Abstract
Neural stem cells (NSCs) are multipotent cells that have the capacity for differentiation into the major cell types of the nervous system, i.e. neurons, astrocytes and oligodendrocytes. Valproic acid (VPA) is a widely prescribed drug for seizures and bipolar disorder in clinic. Previously, a number of researches have been shown that VPA has differential effects on growth, proliferation and differentiation in many types of cells. However, whether VPA can induce NSCs from embryonic cerebral cortex differentiate into neurons and its possible molecular mechanism is also not clear. Wnt signaling is implicated in the control of cell growth and differentiation during CNS development in animal model, but its action at the cellular level has been poorly understood. In this experiment, we examined neuronal differentiation of NSCs induced by VPA culture media using vitro immunochemistry assay. The neuronal differentiation of NSCs was examined after treated with 0.75 mM VPA for three, seven and ten days. RT-PCR assay was employed to examine the level of Wnt-3α and β-catenin. The results indicated that there were more β-tublin III positive cells in NSCs treated with VPA medium compared to the control group. The expression of Wnt-3α and β-catenin in NSCs treated with VPA medium was significantly greater compared to that of control media. In conclusion, these findings indicated that VPA could induce neuronal differentiation of NSCs by activating Wnt signal pathway.
Keywords: Wnt-3α, β-catenin, valproic acid, neural stem cells, differentiation
Introduction
The central nervous system (CNS) was long thought to be largely postmitotic with very limited ability to regenerate. Thus, it came as a superviser when neural stem cells (NSCs) were discovered [1]. NSCs can self-renew to produce more stem cells and have teenage ability, capable of generating neurons, astrocytes and oligodendrocytes [2-6]. So they brought hopes to the patients with CNS disorders.
Previous studies have suggested that the behavior of NSCs was regulated by both intrinsic characteristic and extrinsic signals originating from the culture medium [7]. For example, glial cell line-derived neurotrophic factor (GDNF), neurotrophin-3 (NT-3) and ciliary neurotrophic factor (CNTF) have been shown to promote the differentiation of NSC in culture [8-10]. In addition to these neurotrophic factors, chemicals can also regulate the multi-potential of NSCs.
Valproic acid (VPA) was a pharmacologic agents utilized in the treatment of manic-depressive illness for nearly 50 years in clinic. Recent studies have demonstrated that VPA has neuroprotection effect. The potential mechanism for this effect involved of the expression of the anti-apoptotic protein Bcl-2, upegulated by VPA in rat mouse brain and human SH-SY5Y neuroblastoma cells to produced neurotrophic effects [11,12]. Furthermore, VPA promoted neuronal fate through the induction of NeuroD in adult hippocampal neural progenitor [13]. However, the effect of VPA on NSCs remains unclear. In this study, we showed that VPA induced NSCs to differentiate predominantly into neurons, at least in part, by activating Wnt-3α and β-catenin.
Materials and animals
Neural stem cell culture
All animals used in this study were handled according to the Guidelines of Chongqing Association for Animal Care and Use (SCXK Yu20020003) and the Research Institude of surgery for the management of laboratory animals. Animal Ethic of this study was approved by the Third Military Medical University.
Primary neural stem cells (NSCs) were derived from embryonic 13-15 days Sprague-Dawley (SD) rats as derived previously [14]. Briefly, the telencephalon was rapidly dissected and placed into 2 ml tube containing 0.25% trypsin. The tissue was mechanically dissociated into signal cell suspension. Cell number and viability were assessed by staining a small volume of cell suspension with 0.4% typan blue. Signal-cell suspensions were transferred to growth medium consisting of neural basal (NB) medium and 2% B27 supplemented with 20 ng/ml human recombinant basic fibroblast growth factor (bFGF, Gibico Invitrogen, USA), 20 ng/ml epidermal growth factor (EGF, Gibico Invitrogen, USA) at 5×104 cells/ml. The cells were then planted into culture flasks and maintained under a humidified atmosphere of 5% CO2 in air at 37°C. After 3-5 day in vitro, the neurosphere were dissociated into single-cell suspension and seeded onto 24-cell plates at 1-2 cell per well. The neurosphere subclusters were digested and another passage was performed as before. The cell passage protocol was performed every 5-6 day to obtain neurosphere originating from a single primary cell. Secondary or tertiary neurospheres were used for subsequent experiments. Half of the medium was replaced every other day. For Brdu labeling, NSCs were incubated in medium containing 10 μM Brdu for 18 h prior staining.
The third passage cells were plated on coverslips coated with poly-L-lysine (Sigma) at the density of 1×105 cells/ml, then cultured with NB medium with 2% B27 supplement and fetal bovine serum (2%), including valproic acid with the concentration of 0.75 mM [15]. The cells cultured in NB medium with 2% B27 supplement and fetal bovine serum (2%) were control group. The medium was half-exchanged every other day. At 3 days, 7 days and 10 days after culture, differentiated cells were immunochemistry staining with antibody of β-tublin III (mouse anti-rat IgG, 1:800, Sigma, USA) and glial fibrous acidic protein (GFAP, rabbit anti-rat IgG, 1:400, Sigma, USA).
Identification of NSCs and differentiated neural cells
After 30-min fixation in 4% paraformaldehyde, the NSCs were treated with 1N HCl for 10 min to denature DNA, and then neutralized with 0.1 M sodium borate for another 10 min. Samples were washed three times with 0.01 M PBS. 0.5% Triton-X-100 and 1% BSA prior to an overnight incubation with the primary mouse anti-rat Brdu antibody (1:800, Sigma, USA) and rabbit anti-rat nestin (1:200, Sigma, USA). In order to identify the multiple differentiations of NSCs, the differentiated cells from NSCs were stained with β-tublin III and GFAP antibody. The number of cells was counted with DAPI (sigma) staining. After 3×5 min washed, fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse secondary antibody (1:100, Zhongshan, China) was used for staining β-tublin III and tetramethylrhodamine isothiocyanate (TRITC)-conjugated goat anti-rabbit secondary antibody (1:100, Zhongshan, China) was used for staining GFAP respectively. Subsequently, the sample was stained with DAPI for 3 min and then washed three times with 0.01 M PBS prior to scanning with laser con-focal microscope (Leica, SP2, Germany).
Real time RT-PCR
The procedure was used as describe by Hou et al. [16]. Total RNA was extracted from differentiated NSCs after 3 days, 7 days and 10 days with Trizol (Invitrogen, USA). The separation and precipitation of RNA was accomplished with chloroform and isopropyl alcohol. Total RNA was isolated and purified by an Rneasy minikit (Qiagen, USA) with the addition of Rnasefree DNase I (Qiagen, USA). The two-step SYBR ExScriptTM RT-PCR kit (Perfect Real TimE, TaKaRa, Japan) for real-time RT-PCR was used to analyze the expression of Wnt-3α and β-catenin. Glycealdehyde-3-phosphate dehydrogenase (GAPDH, Santa Cruz, USA) was used as the housekeeping gene. Quantification was achieved using standard curves derived from gene expression relative to the level of GAPDH gene expression.
PCR was performed for 32 cycles (at 92°C for 30 s, at 62°C for 1 min and at 72°C for 30 s) in 50 µL total volume containing (in mM) dNTPs 0.2; Tris-HCl, 13; KCl, 65; MgCl2, 2.6, with Triton 3100 0.13% and 2 units of Taq Polymerase (Promega). An amplification step was performed at 62°C for 10 min. Ten microlitre-PCR samples were loaded on a 2% agarose gel (Life Technologies, Cergy-Pontoise, France) containing Gel Star (Biorad, Hercules, USA) and photographed. The primers used are listed in Table 1.
Table 1.
Primers used for real-time reverse transcription-polymerase chain reaction
| Gene | Forward primer (5, -3,) | Reverse primer (3, -5,) | Length (bp) | Access number |
|---|---|---|---|---|
| Wnt-3α | gccagtcacatgcacctcaa | gctctgtgggcaccttgaag | 220 | XM_220546.5 |
| β-catenin | tgggctgcagaaaatggttg | tcgggtctgtcaggtgaggc | 290 | NM_053357.2 |
| GAPDH | aggttgtctcctgcgacttca | tggtccagggtttcttactcc | 163 | NM_017008.3 |
Statistical analysis
The percentage of positive cells in relation to the total cell number was determined in 5 random fields under microscope for each group in three independent experiments. All data were presented as mean ± SD. Statistical analysis of data was performed using a one-way analysis of variance (ANOVA). P < 0.05 was considered to be statistically significant.
Results
NSCs could proliferate and differentiate into different types of neural cells
On the second day after primary culture, cells cultured in NB medium with B27 supplement and bFGF proliferated and formed neurospheres (Figure 1A and 1B). These neurospheres expressed neural stem cell specific marker Nestin (Figure 1C and 1D). Three days after withdraw bFGF, the cells from differentiated neurospheres can differentiate into neurons and astrocytes (Figure 1E-H).
Figure 1.
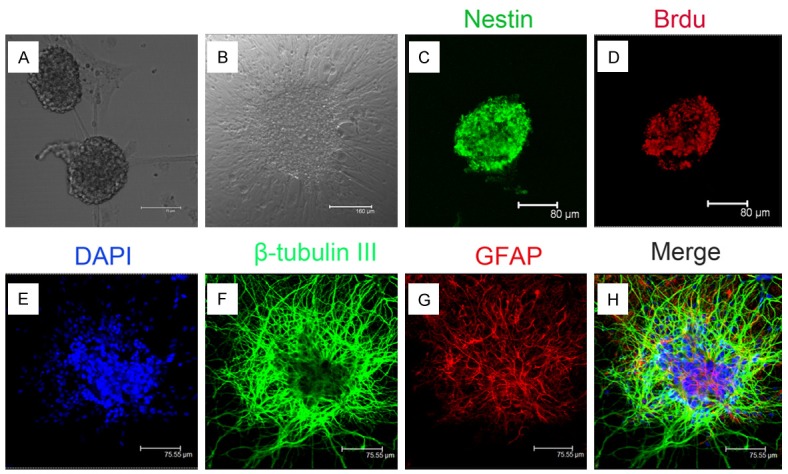
The growth and identification of NSCs in vitro. A. Representative photomicrograph of neurospheres in culture (bar = 75 µm). B. Representative photomicrograph of differentiated NSCs (bar = 160 µm). C. Immunocytochemical staining of purified NSCs with Nestin (bar = 80 µm). D. Immunocytochemical staining of NSCs with anti-Brd-U antibody (bar = 80 µm). E. Nucleus staining of NSCs with DAPI (bar = 75.55 µm). F. Immunocytochemical staining of differentiated neurons from neurospheres (green indicates neuron-specific label β-tubulin III, bar = 75.55 µm). G. Immunocytochemical staining of differentiated astrocytes from neurospheres(red indicates astrocyte-specific label GFAP, bar = 75.55 µm). H. Merge of F and G (bar = 75.55 µm).
Differentiation of NSCs with valproic acid
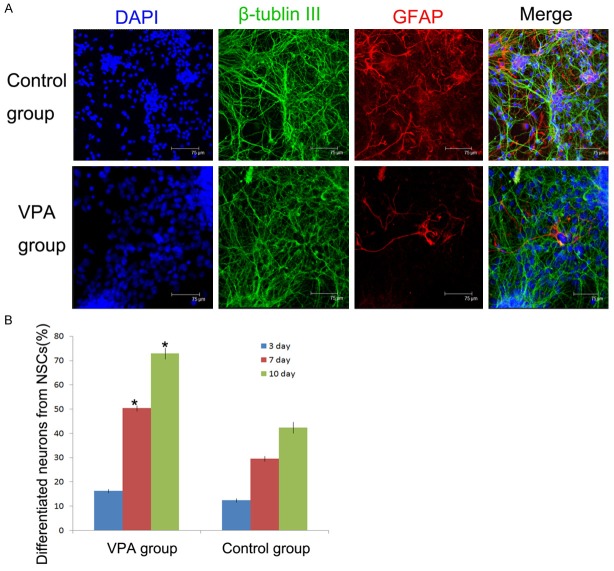
To confirm differentiated cells from NSCs with valproic acid, neurons specific marker and astrocytes marker were used for immunofluorescence staining (Figure 2A). Our results showed that after differentiation 3 days, the number of neuron-like cells in two groups had no significant difference. After 7 days and 10 days, the cells cultured in valproic acid group, the number of neurons differentiated from NSCs were much higher than those in control group. At 10 days, 74.2 ± 2.40% differentiated cells form NSCs were expressed neuron specific marker β-tubulin III, which was significantly higher compared to the control group (46.8 ± 2.36% β-tubulin III positive cells) (Figure 2B).
Figure 2.
Identification and quantification of differentiated neural cells cultured in VPA medium and control medium 7 days in vitro. A. Immunocytochemical staining of differentiated neural cells in different midium. β-tubulin III staining (green) indicates neurons; GFAP staining (red) indicates astrocytes. The nuclei were counterstained with DAPI (blue). (Scale bar = 75 µm). B. Quantification of differentiated neurons in two culture media. *P < 0.05 indicates statistical significance compared with control group.
Wnt-3α and β-catenin expression in differentiation process of NSCs
To confirm the expression of Wnt-3α and β-catenin, Wnt-3α and β-catenin mRNA was examined by RT-PCR at different differentiation time points in vitro. On the 3rd, 7th and 10th day after NSCs differentiation, both mRNA expression of Wnt-3α and β-catenin was higher than control group (P < 0.01). Interestingly, the expression of Wnt-3α and β-catenin in both groups at 7 days and 10 days was lower than that at 3 days (Figure 3A and 3B). That means Wnt-3α and β-catenin mRNA didn’t increase following with the differentiation time. These results suggested that VPA stimulated neuronal differentiation by activating Wnt signal pathway such as Wnt-3α and β-catenin.
Figure 3.
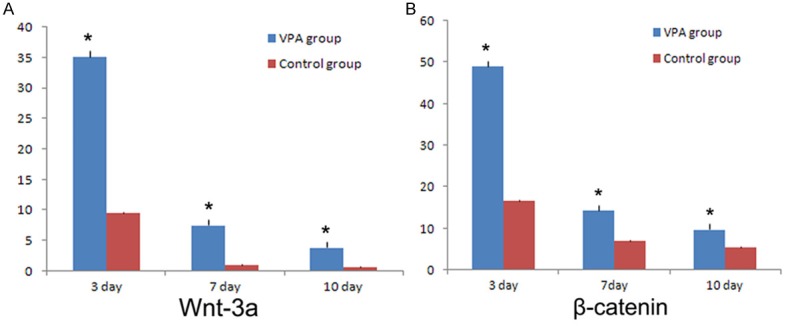
RT-PCR analysis of Wnt-3α and β-catenin expression in NSCs cultured in VPA medium and control medium at 3, 7, 10 days in vitro. A. The expression of Wnt-3α in two groups. B. The expression of β-catenin in two groups. The level of Wnt-3α and β-catenin in VPA treated NSCs was higher than that in the control group. *P < 0.01 indicates statistical significance compared to the control group.
Discussion
NSCs exist in the mammalian developing and adult nervous system and have the ability to self-renew and differentiate into three major neural lineages. Tremendous interest in the potential of NSCs for the treatment of neurodegenerative disease and central nervous system injuries has substantially promoted research on NSCs [17]. Regulation of NSCs self-renew and differentiation is the major challenge for neural stem cell-based cell replacement therapies to ameliorate some of clinic feature of experiment models of neurological disease, including spinal cord trauma, neurogenetic degeneration and stroke [18].
Previous studies suggested that differentiation of NSCs are influenced the effects of intrinsic and extrinsic signals coming from substrates, medium components, and the complex interaction among cells [19]. VPA is an effective and widely used antiepileptic and anticonvulsant drug. Previous studies showed that VPA was shown to protect cultured rat hippocampal neurons against amyloid and glutamate neurotoxicity and can protect cultured cerebral cortical neurons, neural progenitor cell, mesenchymal pluripotent cell, and retinal ganglion cells from apoptosis [20-23]. VPA could also induced proliferation of rat Schwann cells [24]. Conversely, some experiments revealed that a doses exposure to VPA had no changes in cell proliferation for Hela cell [25]. Yuan et al. [12] have further demonstrated that VPA produces similar effects to those of neurotrophic factors, promoting neurite growth for SH-SY5Y cells. Other study revealed that VPA actively suppressed differentiation into astrocytes and oligodendrocytes, while promoting differentiation into neurons by up-regulating the neurogenic bHLH transcription factor NeuroD [13]. So, we asked whether a similar pattern of regulation by VPA is evident in NSCs. And little is known about the molecular mechanisms underlying this event.
In order to investigate the effects of VPA on NSCs differentiation in vitro, our previous data showed that the VPA on NSCs differentiation have concentration and time effect [15]. It indicated that 0.75 mM is a desirable concentration for the differentiation of NSCs. Thus, in this experiment, we chose 0.75 mM VPA to treat NSCs and explored its possible mechanism further. In this series of experiments, we found that treatment with VPA during the NSCs stages resulted in neuronal differentiation, accompanied by increases in neuronal cell numbers and the expression of Wnt-3α and β-catenin. In the first three days, NSCs in all groups, began to adhere and differentiate. But the ratio of neurons differentiated from NSCs had no significant difference. Interestingly, the expression of Wnt-3α and β-catenin began to increase. Seven and ten days after differentiation, NSCs in VPA were more likely to differentiate into neurons than those in control group. Accordingly, the expression of Wnt-3α and β-catenin in VPA groups was higher than that in control group. But the expression of Wnt-3α and β-catenin didn’t increase accompany with the time. We supposed that VPA-mediate promotion of neuronal differentiation was due to the activation of Wnt-3α and β-catenin. Wnt-3α and β-catenin expressed at the early stage of NSCs, and the expression level didn’t rise apparently along with time increasing. That means there was no relation between the expression level of Wnt-3α and β-catenin and the number of differentiated neurons.
Wnt protein was cysteine-rich lipid-modified proteins that play a major role in various processes during cell development including proliferation, differentiation and fate decision [26]. Wnt-3α was the member of canonical pathway and β-catenin was an essential component of the canonical Wnt signaling system. At present, some studies have shown that activation of the Wnt/β-catenin pathway supports pluripotency, whereas other studies indicated a role in promoting differentiation [27]. We presumed that Wnts play different role in specific cell and tissue. Recent studies showed Wnt/β-catenin signaling regulate neuronal differentiation of mesenchymal stem cells, osteoblasts [28,29] and regeneration of neuron in the mouse retina [30]. At the same time others have identified that Wnt-3α is crucial for the differentiation of hippocampus caudomedial progenitor cells and zebrafish cerebellum cell [31,32]. Wnt-3α in combination with bone morphogenetic proteins and sonic hedgehog, induced the differentiation of ESCs into interneurons [33]. Besides, Wnt-3α regulated chondrocyte differentiation via c-Jun/AP-1 pathway [34]. In our experiment, we observed that Wnt-3α and β-catenin anticipate in the differentiation of NSCs as well. It has been identified that Wnt-3α and β-catenin have crosstalk with hedgehog, GSK-3β, c-Jun and Nogo A signal pathway [31-36].
In summary, VPA can promote NSCs differentiated into neurons through activating Wnt-3α and β-catenin. These findings demonstrate that VPA can induce differentiation of NSCs into neurons by activation of Wnt canonical signaling pathways. Our data provides novel information regarding NSCs response to VPA and a potential therapy for inducing differentiation of NSCs for clinical application. Nonetheless, additional studies will be required to determine whether there is crosstalk between Wnt-3α/β-catenin and other signaling pathway. Also more work is required to assess whether these VPA-induced neurons are able to improve neurological functions under disease conditions.
Acknowledgements
This research was supported by a grant from Chongqing National Natural Science Fund (CSTC, No. 2008BB5278). The funder had role in study design, data collection and analysis, decision to publish, and preparation of the manuscript.
Disclosure of conflict of interest
None.
References
- 1.Goh EL, Ma D, Ming GL, Song H. Adult neural stem cells and repair of the adult central nervous system. J Hematother Stem Cell Res. 2003;12:671–679. doi: 10.1089/15258160360732696. [DOI] [PubMed] [Google Scholar]
- 2.Yin X, Li L, Zhang X, Yang Y, Chai Y, Han X, Feng Z. Development of neural stem cells at different sites of fetus brain of different gestational age. Int J Clin Exp Pathol. 2013;6:2757–2764. [PMC free article] [PubMed] [Google Scholar]
- 3.Alvarez-Buylla A, Temple S. Stem cells in the developing and adult nervous system. J Neurobiol. 1998;36:105–110. [PubMed] [Google Scholar]
- 4.Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- 5.Ren H, Chen J, Wang Y, Zhang S, Zhang B. Intracerebral neural stem cell transplantation improved the auditory of mice with presbycusis. Int J Clin Exp Pathol. 2013;6:230–341. [PMC free article] [PubMed] [Google Scholar]
- 6.Temple S. The development of neural stem cells. Nature. 2001;414:112–117. doi: 10.1038/35102174. [DOI] [PubMed] [Google Scholar]
- 7.Liu Y, Liu RR, Wang L, Zeng L, Long ZY, Wu YM. The effects of different phenotype astrocytes on neural stem cells differentiation in co-culture. Neurosci Lett. 2012;508:61–66. doi: 10.1016/j.neulet.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 8.Dovere L, Fera S, Grasso M, Lamberti D, Gargioli C, Muciaccia B, Lustri AM, Stefanini M, Vicini E. The niche-derived glial cell line-derived neurotrophic factor (GDNF) induces migration of mouse spermatogonial stem/progenitor cells. PLoS One. 2013;8:e59431. doi: 10.1371/journal.pone.0059431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma S, Liang S, Jiao H, Chi L, Shi X, Tian Y, Yang B, Guan F. Human umbilical cord mesenchymal stem cells inhibit C6 glioma growth via secretion of dickkopf-1 (DKK1) Mol Cell Biochem. 2014;385:277–286. doi: 10.1007/s11010-013-1836-y. [DOI] [PubMed] [Google Scholar]
- 10.Payne AG. Ciliary neurotrophic factor: its possible role as a stem cell homing beacon in neurological diseases and disorders. Med Hypotheses. 2005;64:880–881. doi: 10.1016/j.mehy.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 11.Corson TW, Woo KK, Li PP, Warsh JJ. Cell-type specific regulation of calreticulin and Bcl-2 expression by mood stabilizer drugs. Eur Neuropsychopharmacol. 2004;14:143–150. doi: 10.1016/S0924-977X(03)00102-0. [DOI] [PubMed] [Google Scholar]
- 12.Yuan PX, Huang LD, Jiang YM, Gutkind JS, Manji HK, Chen G. The mood stabilizer valproic acid activates mitogen-activated protein kinases and promotes neurite growth. J Biol Chem. 2001;276:31674–31683. doi: 10.1074/jbc.M104309200. [DOI] [PubMed] [Google Scholar]
- 13.Hsieh J, Nakashima K, Kuwabara T, Mejia E, Gage FH. Histone deacetylase inhibition-mediated neuronal differentiation of multipotent adult neural progenitor cells. Proc Natl Acad Sci USA. 2004;101:16659–16664. doi: 10.1073/pnas.0407643101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brannen CL, Sugaya K. In vitro differentiation of multipotent human neural progenitors in serum-free medium. Neuroreport. 2000;11:1123–1128. doi: 10.1097/00001756-200004070-00042. [DOI] [PubMed] [Google Scholar]
- 15.Wang L, Wu YM, Liu Y, Nan GX, Long ZY. The dose and time effect of valproic acid on differentiation of neural stem cells. Prog Modern Biomed. 2011;11:2263–2265. [Google Scholar]
- 16.Hou T, Xu J, Wu X, Xie Z, Luo F, Zhang Z, Zeng L. Umbilical cord Wharton’s Jelly: a new potential cell source of mesenchymal stromal cells for bone tissue engineering. Tissue Eng Part A. 2009;15:2325–2334. doi: 10.1089/ten.tea.2008.0402. [DOI] [PubMed] [Google Scholar]
- 17.Qu Q, Shi Y. Neural stem cells in the developing and adult brains. J Cell Physiol. 2009;221:5–9. doi: 10.1002/jcp.21862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang L, Shi J, van Ginkel FW, Lan L, Niemeyer G, Martin DR, Snyder EY, Cox NR. Neural stem/progenitor cells modulate immune responses by suppressing T lymphocytes with nitric oxide and prostaglandin E2. Exp Neurol. 2009;216:177–183. doi: 10.1016/j.expneurol.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 19.Li X, Yang Z, Zhang A. The effect of neurotrophin-3/chitosan carriers on the proliferation and differentiation of neural stem cells. Biomaterials. 2009;30:4978–4985. doi: 10.1016/j.biomaterials.2009.05.047. [DOI] [PubMed] [Google Scholar]
- 20.Matsuda Y, Wakai T, Kubota M, Osawa M, Hirose Y, Sakata J, Kobayashi T, Fujimaki S, Takamura M, Yamaqiwa S, Aoyaqi Y. Valproic acid overcomes transforming growth factor-beta-mediated sorafenib resistance in hepatocellular carcinoma. Int J Clin Exp Pathol. 2014;7:1299–1313. [PMC free article] [PubMed] [Google Scholar]
- 21.Go HS, Seo JE, Kim KC, Han SM, Kim P, Kang YS, Han SH, Shin CY, Ko KH. Valproic acid inhibits neural progenitor cell death by activation of NF-κB signaling pathway and up-regulation of Bcl-XL. J Biomed Sci. 2011;18:48. doi: 10.1186/1423-0127-18-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hatakeyama Y, Hatakeyama J, Takahashi A, Oka K, Tsuruga E, Inai T, Sawa Y. The effect of valproic Acid on mesenchymal pluripotent cell proliferation and differentiation in extracellular matrices. Drug Target Insights. 2011;5:1–9. doi: 10.4137/DTI.S6534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Biermann J, Boyle J, Pielen A, Lagrèze WA. Histone deacetylase inhibitors sodium butyrate and valproic acid delay spontaneous cell death in purified rat retinal ganglion cells. Mol Vis. 2011;5:395–403. [PMC free article] [PubMed] [Google Scholar]
- 24.Fei W, Aixi Y, Danmou X, Wusheng K, Zhengren P, Ting R. The mood stabilizer valproic acid induces proliferation and myelination of rat Schwann cells. Neurosci Res. 2011;70:383–390. doi: 10.1016/j.neures.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 25.Felisbino MB, Tamashiro WM, Mello ML. Chromatin remodeling, cell proliferation and cell death in valproic acid-treated HeLa cells. PLoS One. 2011;6:e29144. doi: 10.1371/journal.pone.0029144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kléber M, Sommer L. Wnt signaling and the regulation of stem cell function. Curr Opin Cell Biol. 2004;16:681–687. doi: 10.1016/j.ceb.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 27.Bräutigam C, Raggioli A, Winter J. The Wnt/β-catenin pathway regulates the expression of the miR-302 cluster in mouse ESCs and P19 cells. PLoS One. 2013;8:e75315. doi: 10.1371/journal.pone.0075315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tamura M, Uyama M, Sugiyama Y, Sato M. Canonical Wnt signaling activates miR-34 expression during osteoblastic differentiation. Mol Med Rep. 2013;8:1807–1811. doi: 10.3892/mmr.2013.1713. [DOI] [PubMed] [Google Scholar]
- 29.Guo Y, Li Z, Ding R, Li H, Zhang L, Yuan W, Wang Y. Parathyroid hormone induces epithelial-to-mesenchymal transition via the Wnt-beta-catenin signaling pathway in human renal proximal tubular cells. Int J Clin Exp Pathol. 2014;7:5978–5987. [PMC free article] [PubMed] [Google Scholar]
- 30.Sanges D, Romo N, Simonte G, Di Vicino U, Tahoces AD, Fernández E, Cosma MP. Wnt/β-catenin signaling triggers neuron reprogramming and regeneration in the mouse retina. Cell Rep. 2013;4:271–286. doi: 10.1016/j.celrep.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 31.Lee SM, Tole S, Grove E, McMahon AP. A local Wnt-3α signal is required for development of the mammalian hippocampus. Development. 2000;127:457–467. doi: 10.1242/dev.127.3.457. [DOI] [PubMed] [Google Scholar]
- 32.Toledo EM, Colombres M, Inestrosa NC. Wnt signaling in neuroprotection and stem cell differentiation. Prog Neurobiol. 2008;86:281–296. doi: 10.1016/j.pneurobio.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 33.Murashov AK, Pak ES, Hendricks WA, Owensby JP, Sierpinski PL, Tatko LM, Fletcher PL. Directed differentiation of embryonic stem cells into dorsal interneurons. FASEB J. 2005;19:252–254. doi: 10.1096/fj.04-2251fje. [DOI] [PubMed] [Google Scholar]
- 34.Hwang SG, Yu SS, Lee SW, Chun JS. Wnt-3α regulates chondrocyte differentiation via c-Jun/AP-1 pathway. FEBS Lett. 2005;579:4837–4842. doi: 10.1016/j.febslet.2005.07.067. [DOI] [PubMed] [Google Scholar]
- 35.Yanai K, Nakamura M, Akiyoshi T, Nagai S, Wada J, Koga K, Noshiro H, Nagai E, Tsuneyoshi M, Tanaka M, Katano M. Crosstalk of hedgehog and Wnt pathways in gastric cancer. Cancer Lett. 2008;263:145–156. doi: 10.1016/j.canlet.2007.12.030. [DOI] [PubMed] [Google Scholar]
- 36.Chung R, Peters AC, Armanious H, Anand M, Gelebart P, Lai R. Biological and clinical significance of GSK-3-beta in mantle cell lymphoma-an immunohistochemical study. Int J Clin Exp Pathol. 2010;3:244–253. [PMC free article] [PubMed] [Google Scholar]