration of Mncl2, dynamic manganese-enhanced MRI (MEMRI) was performed at predetermined time points. The signal-to-noise ratio (SNR) was calculated to evaluate the enhancements of eyes. Results: After topical administration, the maximum concentrations of Mn2+ in the aqueous and vitreous samples were 11.1641 ± 0.7202 (2 hours) and 1.5622 ± 0.1567 (12 hours). In group 3, the maximum enhancement of aqueous humor (SNR = 108.81 ± 10.65) appeared at 2 hours postdose, whereas, no significant changes were detected in vitreous. Conclusion: Mn2+ could distribute into aqueous humor rapidly after topical administration of Mncl2, whereas, the concentration of Mn2+ in vitreous body fluctuated in a narrow range over the course. The uptake of Mn2+ in retina may involve several different pathways.
Keywords: Drug distribution, manganese, topical administration, MEMRI, ICP-MS
Introduction
Manganese (Mn2+) enters cells through voltage gated calcium channels and then moves along axons via axonal transport [1,2]. Given the paramagnetic nature of Mn2+, its uptake and transport in the nervous system is detectable by MRI with T1-weighted imaging (T1WI). Manganese-enhanced MRI (MEMRI) has recently been used as a biomarker and tracer to investigate optic nerve axonal integrity [3-8], retinal projections to the superior colliculus [9], layer-specific calcium-dependent retinal fMRI activation [10,11], cortical response to stimulation or postnatal development and plasticity of retinal and callosal projections [12,13].
When MEMRI is used to investigate the visual system, an intravitreal injection is the common means of delivery of the Mn2+ solution into the ocular space [3,14-16]. However, direct injection of drugs into the vitreous body is stressful for patients and could potentially produce severe adverse events, such as endophthalmitis, retinal detachment, uveitis, ocular hypertension, cataract, intraocular hemorrhage, and hypotony [17]. In an attempt to remedy the invasive intravitreal injection of MnCl2, Sun SW et al. proposed to deliver Mn2+ through topical loading [18,19]. In their study, they considered that the uptake of Mn2+ may do not involve the vitreous body. But the definite conclusion can not be established from the observed lack of vitreous enhancement in previous studies.
To investigate the penetration and distribution of Mn2+ after topical administration of MnCl2, we measured the concentration of Mn2+ in aqueous humor and vitreous body in rabbits. And, MEMRI was performed at same time, to analyze the relation of the enhancement of eyes and Mn2+ concentration in aqueous humor and vitreous body.
Materials and methods
Animals and groups
Forty-eight Chinese white rabbits were divided into three groups, 1) control group (n = 4), to analyze the baseline concentration of Mn2+ in aqueous humor, vitreous body and serum. 2) concentration test group, in which the concentrations of Mn2+ in aqueous humor, vitreous body and serum were analyzed at predetermined time points after topical administration of MnCl2. 3) MEMRI group (n = 4), to visualize the enhancement images after administration of MnCl2.
The rabbits (supplied by Zhengzhou University Experimental Animal Center) of mixed gender and an average age of 15 weeks were reared in room with standard chow and water supply. Animals were treated in accordance with the guidelines of the Association for Research in Vision and Ophthalmology and protocols approved by the Zhengzhou University Animal Ethical Committee for animal research.
MnCl2 administration
For topical administration of MnCl2, 1.0 mol·L-1 MnCl2 in distilled and deionized water was freshly prepared (PH = 6.5). The rabbits were anesthetized with xylazine (5 mg•kg-1) and ketamine (35 mg•kg-1). Utilizing a micropipette, the left eye of rabbits in group 2 (n = 40) and MEMRI group (n = 4) received one topical instillation (20 μl) of MnCl2.
Samples collection
In group 2 (n = 40), at predetermined time points (0.5, 1, 2, 4, 6, 12, 24, 48, 72 and 168 hours postdose), 4 rabbits were first anesthetized with xylazine (5 mg•kg-1) and ketamine (35 mg•kg-1). A sample of arterial blood was drawn from the central artery of the ears just before the euthanasia. Rabbits were then sacrificed with pentobarbital overdose (1.2 ml•kg-1). The left eyes were enucleated and then rinsed with an isotonic saline solution. The aqueous humor (0.15 ml) was removed from the eye using a 1-ml syringe attached to a 27-gauge needle. Vitreous body (0.15 ml) was aspired with a 15-gauge needle mounted on a 5-ml syringe. Serum was obtained by allowing the blood sample to clot at room temperature for 1 hour followed by centrifugation. All samples were diluted to 15 ml with distilled and deionized water. The 4 rabbits in group 3 were sacrificed and samples were obtained as mentioned above.
Mass spectrometry analysis of Mn2+
The concentrations of Mn2+ were analyzed on a PE/SCIEX Elan 6100 inductively coupled plasma-mass spectrometer (ICP-MS, Perkin Elmer Life & Analytical Sciences, Shelton, Connecticut, USA). All samples were vortexed and aspirated into a pneumatic nebulizer. The resulting aerosol was directed to the hot plasma discharge by a flow of argon. Instrumentation response was defined by the linear relationship of analyte concentration vs ion counts. Analyte concentrations were derived by reading the ion count ratio for each mass of interest.
Memri
In group 3 (n = 4), T1WI was repeated at predetermined time points (0.5, 1, 2, 4, 6, 12, 24, 48, 72 and 168 hours postdose). MRI was performed using a clinical 3.0 Tesla scanner (Siemens, Germany), with a 3-inch surface coil as a receiver. The employed sequence acquired data with the following parameters: voxel size, 0.2 × 0.2 × 2 mm, matrix 448 × 314, echo time TE = 16 ms, repetition time TR = 600 ms, band width = 199 Hz/px, a total TA (acquisition time) of approximately 8 minutes was achieved.
Manually drawn regions of interest (ROIs) were placed in oblique 2D slices. The mean signal intensities of aqueous humor and vitreous body were measured. The signal-to-noise ratio (SNR) was calculated with the following formula: SNR = S/SDair. Where S represents the signal intensity in the ROI of the Mn2+ enhanced area, and SDair is the mean value of the SD in three ROIs in air.
Statistical analysis
Data were given as mean ± standard error and analyzed by SPSS15.0. The concentration of Mn2+ and SNR at predetermined time points were compared to the baseline concentration and SNR of rabbit eyes in control group, using independent sample t-test. P < 0.01 was considered statistically significant.
Results
Measurement of Mn2+ concentration
The concentrations of Mn2+ versus time profiles in aqueous humor, vitreous body and serum after topical administration are shown in Table 1. The concentration of Mn2+ in aqueous humor increased rapidly 0.5 hours after topical administration, and the maximum was measured at 2 hours (11.1641 mg•L-1). Then the concentration decayed rapidly, and the concentration decreased to 2.2523 mg•L-1 at 6 hours. The concentrations of Mn2+ in aqueous humor at predetermined time points (0.5, 1, 2, 4, 6, 12, 24, 48, and 72 hours postdose) were significantly different from the control group. (P < 0.01) In vitreous body, the concentration of Mn2+ increased slowly 0.5 hours postdose, the maximum concentration was measured at 12 hours (1.5622 mg•L-1). The concentration of Mn2+ in aqueous humor and vitreous body decreased to the baseline at 168 hours. Whereas, no obvious changes of Mn2+ concentration were detected in serum at all times throughout the study (Figure 1).
Table 1.
The time course of Mn2+ Concentrations in aqueous humor, vitreous body and serum after topical administration of MnCl2 (1 mol•L-1)
| Time (h) | Aqueous humor (mg•L-1) | Vitreous body (mg•L-1) | Serum (mg•L-1) |
|---|---|---|---|
| Control | 0.2136 ± 0.0183 | 0.2234 ± 0.0154 | 0.2379 ± 0.0093 |
| 0.5 | 5.3062 ± 0.3011☆ | 0.2713 ± 0.0154☆ | 0.2435 ± 0.0164 |
| 1 | 7.9690 ± 0.4721☆ | 0.3011 ± 0.0148☆ | 0.2431 ± 0.0161 |
| 2 | 11.1641 ± 0.7202☆ | 0.5012 ± 0.0125☆ | 0.2309 ± 0.0137 |
| 4 | 5.4838 ± 0.6073☆ | 1.2195 ± 0.0977☆ | 0.2341 ± 0.0124 |
| 6 | 2.2523 ± 0.1921☆ | 1.2786 ± 0.1504☆ | 0.2401 ± 0.0152 |
| 12 | 1.9814 ± 0.2019☆ | 1.5622 ± 0.1567☆ | 0.2236 ± 0.0141 |
| 24 | 1.6626 ± 0.2833☆ | 1.0453 ± 0.1336☆ | 0.2283 ± 0.0129 |
| 48 | 0.8640 ± 0.1750☆ | 0.7322 ± 0.1480☆ | 0.2309 ± 0.0168 |
| 72 | 0.4936 ± 0.1702☆ | 0.3612 ± 0.0755☆ | 0.2256 ± 0.0114 |
| 168 | 0.2301 ± 0.0209 | 0.2435 ± 0.0206 | 0.2321 ± 0.0132 |
P < 0.01, compared with control group.
Figure 1.
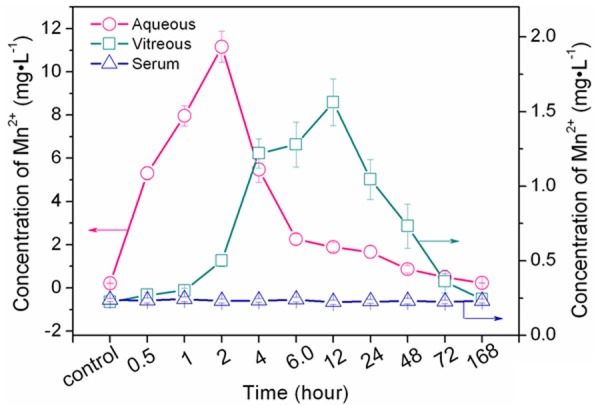
Mn2+ concentrations versus time profiles in aqueous humor, vitreous body and serum after topical administration of MnCl2 (20 μL, 1 mol•L-1). The maximum concentrations of Mn2+ in aqueous humor and vitreous body were 11.1641 ± 0.7202 mg•L-1 (2 hours) and 1.5622 ± 0.1567 mg•L-1. The concentration of Mn2+ decreased rapidly. The concentration in vitreous body fluctuated in a narrow range, and no obvious changes were detected in serum.
MEMRI of eyes
In group 3, we obtained the repeated T1WIs of eyes, at predetermined time points (0.5, 1, 2, 4, 6, 12, 24, 48, 72 and 168 hours postdose) after MnCl2 (20 μL, 1 mol•L-1) loading. Data collected from right eyes without Mn2+ treatments were added as a control. The aqueous humor showed initial significant enhancement 0.5 hour after MnCl2 loading, and reached the peak at 2 hours postdose. The enhancement of aqueous humor began to decrease after 2 hours postdose. Whereas, there were no obvious enhancement of vitreous body during the follow up (Figure 2).
Figure 2.
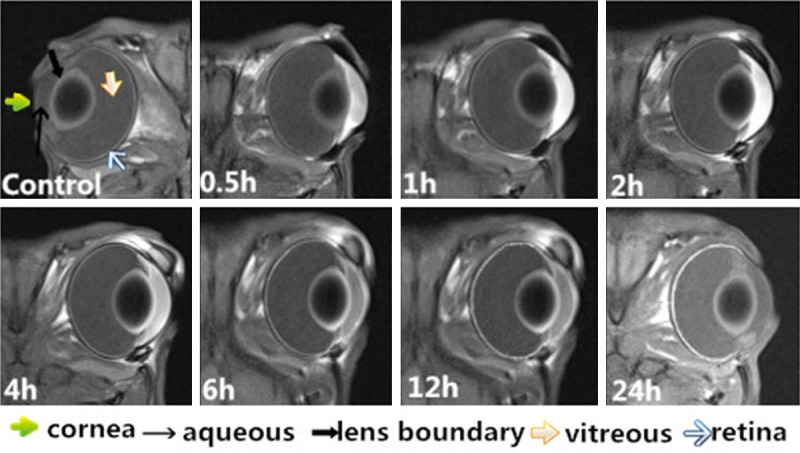
Dynamic images of the rabbit eyes (T1WI) after topical administration of MnCl2 (1 mol•L-1) at predetermined time points (0.5, 1, 2, 4, 6, 12 and 24 hours postdose). The enhancement of cornea and aqueous humor began at 0.5 hour, the maximum enhancement appeared at 2 hours, and then gradually decreased. There were no obvious enhancement of vitreous body during the follow up.
The SNR of different tissue versus time profiles are shown in Table 2. The SNRs of aqueous humor at 0.5, 1, 2, 4, 6, 12 and 24 hours postdose were significantly different from the control eyes. (P < 0.01). Whereas, the vitreous body did not show significant changes in the entire temporal evaluation.
Table 2.
The SNR of eyes at predetermined time points after topical administration of MnCl2 (1 mol•L-1)
| Time (h) | Aqueous humor | Vitreous body |
|---|---|---|
| Control | 27.12 ± 1.88 | 28.45 ± 1.81 |
| 0.5 | 99.77 ± 2.26※ | 29.26 ± 2.24 |
| 1 | 105.21 ± 3.92※ | 25.33 ± 1.05 |
| 2 | 108.81 ± 10.65※ | 29.03 ± 1.97 |
| 4 | 85.66 ± 5.08※ | 30.23 ± 1.87 |
| 6 | 75.25 ± 6.30※ | 30.44 ± 1.79 |
| 12 | 62.61 ± 5.41※ | 31.13 ± 2.05 |
| 24 | 37.54 ± 3.95※ | 29.95 ± 2.38 |
| 48 | 31.02 ± 5.09 | 32.36 ± 2.33 |
| 72 | 28.08 ± 5.76 | 28.38 ± 2.64 |
| 168 | 28.74 ± 4.03 | 30.55 ± 1.83 |
P < 0.01, compared with control group, (SNR, Signal-to-Noise ratio).
Discussion
There is increasing interest in developing MEMRI as a technique for functional and molecular imaging of biological processes, and Mn2+ is now a well-established contrast agent in this technique [7-9]. Recently, Sun SW et al have demonstrated the feasibility of using topical administration of Mn2+ for MEMRI [18]. The penetration and distribution of Mn2+ is a complicated process, for further study of topical administration of Mn2+ for MEMRI, we evaluated the concentration of Mn2+ in aqueous humor and vitreous body using ICP-MS, and analyzed the relation of Mn2+ concentration and enhancement of eyes in rabbits.
ICP-MS is a specific detector for metals at extremely low level. It plays an increasingly major role in trace elements analysis in different aspects [20-23]. In the study, the Mn2+ concentration in aqueous humor began to increase 0.5 hour after topical administration of MnCl2. The concentration increased rapidly with an initial burst effect at the early stage, and then reached maximum concentration at 2 hours. MEMRI showed the enhancement of aqueous humor synchronized with the fluctuation of the Mn2+ concentration in aqueous humor. The maximum SNR of the aqueous humor emerged at 2 hours (108.81 ± 10.65). The releasing model of Mn2+ was consistent with many topical administration drugs [24-27].
In previous study, MEMRI showed there were no detectable changes of signal intensity in vitreous body after topical administration of MnCl2. SUN SW et al concluded that the uptake of Mn2+ might do not involve the vitreous body [18]. But they did not measure the Mn2+ concentration in vitreous body. In the study, although the vitreous body did not show obvious enhancement of MEMRI, the concentration of Mn2+ fluctuated during the follow up times. We found that the Mn2+ concentration in vitreous body began to increase slowly 0.5 hour after administration of MnCl2, and the maximum concentration was measured on 12 hours (1.5622 mg•L-1).
In the study, the Mn2+ concentration was very low in vitreous body. These findings suggested that the uptake of Mn2+ in retina may do not involve the vitreous body or the Mn2+ enters the retina via other different routes. The specific transport pathways across eye tissues can alter the passive permeation, such as ion channels or membrane transporters. The previous studies confirmed that the enhancement depended on the health retina ganglia cells (RGCs), the loss of RGCs can minimize the amount of Mn2+ that enters the cells [18,28-30]. As a calcium analog, the calcium channels in cells may affect the absorption of Mn2+ in retina, and cause the significant enhancement of retina.
The presented study evaluated the concentration of Mn2+ in aqueous humor and vitreous body after topical administration of MnCl2, and analyze the relationship of the enhancement of MEMRI and Mn2+ concentration of eyes in rabbits. Whereas, our study was compromised by several limitations. In the study, we just investigated the concentration of Mn2+ in aqueous humor and vitreous body, but the concentrations of Mn2+ in cornea, lens boundary, retina and optic nerve were not included.
In conclusion, after topical administration of MnCl2, Mn2+ could distribute into aqueous humor rapidly, whereas, the Mn2+ concentration in vitreous body fluctuated in a narrow range over the course. There were no significant enhancement in vitreous body. Maybe the uptake of Mn2+ in retina did not involve vitreous body, or it involved several different pathways.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (Grant NO. 81371017).
Disclosure of conflict of interest
None.
References
- 1.Pautler RG. In vivo, trans-synaptic tract-tracing utilizing manganese-enhanced magnetic resonance imaging (MEMRI) NMR Biomed. 2004;17:595–601. doi: 10.1002/nbm.942. [DOI] [PubMed] [Google Scholar]
- 2.Sloot WN, Gramsbergen JB. Axonal transport of manganese and its relevance to selective neurotoxicity in the rat basal ganglia. Brain Res. 1994;657:124–132. doi: 10.1016/0006-8993(94)90959-8. [DOI] [PubMed] [Google Scholar]
- 3.Boretius S, Gadjanski I, Demmer I, Bahr M, Diem R, Michaelis T, Frahm J. MRI of optic neuritis in a rat model. Neuroimage. 2008;41:323–334. doi: 10.1016/j.neuroimage.2008.02.021. [DOI] [PubMed] [Google Scholar]
- 4.Gadjanski I, Boretius S, Williams SK, Lingor P, Knoferle J, Sattler MB, Fairless R, Hochmeister S, Suhs KW, Michaelis T, Frahm J, Storch MK, Bahr M, Diem R. Role of n-type voltage-dependent calcium channels in autoimmune optic neuritis. Ann Neurol. 2009;66:81–93. doi: 10.1002/ana.21668. [DOI] [PubMed] [Google Scholar]
- 5.Thuen M, Olsen O, Berry M, Pedersen TB, Kristoffersen A, Haraldseth O, Sandvig A, Brekken C. Combination of Mn (2+)-enhanced and diffusion tensor MR imaging gives complementary information about injury and regeneration in the adult rat optic nerve. J Magn Reson Imaging. 2009;29:39–51. doi: 10.1002/jmri.21606. [DOI] [PubMed] [Google Scholar]
- 6.Berkowitz BA, Bissg D, Dutczak O, Corbett S, North R, Roberts R. MRI biomarkers for evaluation of treatment efficacy in preclinical diabetic retinopathy. Expert Opin Med Diagn. 2013;7:393–403. doi: 10.1517/17530059.2013.814639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nair G, Pardue MT, Kim M, Duong TQ. Manganese-Enhanced MRI Reveals Multiple Cellular and Vascular Layers in Normal and Degenerated Retinas. J Magn Reson Imaging. 2011;34:1422–1429. doi: 10.1002/jmri.22719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan KC, Fu QL, Hui ES, So KF, Wu EX. Evaluation of the retina and optic nerve in a rat model of chronic glaucoma using in vivo manganese-enhanced magnetic resonance imaging. Neuroimage. 2008;40:1166–1174. doi: 10.1016/j.neuroimage.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Chan KC, Li J, Kau P, Zhou IY, Cheung MM, Lau C, Yang J, So KF, Wu EX. In vivo retinotopic mapping of superior colliculus using manganese-enhanced magnetic resonance imaging. Neuroimage. 2011;54:389–395. doi: 10.1016/j.neuroimage.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 10.De La Garza BH, Li G, Shih YY, Duong TQ. Layer-specific manganese enhanced MRI of the retina in light and dark adaptation. Invest Ophthalmol Vis Sci. 2012;53:4352–4358. doi: 10.1167/iovs.11-8826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berkowitz BA, Roberts R, Goebel DJ, Luan H. Noninvasive and simultaneous imaging of layer-specific retinal functional adaptation by manganese-enhanced MRI. Invest Ophthalmol Vis Sci. 2006;47:2668–2674. doi: 10.1167/iovs.05-1588. [DOI] [PubMed] [Google Scholar]
- 12.Bissig D, Berkowitz BA. Manganese-enhanced MRI of layer-specific activity in the visual cortex from awake and free-moving rats. Neuroimage. 2009;44:627–635. doi: 10.1016/j.neuroimage.2008.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan KC, Cheng JS, Fan S, Zhou IY, Yang J, Wu EX. In vivo evaluation of retinal and callosal projections in early postnatal development and plasticity using manganese-enhanced MRI and diffusion tensor imaging. Neuroimage. 2012;59:2274–2283. doi: 10.1016/j.neuroimage.2011.09.055. [DOI] [PubMed] [Google Scholar]
- 14.Lindsey JD, Scadeng M, Dubowitz DJ, Crowston JG, Weinreb RN. Magnetic resonance imaging of the visual system in vivo: transsynaptic illumination of V1 and V2 visual cortex. Neuroimage. 2007;34:1619–1626. doi: 10.1016/j.neuroimage.2006.07.048. [DOI] [PubMed] [Google Scholar]
- 15.Olsen O, Thuen M, Berry M, Kovalev V, Petrou M, Goa PE, Sandvig A, Haraldseth O, Brekken C. Axon tracing in the adult rat optic nerve and tract after intravitreal injection of MnDPDP using a semiautomatic segmentation technique. J Magn Reson Imaging. 2008;27:34–42. doi: 10.1002/jmri.21234. [DOI] [PubMed] [Google Scholar]
- 16.Berkowitz BA, Roberts R, Goebel DJ, Luan H. Noninvasive and simultaneous imaging of layer-specific retinal functional adaptation by manganese-enhanced MRI. Invest Ophthalmol Vis Sci. 2006;47:2668–2674. doi: 10.1167/iovs.05-1588. [DOI] [PubMed] [Google Scholar]
- 17.Jager RD, Aiello LP, Patel SC, Cunningham ET Jr. Risks of intravitreous injection: a comprehensive review. Retina. 2004;24:676–698. doi: 10.1097/00006982-200410000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Sun SW, Campbell B, Lunderville C, Won E, Liang HF. Noninvasive topical loading for manganese-enhanced MRI of the mouse visual system. Invest Ophthalmol Vis Sci. 2011;52:3914–3920. doi: 10.1167/iovs.10-6363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun SW, Thiel T, Liang HF. Impact of Repeated Topical-Loaded Manganese-Enhanced MRI on the Mouse Visual System. Invest Ophthalmol Vis Sci. 2012;53:4699–4709. doi: 10.1167/iovs.12-9715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Konz I, Fernandez B, Fernandez ML, Pereiro R, Gonzalez-Iglesias H, Coca-Prados M, Sanz-Medel A. Quantitative bioimaging of trace elements in the human lens by LA-ICP-MS. Anal Bioanal Chem. 2014;406:2343–2348. doi: 10.1007/s00216-014-7617-y. [DOI] [PubMed] [Google Scholar]
- 21.Krachler M. Environmental applications of single collector high resolution ICP-MS. J Environ Monit. 2007;9:790–804. doi: 10.1039/b703823m. [DOI] [PubMed] [Google Scholar]
- 22.Balcaen L, Bolea-Fernandez E, Resano M, Vanhaecke F. Accurate determination of ultra-trace levels of Ti in blood serum using ICP-MS/MS. Analytica Chimica Acta. 2014;809:1–8. doi: 10.1016/j.aca.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 23.Erie JC, Butz JA, Good JA, Erie EA, Burritt MF, Cameron JD. Heavy metal concentrations in human eyes. Am J Ophthalmol. 2005;139:888–893. doi: 10.1016/j.ajo.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 24.Ozturk F, Kortunay S, Kurt E, Ilker SS, Basci NE, Bozkurt A. Penetration of topical and oral ciprofloxacin into the aqueous and vitreous humor in inflamed eyes. Retina. 1999;19:218–222. doi: 10.1097/00006982-199903000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Goldblum D, Fausch K, Frueh BE, Theurillat R, Thormann W, Zimmerli S. Ocular penetration of caspofungin in a rabbit uveitis model. Graefe’s Arch Clin Exp Ophthalmol. 2007;245:825–833. doi: 10.1007/s00417-006-0460-x. [DOI] [PubMed] [Google Scholar]
- 26.Sakarya R, Sakarya Y, Ozcimen M, Kesli R, Alpfidan I, Kara S. Ocular penetration of topically applied 1% daptomycin in a rabbit model. J Ocul Pharmacol Ther. 2013;29:75–78. doi: 10.1089/jop.2012.0111. [DOI] [PubMed] [Google Scholar]
- 27.Zhang JJ, Wang LY, Zhou J, Zhang L, Xia HY, Zhou TY. Ocular penetration and pharmacokinetics of topical clarithromycin eye drops to rabbits. J Ocul Pharmacol Ther. 2014;30:42–48. doi: 10.1089/jop.2013.0042. [DOI] [PubMed] [Google Scholar]
- 28.Zheng L, Gong BD, Hatala DA, Kern TS. Retinal ischemia and reperfusion causes capillary degeneration: similarities to diabetes. Invest Ophthalmol Vis Sci. 2007;48:361–367. doi: 10.1167/iovs.06-0510. [DOI] [PubMed] [Google Scholar]
- 29.Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, Neufeld AH. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage. 2003;20:1714–1722. doi: 10.1016/j.neuroimage.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 30.Feng Y, Luo LS, Ma ZZ, Sun XD, Hu YT. In vivo detection of severity of optic nerve crush using manganese-enhanced magnetic resonance imaging in rats. Chin Med J (Engl) 2014;127:522–527. [PubMed] [Google Scholar]


