Abstract
The imperfections of scaffold materials have hindered the clinical application of cartilage tissue engineering. The recently developed cell-sheet technique is adopted to engineer tissues without scaffold materials, thus is considered being potentially able to overcome the problems concerning the scaffold imperfections. This study constructed monolayer and bilayer chondrocyte cell sheets and harvested the sheets with cell scraper instead of temperature-responsive culture dishes. The properties of the cultured chondrocyte cell sheets and the feasibility of cartilage engineering using the chondrocyte cell sheets was further investigated via in vitro and in vivo study. Primary extracellular matrix (ECM) formation and type II collagen expression was detected in the cell sheets during in vitro culture. After implanted into nude mice for 8 weeks, mature cartilage discs were harvested. The morphology of newly formed cartilage was similar in the constructs originated from monolayer and bilayer chondrocyte cell sheet. The chondrocytes were located within evenly distributed ovoid lacunae. Robust ECM formation and intense expression of type II collagen was observed surrounding the evenly distributed chondrocytes in the neocartilages. Biochemical analysis showed that the DNA contents of the neocartilages were higher than native human costal cartilage; while the contents of the main component of ECM, glycosaminoglycan and hydroxyproline, were similar to native human costal cartilage. In conclusion, the chondrocyte cell sheet constructed using the simple and low-cost technique is basically the same with the cell sheet cultured and harvested in temperature-responsive culture dishes, and can be used for cartilage tissue engineering.
Keywords: Cell sheet, tissue engineering, cartilage, chondrocyte, extracellular matrix, type II collagen
Introduction
Current approaches for external ear reconstruction for patients with microtia or acquired auricular defect contain of autologous costal cartilage sculpturing and transplantation, alloplastic implants and prosthetic ears. However, these approaches often result in suboptimal aesthetic outcomes and may lead to various complications [1], thus more reasonable alternative strategies are in need.
Cartilage tissue engineering, to artificially construct cartilaginous tissue using autologous chondrocytes or stem cells and biodegradable scaffold material, seems to be an ideal strategy for cartilage reconstruction [2]. Numerous studies have demonstrated its feasibility both in vitro and in vivo [2,3]. However, many obstacles have yet to be overcome, problems relating to cell sources and scaffold imperfections remain. Synthetic polymers and natural materials have been adopted to deliver cells into cartilage defect sites, and to reinforce the mechanical stability of three-dimensional tissue engineered chondral grafts [4-8]. Some scaffolds have been successfully applied for the cartilage engineering; however, there are problems with biocompatibility and cellular viability. Furthermore, the exogenous scaffold materials may generate degradation products that cause an inflammatory reaction and negatively affect neocartilage formation in vivo.
In the 1990s, a cell-sheet technique based on a temperature-responsive culture dish was developed [9,10], which was thereafter adopted to engineer tissues without scaffold materials. The new strategy has been considered being potentially able to overcome the problems concerning the scaffold imperfections. Although the temperature-responsive culture dishes (UpCell™ CellSeed Inc., Tokyo, Japan) is now commercially available, it is quite expensive. Moreover, the temperature-responsive coating polymer, poly (N-isopropylacrylamide) (PNIAM) is another extrinsic material, which is potentially associated with the problems similar to the scaffold materials. In this study, we tried another cell-sheet culturing method without the temperature-responsive culture dishes. The purpose of this study was to investigate the feasibility of human chondrocyte-sheet culture using conventional culture dishes and further investigate the properties of the cultured chondrocyte-sheets in vitro and in vivo.
Materials and methods
Cell source
A patient with microtia, female, 14-year old, was subjected to total auricle reconstruction. Autologous costal cartilage was harvested and sculptured and satisfactory auricle was reconstructed. The leftover cartilage chippings was collected and used for primary chondrocyte isolation. This study was performed in compliance with the Helsinki Declaration, and was approved by the Ethics Committee of the 306th Hospital of PLA. Informed consents of the laboratory usage of their tissues for scientific study were signed by the two parents. The animal study was approved by the Institutional Animal Care and Use Committee of the 306th Hospital of PLA and all procedures were performed according to the Guidelines for the Care and Use of Laboratory Animals of the 306tth Hospital of PLA.
Chondrocyte culture and cell sheet construction
The costal cartilage chippings (4.8 g wet weight) were cut into 1 mm3 fragments and treated with 0.1% collagenase type II (Invitrogen Corporation, Grand Island, NY, USA) in a D-MEM/F-12 medium (Gibco, Grand island, NY, USA) with 1% antibiotic/antimycotic solution (Beijing Solarbio Science & Technology Co., Ltd, Beijing, China) at 37°C for 16 h. The digested cell suspension was filtered through a cell strainer of 100 μm pore size (BD Biosciences, Bedford, MA, USA), and then the chondrocytes were isolated. The chondrocytes were then plated into 10 cm cell culture dish (430167, Corning Incorporated life sciences, Tewksbury, MA, USA) at a density of 10,000 cells/cm2 and cultured with D-MEM/F-12 medium medium supplemented with 10% fetal bovine serum (Hangzhou Sijiqing Biological Engineering Materials Co., Ltd., Hangzhou, China), 100U/mL penicillin and 100 mg/mL streptomycin (Beijing Solarbio Science & Technology Co., Ltd, Beijing, China), 292 mg/mL L-glutamine (Sigma-Aldrich, St. Louis, MO, USA), 0.1 mM nonessential amino acids (Beijing Solarbio Science & Technology Co., Ltd, Beijing, China), and 50 mg/mL L-ascorbic acid (Sigma-Aldrich, St. Louis, MO, USA). Upon reaching confluence, the chondrocytes were trypsinized with 0.25% trypsin-EDTA (Beijing Solarbio Science & Technology Co., Ltd, Beijing, China). Cell passaging was performed in a dilution of 1:4. The third passage cells were used for fabrication of chondrocyte cell sheets. Continuous culture was allowed after the chondrocytes had reached 100% confluent. Two weeks later, a thin film formed in the cell culture dish, which was found containing a single layered chondrocytes and gelatinous chondroid extracellular matrix (ECM) under inverted microscope. To create bilayer cell sheets, frozen second passage chondrocytes were thawed and seeded at a density of 4 × 104 cells/cm2 over the third passage chondrocytes upon 100% confluent reached.
The cell sheet was released from the bottom of the dish with a sterile cell scraper (Corning Incorporated life sciences, Tewksbury, MA, USA). The released cell sheets were cultured in vitro for 12 days with chondrocyte growth medium. During the in vitro culturing, the cell sheet gradually curled up and became a gelatinous chondroid mass (Figure 1). At the end of 12-day in vitro culturing, six monolayer and six bilayer chondrocyte cell sheets were harvested and processed for histological, immunohistological, and biochemical evaluation.
Figure 1.
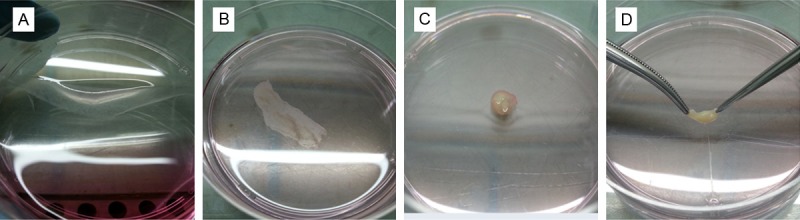
Cell sheet harvest and in vitro culture. A: Half of the cell sheet was released from the bottom of the dish; B: In vitro culture of the released cell sheet at day 2; C & D: In vitro culture of the released cell sheet at day 14.
Construct implantation
Twenty four gelatinous chondroid constructs, 12 comprised of monolayer chondrocyte cell sheet, 12 comprised of bilayer chondrocyte cell sheet, were implanted subcutaneously on the backs of 6 8-week-old female BALB nude mice (Vital River Laboratories, Beijing, China), 4 constructs per mouse. General anesthesia was achieved with intramuscular injection of ketamine 20 mg/kg (Gutian Pharmaceutical co., LTD, Fuzhou, Fujian Province, China) and xylazine hydrochloride 0.3 ml/kg (Huamu Animal Health products co., LTD, Changchun, Jilin Province, China). Under aseptic conditions, 3 separated subcutaneous pockets were created through horizontal incisions and blunt dissections. A gelatinous chondroid construct was carefully inserted into the subcutaneous pocket and then sutured with non-absorbable monofilament suture, which was removed after 7 days.
Gross evaluation and histology
Eight weeks after the implantation, the mice were sacrificed by cervical dislocation euthanasia. The specimens were harvested and carefully dissected from the surrounding tissue. Each sample was split into two halves; one half was processed for histological evaluation, the other half was stored at -80°C and used for biochemical testing. Samples for histology were fixed in 10% neutral buffered formalin for longer than 24 hours and then embedded in paraffin wax according to embedding machine manufactures instructions. Sections of 5 µm thickness were prepared on the microtome (Leica). The sections were stained with hematoxylin and eosin; ECM formation in the newly formed cartilage tissue was evaluated with toluidine blue and safranin O staining.
Immunohistochemistry
Sections were heated in tissue-drying oven for 45 minutes at 60°C, and then deparaffinized in sequential xylene and rehydrated in graded ethanol baths. Antigen retrieval was performed in 10 mM sodium citrate buffer, pH 6.0 at 95-100°C for 20 minutes. Background signal was blocked using 5% goat serum for 30 minutes at room temperature. Sections were incubated with rabbit polyclonal anti-Collagen II antibody (ab34712, Abcam, Cambridge, MA) 45 minutes at room temperature, and horseradish peroxidase conjugated goat anti-rabbit IgG (Boster Biological Technologies, Wuhan, Hubei Province, China) 30 minutes at room temperature. The color of the antibody staining was revealed using diaminobenzidine substrate solution (ZSGB Biological Technologies, Beijing, China), and counterstained with hematoxylin.
Quantitative DNA and ECM analyses
Frozen samples from the in vitro and in vivo studies were used for the quantitative DNA and ECM analyses. Half of the samples were weighed, minced and digested with 10% proteinase K (Sigma-Aldrich) at 56°C overnight. The DNA extraction and purification was performed using a Qiagen DNeasy kit (Qiagen, Inc., Valencia, CA) according to the manufacturer’s instructions. Total DNA content was detected using a PicoGreen dsDNA assay. To investigate the components of the ECM, the other half of the samples were lyophilized, weighed and digested with papain solution (125 mg/mL papain type III, 100 mM phosphate, 10 mM l-cysteine, and 10 mM ethylenediaminetetraacetic acid, pH 6.3) at 60°C for 16 h. Aliquots of these digests were used for glycosaminoglycan (GAG) and hydroxyproline (HP) content assay. GAG content was spectrophotometrically measured using dimethylmethylene blue dye (Biocolor Ltd., Carrickfergus, United Kingdom) with chondroitin sulfate as a standard. HP content was measured using Stegemann’s hydroxyproline assay. The content of the DNA and ECM components of the native human costal cartilage was also measured using the same methods.
Statistical analysis
The biochemical analyses values are expressed as mean ± standard deviation. Statistical analyses were performed using SPSS 17.0 (SPSS, Chicago, IL). Comparison of means was assessed by a one-way analysis of variance and the Tukey HSD test was used for multiple comparisons (P < 0.05 was considered significant).
Results
The primary chondrocyte isolation had a yield of 2 million cells per gram of costal cartilage. In the in vitro cultures, the costal chondrocytes secreted rich gelatinous ECM upon reaching high confluence. Both the monolayer and bilayer chondrocyte cell sheets formed soft tremelloid tissues. No apparent difference was noted. The tissues showed high fluidity and gelling property. Condensed arranged cells were observed under inverted microscope. Histologically intact thin films comprise of layered chondrocytes and ECM was observed (Figure 2).
Figure 2.
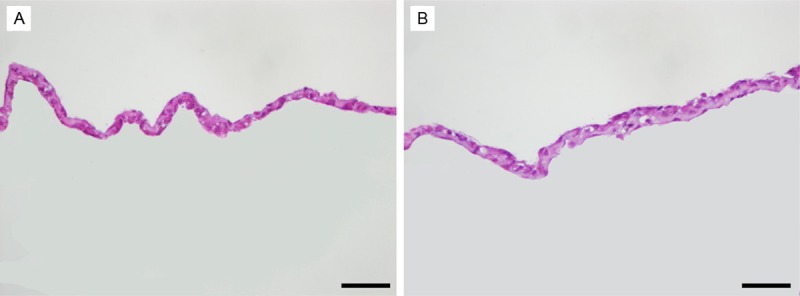
Histology of the chondrocyte cell sheets. A: Monolayer chondrocyte cell sheet; B: Bilayer chondrocyte cell sheet. Original magnification: ×400. Scale bar, 50 μm.
By the end of the 12-day in vitro culturing, the chondrocyte cell sheets shrank and became a gelatinous chondroid mass (Figure 1C and 1D). Although the shrunk cartilage-like tissues showed lower fluidity but still appeared pulpy and fragile non-transparent gel. Histological examination of the neutral formalin fixed in vitro cultured samples indicated that folded chondrocyte cell sheet stack up. The folded sheet became thicker. The neighboring sheets adhered to each other due to consecutive ECM secretion. Primary ECM formation was detected by safranin O and toluidine blue staining (Figure 3). Immunohistochemistry staining indicated that type II collagen expression in the ECM was initiated during the in vitro culturing of the chondrocyte cell sheets (Figure 3).
Figure 3.
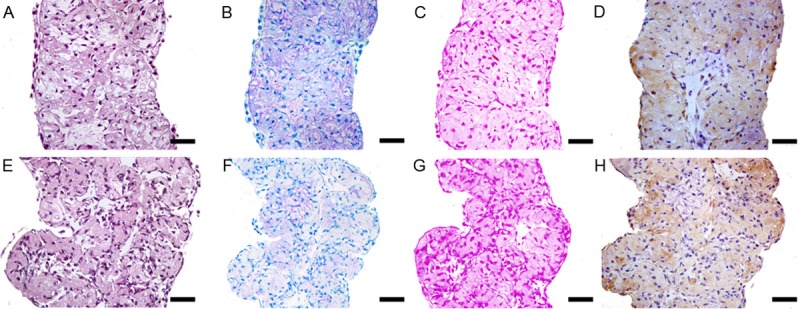
Histology and immunohistochemical examination of the in vitro cultured cell sheet. A-D: Monolayer chondrocyte cell sheet; E-H: Bilayer chondrocyte cell sheet. A & E: HE staining; B & F: Toluidine blue staining; C & G: Safranin O staining; D & H: Immunohistochemical staining of type II collagen. Original magnification: ×400. Scale bar, 50 μm.
After these cultured cell sheets together with the gelatinous chondroid matrix were implanted subcutaneously into nude mice for 8 weeks, mature cartilage discs were harvested. All constructs were surrounded by fibrous tissue that could be easily removed. Grossly, the tissue resembled cartilage discs, both monolayer and bilayer chondrocyte cell sheet originated, were flexible. There was no obvious difference between the volumes of cartilage blocks made from the bilayer chondrocyte cell sheets and those from monolayer chondrocyte cell sheets (Figure 4).
Figure 4.
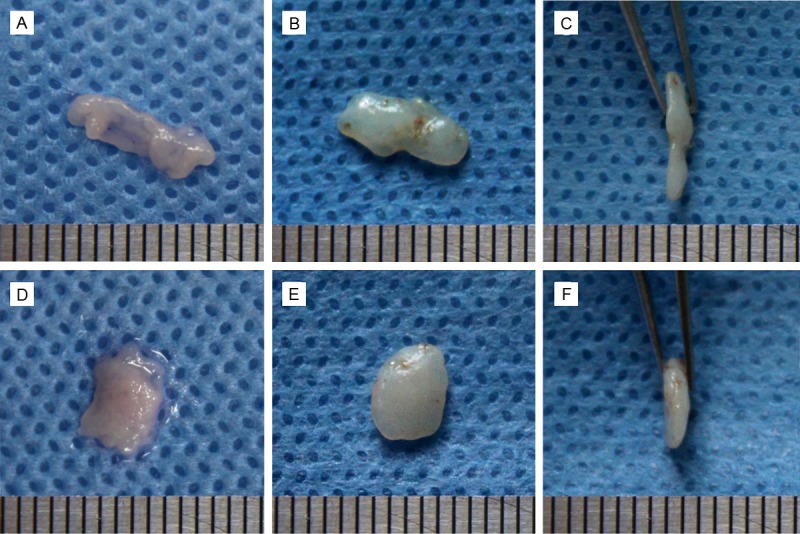
Gross views of the in vitro and in vivo samples. A: Monolayer chondrocyte cell sheet in vitro cultured for 2 weeks; B & C: Cartilage originated from monolayer chondrocyte cell sheet, after 8 weeks implanted in nude mice; D: Bilayer chondrocyte cell sheet in vitro cultured for 2 weeks; E & F: Cartilage originated from monolayer chondrocyte cell sheet, after 8 weeks implanted in nude mice.
The morphology of newly formed cartilage was similar in the constructs originated from monolayer and bilayer chondrocyte cell sheet. The chondrocytes were located within evenly distributed ovoid lacunae. Robust ECM formation in the neocartilages was demonstrated with safranin O and toluidine blue staining, which indicated the presence of abundant sulfated GAG. Immunohistochemistry staining demonstrated that type II collagen, a specific protein of hyaline cartilage, was intensely expressed in the ECM (Figure 5).
Figure 5.
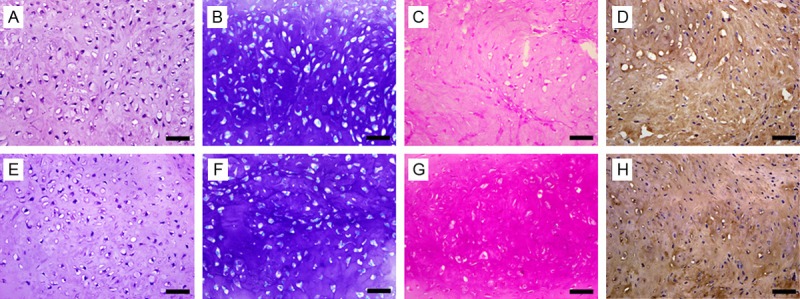
Histology and immunohistochemical examination of the engineered cartilage. A-D: Cartilage originated from monolayer chondrocyte cell sheet; E-H: Cartilage originated from bilayer chondrocyte cell sheet. A & E: HE staining; B & F: Toluidine blue staining; C & G: Safranin O staining; D & H: Immunohistochemical staining of type II collagen. Original magnification: ×400. Scale bar, 50 μm.
The DNA content of the engineered cartilage tissues was similar in the constructs originated from monolayer and bilayer chondrocyte cell sheet, and greater than that of native human costal cartilage (Figure 6, P = 0.039), which indicated that the density of chondrocyte in the neocartilage was higher than that in the native costal cartilage. The content of GAG in the two sorts of engineered constructs was similar. The GAG content of engineered cartilage was similar to that of native human costal cartilage (Figure 7, P = 0.242). Also, no significant difference was found in the HP content of the engineered cartilage tissues and native human costal cartilage (Figure 8, P = 0.375).
Figure 6.
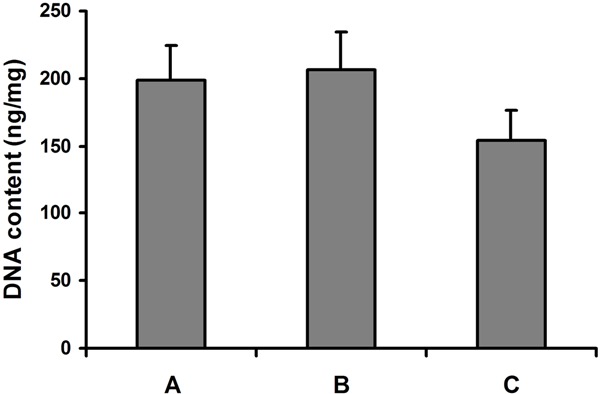
DNA content of the engineered cartilage and native human costal cartilage. A: Cartilage originated from monolayer chondrocyte cell sheet; B: Cartilage originated from bilayer chondrocyte cell sheet; C: Native human costal cartilage.
Figure 7.
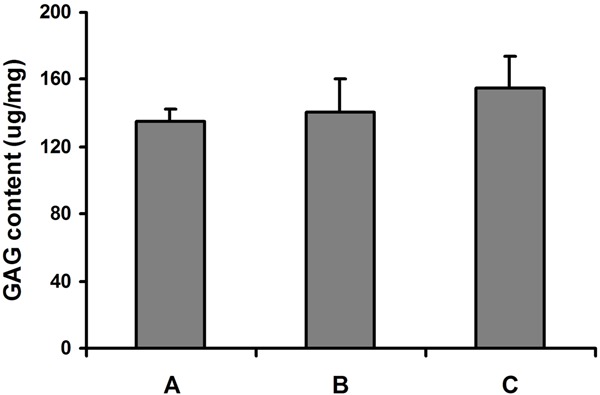
Glycosaminoglycan content of the engineered cartilage and native human costal cartilage. A: Cartilage originated from monolayer chondrocyte cell sheet; B: Cartilage originated from bilayer chondrocyte cell sheet; C: Native human costal cartilage.
Figure 8.
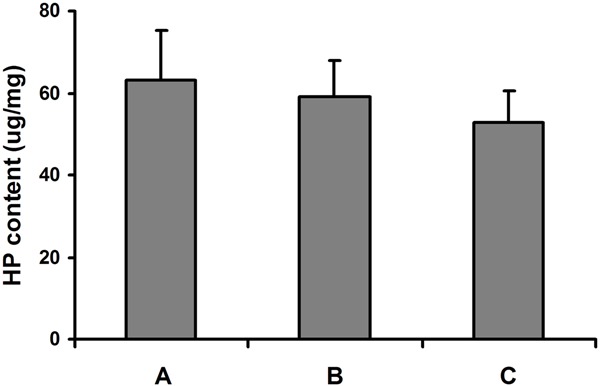
Hydroxyproline content of the engineered cartilage and native human costal cartilage. A: Cartilage originated from monolayer chondrocyte cell sheet; B: Cartilage originated from bilayer chondrocyte cell sheet; C: Native human costal cartilage.
Discussion
Classically, scaffold materials have been an essential in tissue engineering [2,3]. Over decades, various biomaterials have been utilized for cartilage tissue engineering. However, imperfections remain in all kinds of the scaffold materials, either synthetic polymers or natural materials, which lead to unsatisfactory outcomes that are far from achieving native cartilage architecture and function. The recently developed cell-sheet technique, based on a temperature-responsive culture dish, is considered being potentially able to overcome the imperfections of scaffold materials. Since the new technique introduced a new strategy for cartilage regeneration without a scaffold [9,10].
The key element of the temperature-responsive culture dish is a temperature-responsive polymer, poly (nisoproplyacrylamide), which is coated on the surface of the dishes for cell culturing. The polymer is reversible hydrophobic-hydrophilic across the threshold temperature of 32°C. At the standard culture temperature of 37°C, the surface of the polymer coating is hydrophobic, thus allow the seeded chondrocytes adhering to via ECM proteins and cell membrane proteins. After the chondrocytes are cultured to confluence, they attach to the neighboring cells via cell-to-cell junction proteins and ECM proteins. Below the critical responsive temperature of 32°C, the polymer surface become hydrophilic and protein non-adhesive, leading to spontaneous cell detachment with intact cell-ECM structure, which is known as cell-sheet [9,10].
In this study, conventional cell culture dish was used for chondrocyte cultivation and cell scraper was adopted to harvest the chondrocyte cell sheet. The results have demonstrated that the chondrocyte cell sheet in this study was basically the same with the cell sheet cultured and harvested in temperature-responsive culture dishes. We performed additional two-week cultivation after confluence to allow more ECM secretion. Thus the chondrocyte cell sheet could withstand the shearing force when scraping harvest. However, since the cell sheet is harvested by mechanical force, it is vulnerable to damage or breakage. Therefore, careful handling is of great importance to obtain an intact cell sheet without damage.
Different to the classical cartilage tissue engineering that constructing cartilage via seeding isolated chondrocytes or stem cells into scaffold materials, the cell-sheet technique maintains the cell-cell junction and ECM proteins, and allows tissue formation in a natural way. In contrast to cell injection [11], the cell sheet provides a 3-dimensional environment and minimizes cell loss. The thin chondral sheet can be potentially used to repair tissue defects [12-14], such as defects on the surface of articular cartilage [15,16]. The primary chondrocyte cell sheet has good liquidity, thus can be used for injecting transplantation when being cut into small pieces. Since there is no scaffold material to help maintaining 3-dimensional shape, it is difficult to construct specific shaped tissue or organ using the cell sheet technique. This problem can be solved by application of molding technique.
Compared to the temperature-responsive culture system, our technique is much more low-cost and easy to operate. The feasibility of construction cartilage tissue using the new cell sheet technique has been proved by the in vitro and in vivo study. Moreover, this study demonstrated that bilayered cell sheet could be constructed by onlay cell seeding. In the future study, we are going to investigate cell sheets with more layers and try to construct cartilage block with larger volume.
Acknowledgements
This work was supported by the General Financial Grant from the China Postdoctoral Science Foundation (Grant No. 2014M552651) and the National Natural Science Foundation of China (Grant No. 81401609).
Disclosure of conflict of interest
None.
References
- 1.Bauer BS. Reconstruction of microtia. Plast Reconstr Surg. 2009;124:14e–26e. doi: 10.1097/PRS.0b013e3181aa0e79. [DOI] [PubMed] [Google Scholar]
- 2.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 3.Bhardwaj N, Devi D, Mandal BB. Tissue-Engineered Cartilage: The Crossroads of Biomaterials, Cells and Stimulating Factors. Macromol Biosci. 2014 doi: 10.1002/mabi.201400335. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 4.Zhou L, Pomerantseva I, Bassett EK, Bowley CM, Zhao X, Bichara DA, Kulig KM, Vacanti JP, Randolph MA, Sundback CA. Engineering ear constructs with a composite scaffold to maintain dimensions. Tissue Eng Part A. 2011;17:1573–1581. doi: 10.1089/ten.TEA.2010.0627. [DOI] [PubMed] [Google Scholar]
- 5.Cao Y, Vacanti JP, Paige KT, Upton J, Vacanti CA. Transplantation of chondrocytes utilizing a polymercell construct to produce tissue-engineered cartilage in the shape of a human ear. Plast Reconstr Surg. 1997;100:297–302. doi: 10.1097/00006534-199708000-00001. discussion 303-304. [DOI] [PubMed] [Google Scholar]
- 6.Kusuhara H, Isogai N, Enjo M, Otani H, Ikada Y, Jacquet R, Lowder E, Landis WJ. Tissue engineering a model for the human ear: assessment of size, shape, morphology, and gene expression following seeding of different chondrocytes. Wound Repair Regen. 2009;17:136–146. doi: 10.1111/j.1524-475X.2008.00451.x. [DOI] [PubMed] [Google Scholar]
- 7.Kim WS, Vacanti JP, Cima L, Mooney D, Upton J, Puelacher WC, Vacanti CA. Cartilage engineered in predetermined shapes employing cell transplantation on synthetic biodegradable polymers. Plast Reconstr Surg. 1994;94:233–237. discussion 238-240. [PubMed] [Google Scholar]
- 8.Chung C, Mesa J, Randolph MA, Yaremchuk M, Burdick JA. Influence of gel properties on neocartilage formation by auricular chondrocytes photoencapsulated in hyaluronic acid networks. J Biomed Mater Res A. 2006;77:518–525. doi: 10.1002/jbm.a.30660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamada N, Okano T, Sakai H, Karikusa F, Sawasaki Y, Sakurai Y. Thermo-responsive polymeric surfaces; control of attachment and detachment of cultured cells. Makromol Chem Rapid Commun. 1990;11:571–576. [Google Scholar]
- 10.Kwon OH, Kikuchi A, Yamato M, Sakurai Y, Okano T. Rapid cell sheet detachment from poly (N-isopropylacrylamide)-grafted porous cell culture membranes. J Biomed Mater Res. 2000;50:82–89. doi: 10.1002/(sici)1097-4636(200004)50:1<82::aid-jbm12>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 11.Gillogly SD, Voight M, Blackburn T. Treatment of articular cartilage defects of the knee with autologous chondrocyte implantation. J Orthop Sports Phys Ther. 1998;28:241–251. doi: 10.2519/jospt.1998.28.4.241. [DOI] [PubMed] [Google Scholar]
- 12.Shimizu T, Yamato M, Kikuchi A, Okano T. Two-dimensional manipulation of cardiac myocyte sheets utilizing temperature-responsiveculture dishes augments the pulsatile amplitude. Tissue Eng. 2001;7:141–151. doi: 10.1089/107632701300062732. [DOI] [PubMed] [Google Scholar]
- 13.Kanai N, Yamato M, Okano T. Cell sheets engineering for esophageal regenerative medicine. Ann Transl Med. 2014;2:28. doi: 10.3978/j.issn.2305-5839.2014.03.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Viravaidya-Pasuwat K, Wong-in S, Sakulaue P, Siriwatwechakul W. Construction of a chondrocyte cell sheet using temperature-responsive poly (N-isopropylacrylamide)-co-acrylamide. Conf Proc IEEE Eng Med Biol Soc. 2013;2013:6969–6972. doi: 10.1109/EMBC.2013.6611161. [DOI] [PubMed] [Google Scholar]
- 15.Sato M, Yamato M, Hamahashi K, Okano T, Mochida J. Articular cartilage regeneration using cell sheet technology. Anat Rec (Hoboken) 2014;297:36–43. doi: 10.1002/ar.22829. [DOI] [PubMed] [Google Scholar]
- 16.Kaneshiro N, Sato M, Ishihara M, Mitani G, Sakai H, Kikuchi T, Mochida J. Cultured articular chondrocytes sheets for partial thickness cartilage defects utilizing temperature-responsive culture dishes. Eur Cell Mater. 2007;13:87–92. doi: 10.22203/ecm.v013a09. [DOI] [PubMed] [Google Scholar]


