Abstract
The aim of the present study is to investigate whether there is a relationship between miR-18a expression and radiosensitization of non-small-cell lung caner (NSCLC). The relationship between miR-18a expression and clinicopathological characteristics was investigated. To determine whether the miR-18a expression levels were associated with radiotherapeutic efficacy, therapeutic response was evaluated by radiologic Response Evaluation Criteria in Solid Tumors (RECIST). To determine whether miR-18a was required for lung cancer cell radioresistance, A549 cells were treated with different doses of ionizing radiation, following transfection with inhibitor miR-18a or inhibitor NC. We found that the level of miR-18a in NSCLC was strongly correlated with tumor differentiation (P = 0.026), regional lymph node metastasis (P = 0.013) and clinical TNM stage (P = 0.005). According to RECIST, miR-18a expression level was significantly associated with therapeutic response, exhibiting higher expression level in non-responsive patients. Furthermore, the depletion of miR-18a increased A549 cell radiosensitivity. In conclusion, we provide the evidence that down-regulation of miR-18a sensitizes NSCLC to radiation treatment, and it may help to develop a new approach to sensitizing radioresistant lung cancer cells by targeting miR-18a.
Keywords: MicroRNA, miR-18a, NSCLC, lung cancer, radiosensitivity
Introduction
Lung cancer is the leading cause of cancer-related mortality in the word and non-small-cell lung caner (NSCLC) is the most common form of lung cancer [1]. Radiotherapy remains an important form of local and regional cancer therapy [2]. Improving lung cancer radiation sensitivity can increase the local control and radiation effect [3,4]. Cellular radiosensitivity has been show to correlate with cell apoptosis, cell cycle, and repair of DNA damage. Therefore, identifying markers of radioresistance is an unmet need in the therapeutic management of lung cancer patients [5-7].
MicroRNAs (miRNAs) are a group of small non-coding RNAs which suppress their target expression by binding to the 3’ untranslated region (3’UTR). In previous decades, accumulating studies have demonstrated that miRNAs act as key regulators in the development and progression of various types of cancer, including lung cancer [8,9]. Previously, researchers have found that the expression of miR-18a was increased in lung cancer [10]. We are interested in whether there is a relationship between miR-18a expression and radiosensitization of NSCLC.
Materials and methods
Patients and tissue samples
A total of 120 pairs of fresh NSCLC and matched adjacent normal tissue specimens were collected from patients who underwent surgery at the department of thoracic surgery, Jinling Hospital. The fresh tissue specimens were collected and immediately placed in liquid nitrogen and then stored at -80°C until the isolation of RNA. The pathologic diagnosis was conducted by two pathologists, and any different conclusions were resolved by careful study and discussion. Tumor stage was determined according to the 2009 TNM staging classification system. Clinicopathological features of patients are summarized in Table 1. This study was approved by the Research Ethics Committee of Jinling Hospital. Written informed consent was obtained from all of the patients. All specimens were handled and made anonymous according to the ethical and legal standards.
Table 1.
Clinicopathological variables and the expression of miR-18a in total NSCLC patients
| Clinicopathological variables | Case number | miR-18a expression | P value | |
|---|---|---|---|---|
|
| ||||
| High (n = 58) | Low (n = 62) | |||
| Sex distribution | ||||
| Female | 54 | 26 | 28 | 0.551 |
| Male | 66 | 32 | 34 | |
| Age (years) | ||||
| < 60 | 72 | 31 | 41 | 0.214 |
| ≥ 60 | 48 | 27 | 21 | |
| Smoking history | ||||
| Current | 25 | 12 | 13 | 0.593 |
| Former | 83 | 41 | 42 | |
| Never | 12 | 5 | 7 | |
| Differentiation | ||||
| Poor | 33 | 22 | 11 | 0.026 |
| Well/Moderate | 87 | 36 | 51 | |
| TNM stage | ||||
| I-II | 71 | 52 | 19 | 0.005 |
| IIIa | 49 | 6 | 43 | |
| Regional lymph node involvement | ||||
| Absent | 78 | 39 | 39 | 0.013 |
| Present | 42 | 19 | 23 | |
Cell line
The human lung adenocarcinoma cell line A549 was purchased from the Cell Bank of Chinese Academy of Sciences (Shanghai, China). A549 cell line was cultured in DMEM containing 10% fetal bovine serum (Gibco®, Invitrogen, Carlsbad, CA, USA), 100 units/ml penicillin, and 100 μg/ml streptomycin at 37°C in a 5% CO2 humidified incubator to the log phase of proliferation before harvesting the cells. Normal human bronchial epithelial cells (NHBE) (Clonetics™) were maintained in a culture medium according to the protocol provided by Clonetics™.
MicroRNA isolation and real-time quantitative RT-PCR assay
Total miRNA from cultured cells and tissues was extracted using the mirVana miRNA Isolation Kit (Ambion, Austin, TX, USA) according to the manufacturer’s instructions. The purity and concentration of RNA were determined using NanoDrop 1000 spectrophotometer (Thermo Scientific, Wilmington, DE, USA). The differentially expressed amount of the miR-18a was validated in triplicate by quantitative reverse-transcription polymerase chain reaction (qRT-PCR). Briefly, 2 μg of RNA was added to RT reaction, and then, the cDNA served as the template for amplification of PCR with sequence-specific primers (Sangon Biotech, Shanghai, China) using SYBR PrimeScript miRNA RT-PCR kit (Takara Biotechnology Co. Ltd, Dalian, China) on the 7500 Real-Time PCR systems (Applied Biosystems, Carlsbad, CA, USA). The PCR cycling profile was denatured at 95°C for 30 s, followed by 40 cycles of annealing at 95°C for 5 s, and extension at 60°C for 34 s. Small nucleolar RNA U6 was used as an internal standard for normalization. The cycle threshold (CT) value was calculated. The 2-ΔCT (ΔCT = CTmiR18a-CTU6 RNA) method was used to quantify relative amount of miR-18a.
Cell transfection
For the transfection, 1 × 106 cells were treated with 50 nM GMR-miRTM inhibitor miR-18a/inhibitor NC (Shanghai GenePharma) in 1 μl of LipofectamineTM2000 (Invitrogen, USA) according to the manufacturer’s instructions.
Radiation exposure
The cells were seeded into 96-well plates and treated with a range of radiation doses (0-10 Gy) using a 250-kV orthovoltage unit the following day (Philips, Amsterdam, The Netherlands).
Colony-forming assay
A colony-forming assay was performed to determine the radiosensitivity of cells. Cells (1 × 105/well) were plated in a 24-well plate and transfected with inhibitor miR-18a/inhibitor NC at 20 nmol/l by using Lipofectamine TM2000 (Invitrogen). After 48 h, the cells were collected and seeded (500-1,000/well) in a fresh six-well plates. After 12 days, visible colonies were fixed and stained with crystal violet. A population of > 50 cells was counted as one colony. The number of clones was examined using macroscopic observation.
Statistical analysis
The quantitative values were expressed as mean ± standard deviation (SD), and the hypothesis test for significance between two groups utilized the Student’s t test. The chi-square test and Fishers exact tests were used to examine the associations between miR-18a expression and the clinicopathological characters. Statistical significance was set as P < 0.05. Statistical analyses were performed using SPSS 18.0 soft-ware (Chicago, Ill., USA) and GraphPad Prism 5 (GraphPad Software Inc., CA, USA).
Results
Expression of miR-18a in lung cancer tissues and A549 cell line
To define the role of miR-18a in human lung cancer tumorigenesis, we compared the expression levels of miR-18a in tissue samples, A549 lung cancer cell, and NHBE cell line. As shown in Figure 1, the expression levels of miR-18a were up-regulated in lung cancer tissues as compared to matched normal tissues from the same patients. The expression of miR-18a in the A549 lung cancer cell line was significantly increased as compared to NHBE cell line (shown in Figure 2).
Figure 1.
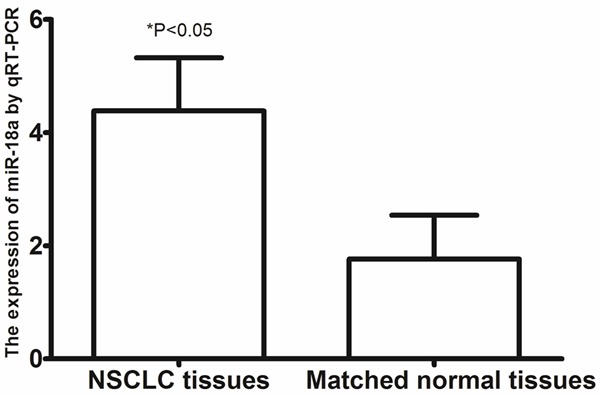
The expression of miR-18a in lung cancer tissues and matched normal tissues.
Figure 2.
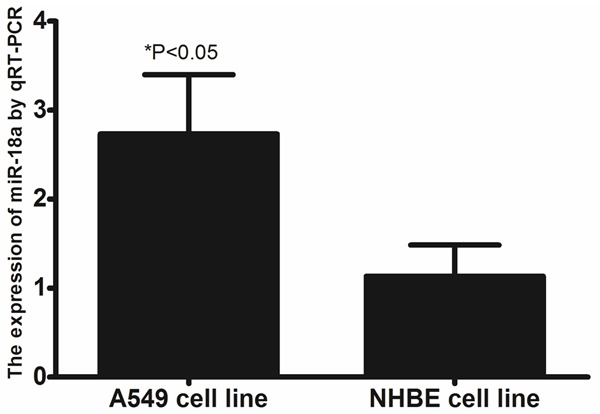
The expression of miR-18a in A549 lung cancer cell line, and NHBE cell line.
Relationships between expression of miR-18a and clinical parameters in lung cancer patients
The relationship between miR-18a expression and clinicopathologic parameters of 120 patients with NSCLC was evaluated. As shown in Table 1, the level of miR-18a in NSCLC was strongly correlated with tumor differentiation (P = 0.026), regional lymph node metastasis (P = 0.013) and clinical TNM stage (P = 0.005). However, there were no significant associations between miR-18a expression and other clinical features including sex (P = 0.551), age (P = 0.214), and smoking status (P = 0.593).
MiR-18a expression correlated with radioresistance of lung cancer patients
To determine whether the miR-18a expression levels were associated with radiotherapeutic efficacy, therapeutic response was evaluated by radiologic Response Evaluation Criteria in Solid Tumors (RECIST). According to RECIST, 65% patients responded to radiotherapy with complete response or partial response; 35% patients were not responsive with stable disease or disease progression. The result showed that miR-18a was significantly associated with therapeutic response, exhibiting higher expression level in non-responsive patients (P = 0.019, shown in Table 2).
Table 2.
Correlation of miR-18a level and response to radiotherapy
| miR-18a expression | ||||
|---|---|---|---|---|
|
|
||||
| Treatment response | All case (n = 120) | High (n = 58) | Low (n = 62) | P value |
| Complete response + partial response | 78 | 30 | 48 | 0.019 |
| Stable disease + disease progression | 42 | 28 | 14 | |
MiR-18a regulated sensitivity of A549 lung cancer cells to radiation
To determine whether miR-18a was required for lung cancer cell radioresistance, A549 cells were treated with different doses of ionizing radiation, from 0 to 10 Gy, following transfection with inhibitor miR-18a or inhibitor NC. Ionizing radiation treatment exhibited a dose-dependent inhibitory effect on the growth of the A549 cells, and the miR-18a depletion enhanced this effect, suggesting that the depletion of miR-18a increased A549 cell radiosensitivity (shown in Figure 3).
Figure 3.
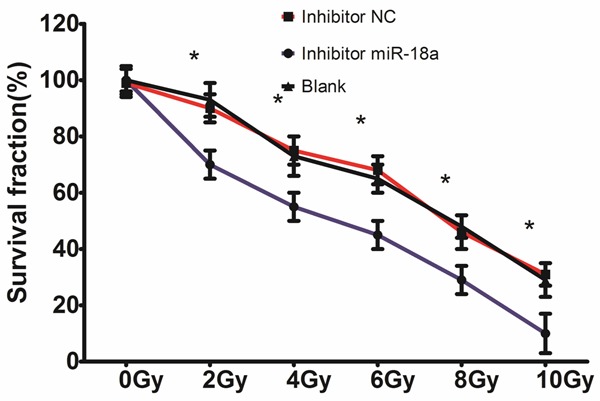
MiR-18a-regulating sensitivity of A549 cells to radiation. The A549 cells transfected with inhibitor miR-18a or inhibitor NC were treated with 0, 2, 4, 6, 8, and 10 Gy irradiation, and survival curves were determined using a colony-forming assay. *P < 0.05.
Discussion
Radiation therapy is one of the most important methods in cancer treatment. However, many types of cancer show radioresistance, thereby affecting the efficacy of radiotherapy in clinical practice. Methods for improving radiosensitivity and reducing radioresistance are accordingly of great interest in cancer radiotherapy [11]. The factors that affect the consequences of radiotherapy include the capability of cancer cells to develop radioresistance, the degree of tumor tissue hypoxia, and the survival ability of the remaining cancer cells postradiotherapy [12]. Thus, overcoming cancer radioresistance is important for achieving better clinical outcomes for patients.
Several previous research studies have shown that miRNAs were closely related to tumor radiosensitivity. This is because miRNAs have the ability to increase and decrease radiosensitivity of tumors [13-15]. Given that miRNAs have the ability to regulate multiple oncogenic processes such as responsiveness to therapy, we must explore the role of miRNAs in radiation resistance.
miR-18a belongs to the miR-17-92 cluster located in the 13q31.1 region, an area partly regulated by the oncogenic transcription factor c-Myc [16]. miR-17-92 cluster consists of seven miRNAs: miR-17-3p, miR-17-5p, miR-18a, miR-19a, miR-19b, miR-20a, and miR-92a. Previous studies have confirmed that the miR-17-92 cluster is highly expressed in several types of cancers, such as gastric cancer, breast cancer, melanoma, osteosarcoma, and so on [17-21]. The oncogenic role of the miR-17-92 cluster has also been well-documented, with its overexpression linked to accelerated tumour growth, cell proliferation and progression. Previously, Krysan et al found that prostaglandin E2, abundantly produced by NSCLC and inflammatory cells in the tumor microenvironment, was able to stimulate cell proliferation and promote resistance to pharmacologically induced apoptosis in a c-Myc and miR-17-92-dependent manner [22]. In the present study, we investigated if miR-18a could play an important role in the development of radiosensitization in NSCLC. We found that the expression levels of miR-18a were up-regulated in lung cancer tissues and the A549 lung cancer cell line. Furthermore, the level of miR-18a in NSCLC was strongly correlated with tumor differentiation, regional lymph node metastasis and clinical TNM stage. To determine whether the miR-18a expression levels were associated with radiotherapeutic efficacy, therapeutic response was evaluated by RECIST. According to RECIST, 65% patients responded to radiotherapy with complete response or partial response; 35% patients were not responsive with stable disease or disease progression. The result showed that miR-18a was significantly associated with therapeutic response, exhibiting higher expression level in non-responsive patients. To determine whether miR-18a was required for lung cancer cell radioresistance, A549 cells were treated with different doses of ionizing radiation, from 0 to 10 Gy, following transfection with inhibitor miR-18a or inhibitor NC. Ionizing radiation treatment exhibited a dose-dependent inhibitory effect on the growth of the A549 cells, and the miR-18a depletion enhanced this effect, suggesting that the depletion of miR-18a increased A549 cell radiosensitivity. Previously, Song et al found an important link between miR-18a-impaired DNA damage response and downregulation of ataxia-telangiectasia mutated (ATM) kinase in breast cancer. Their findings revealed the significance of miR-18a in regulating cell cycle checkpoints, double strand breaks (DSBs) repair and radiosensitivity in breast cancer. To the best of our knowledge, our study was the first to confirm the effect of miR-18a overexpression on the radiosensitivity of NSCLC.
In conclusion, we provide the evidence that down-regulation of miR-18a sensitizes NSCLC to radiation treatment, and it may help to develop a new approach to sensitizing radioresistant lung cancer cells by targeting miR-18a.
Disclosure of conflict of interest
None.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Webb S. Advances in three-dimensional conformal radiation therapy physics with intensity modulation. Lancet Oncol. 2000;1:30–36. doi: 10.1016/S1470-2045(00)00110-8. [DOI] [PubMed] [Google Scholar]
- 3.Hennon MW, Yendamuri S. Advances in lung cancer surgery. J Carcinog. 2012;11:21. doi: 10.4103/1477-3163.105341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saadeddin A. Radiotherapy for NSCLC: review of conventional and new treatment techniques. J Infect Public Health. 2012;5:S45–49. doi: 10.1016/j.jiph.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 5.Joubert A, Foray N. [Intrinsic radiosensitivity and DNA double-strand breaks in human cells] . Cancer Radiother. 2007;11:129–142. doi: 10.1016/j.canrad.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 6.Ding J, Miao ZH, Meng LH, Geng MY. Emerging cancer therapeutic opportunities target DNA-repair systems. Trends Pharmacol Sci. 2006;27:338–344. doi: 10.1016/j.tips.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 7.Hinz JM, Yamada NA, Salazar EP, Tebbs RS, Thompson LH. Influence of double-strand-break repair pathways on radiosensitivity throughout the cell cycle in CHO cells. DNA Repair. 2005;4:782–792. doi: 10.1016/j.dnarep.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 8.Del Vescovo V, Grasso M, Barbareschi M, Denti MA. MicroRNAs as lung cancer biomarkers. World J Clin Oncol. 2014;5:604–620. doi: 10.5306/wjco.v5.i4.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y, Yang Q, Wang S. MicroRNAs: a new key in lung cancer. Cancer Chemother Pharmacol. 2014;74:1105–1111. doi: 10.1007/s00280-014-2559-9. [DOI] [PubMed] [Google Scholar]
- 10.Mairinger FD, Ting S, Werner R, Walter RF, Hager T, Vollbrecht C, Christoph D, Worm K, Mairinger T, Sheu-Grabellus SY, Theegarten D, Schmid KW, Wohlschlaeger J. Different micro-RNA expression profiles distinguish subtypes of neuroendocrine tumors of the lung: results of a profiling study. Mod Pathol. 2014;27:1632–1640. doi: 10.1038/modpathol.2014.74. [DOI] [PubMed] [Google Scholar]
- 11.Chatterjee S, Willis N, Locks SM, Mott JH, Kelly CG. Dosimetric and radiobiological comparison of helical tomotherapy, forward-planned intensity-modulated radiotherapy and two-phase conformal plans for radical radiotherapy treatment of head and neck squamous cell carcinomas. Br J Radiol. 2011;84:1083–1090. doi: 10.1259/bjr/53812025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joubert A, Vogin G, Devic C, Granzotto A, Viau M, Maalouf M, Thomas C, Colin C, Foray N. [Radiation biology: major advances and perspectives for radiotherapy] . Cancer Radiotherapie. 2011;15:348–354. doi: 10.1016/j.canrad.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Deng X, Ma L, Wu M, Zhang G, Jin C, Guo Y, Liu R. miR-124 radiosensitizes human glioma cells by targeting CDK4. J Neurooncol. 2013;114:263–274. doi: 10.1007/s11060-013-1179-2. [DOI] [PubMed] [Google Scholar]
- 14.Babar IA, Czochor J, Steinmetz A, Weidhaas JB, Glazer PM, Slack FJ. Inhibition of hypoxia-induced miR-155 radiosensitizes hypoxic lung cancer cells. Cancer Biol Ther. 2011;12:908–914. doi: 10.4161/cbt.12.10.17681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li G, Wang Y, Liu Y, Su Z, Liu C, Ren S, Deng T, Huang D, Tian Y, Qiu Y. miR-185-3p regulates nasopharyngeal carcinoma radioresistance by targeting WNT2B in vitro. Cancer Sci. 2014;105:1560–1568. doi: 10.1111/cas.12555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Donnell KA, Wentzel EA, Zeller KI, Dang CV, Mendell JT. c-Myc-regulated microRNAs modulate E2F1 expression. Nature. 2005;435:839–843. doi: 10.1038/nature03677. [DOI] [PubMed] [Google Scholar]
- 17.Strickertsson JA, Rasmussen LJ, Friis-Hansen L. Enterococcus faecalis Infection and Reactive Oxygen Species Down-Regulates the miR-17-92 Cluster in Gastric Adenocarcinoma Cell Culture. Genes. 2014;5:726–738. doi: 10.3390/genes5030726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liao YC, Lin TH, Chen CY, Lin SB, Au LC. The antileukemia activity of natural product HQ17(3) is possibly associated with downregulation of miR-17-92 cluster. BioMed Res Int. 2014;2014:306718. doi: 10.1155/2014/306718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arabi L, Gsponer JR, Smida J, Nathrath M, Perrina V, Jundt G, Ruiz C, Quagliata L, Baumhoer D. Upregulation of the miR-17-92 cluster and its two paraloga in osteosarcoma - reasons and consequences. Genes Cancer. 2014;5:56–63. doi: 10.18632/genesandcancer.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greenberg E, Hajdu S, Nemlich Y, Cohen R, Itzhaki O, Jacob-Hirsch J, Besser MJ, Schachter J, Markel G. Differential regulation of aggressive features in melanoma cells by members of the miR-17-92 complex. Open Biol. 2014;4:140030. doi: 10.1098/rsob.140030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jin L, Lim M, Zhao S, Sano Y, Simone BA, Savage JE, Wickstrom E, Camphausen K, Pestell RG, Simone NL. The metastatic potential of triple-negative breast cancer is decreased via caloric restriction-mediated reduction of the miR-17~92 cluster. Breast Cancer Res Treat. 2014;146:41–50. doi: 10.1007/s10549-014-2978-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krysan K, Kusko R, Grogan T, O’Hearn J, Reckamp KL, Walser TC, Garon EB, Lenburg ME, Sharma S, Spira AE, Elashoff D, Dubinett SM. PGE2-driven expression of c-Myc and oncomiR-17-92 contributes to apoptosis resistance in NSCLC. Mol Cancer Res. 2014;12:765–774. doi: 10.1158/1541-7786.MCR-13-0377. [DOI] [PMC free article] [PubMed] [Google Scholar]


